Standardization of Transfer across Labs between Flow Cytometers for Detection of Lymphocytes in Japanese Encephalitis Vaccinated Children
Summary
In this study, a method was developed to facilitate the transfer of experimental settings and analysis templates between two flow cytometers in two laboratories for the detection of lymphocytes in Japanese encephalitis-vaccinated children. The standardization method for the flow cytometer experiments will allow research projects to be effectively conducted in multiple centers.
Abstract
An increasing number of laboratories need to collect data from multiple flow cytometers, especially for research projects performed across multiple centers. The challenges of using two flow cytometers in different labs include the lack of standardized materials, software compatibility issues, inconsistencies in instrument setup, and the use of different configurations for different flow cytometers. To establish a standardized flow cytometry experiment to achieve the consistency and comparability of experimental results across multiple centers, a rapid and feasible standardization method was established to transfer parameters across different flow cytometers.
The methods developed in this study allowed the transfer of experimental settings and analysis templates between two flow cytometers in different laboratories for the detection of lymphocytes in Japanese encephalitis (JE)-vaccinated children. A consistent fluorescence intensity was obtained between the two cytometers using fluorescence standard beads to establish the cytometer settings. Comparable results were obtained in two laboratories with different types of instruments. Using this method, we can standardize analysis for evaluating the immune function of JE-vaccinated children in different laboratories with different instruments, diminish the differences in data and results among flow cytometers in multiple centers, and provide a feasible approach for the mutual accreditation of laboratory results. The standardization method of flow cytometer experiments will ensure the effective performance of research projects across multiple centers.
Introduction
The standardization of flow cytometry is useful for the comparability of results obtained from different cytometers across different laboratories and study centers, and conducive to the mutual recognition of results to improve work efficiency. An increasing number of scenarios require standardization. During the drug development process, flow cytometry standardization is important, as a developed and validated assay will support the whole drug development process from preclinical to clinical analysis. Flow cytometric methods are frequently transferred between the pharmaceutical industry and collaborating laboratories1. Moreover, it is essential to obtain comparable data from multicentric clinical studies. For example, a standardization workflow was developed in the Systemic Autoimmune Diseases Multicenter Clinical Research Project to obtain comparable data from multicentric flow cytometry2.
The standardization of flow cytometry methods is challenging. The challenges experienced across labs are attributed to the lack of standardized materials, software compatibility issues, inconsistencies in instrument setup, and the use of different configurations among different flow cytometers and divergent gating strategies between the centers3,4. Therefore, it is important to conduct a gap analysis between laboratories. Sample access, quality systems, personnel qualifications, and instrument configuration must be reviewed to ensure that the requirements are met.
At present, children vaccinated with the Japanese encephalitis (JE) vaccine have a significantly reduced incidence of JE5. Monitoring peripheral blood immune cells can help understand the changes in cell-mediated adaptive immunity after vaccination, and the correlation between the changes in peripheral blood lymphocyte subsets and the effects of vaccination. Due to the limited stability of whole blood samples, evaluations of vaccine efficacy are often performed in multiple centers. For this analysis, we defined naïve CD8+ or CD4+ T cells as CD27+ CD45RA+, central memory T cells (TCM) as CD27+ CD45RA–, effector memory T cells (TEM) as CD27– CD45RA–, and terminally differentiated effector memory T cells (TEMRA) as CD27– CD45RA+. CD19+ B cells can be separated into populations that express CD27 versus IgD6,7, naïve B cells express CD27n memory B cells (mBCs) can be identified based on the expression of IgD6, and regulatory T cells (Tregs) can be identified as CD4+CD25++CD127low 8. To establish a standardized flow cytometry experiment to achieve the consistency and comparability of experimental results in multiple centers, a rapid and feasible standardization method was established to facilitate the transfer of protocols across different flow cytometers for the detection of lymphocytes in the whole blood of JE-vaccinated children. Six healthy children (2 years old) were recruited from Beijing Children's Hospital, Capital Medical University. After receiving a prime and boost vaccination with a live-attenuated JE SA14-14-2 vaccine less than 6 months prior, peripheral blood samples were collected from the volunteers. Highly comparable data were obtained from different instruments following standardized procedures, which is helpful for multicenter assessments.
Protocol
The study was approved by the Ethics Committee of Beijing Children's Hospital, Capital Medical University (Approval Number: 2020-k-85). Informed consent of human subjects was waived as only residual samples after clinical testing were used in this study. Two labs are involved in this study. The transferring lab is where the standardized method was developed using one flow cytometer. The cytometer in this lab is hereinafter referred to as cytometer A. The test method lab is the laboratory that receives methods using another flow cytometer, and the cytometer in this lab is hereinafter referred to as cytometer B.
1. Peripheral blood sample collection and cell preparation
- Collect peripheral blood samples (2 mL) from JE-vaccinated children and unvaccinated children in EDTA-K2-anticoagulated tubes by standard venipuncture.
- Mix the whole blood sample by turning the tubes upside down 10x. Add 100 µL of whole blood to a 12 mm x 75 mm tube. Make each sample in duplicate.
- Add 10 µL of brilliant stain buffer (BV buffer) to a 12 mm x 75 mm tube. Add 5 µL of each antibody (CD45, CD3, CD4, CD8, CD127, CD27, CD19, IgD; dilution factor: 1:20) and 20 µL of antibody (CD25, CD45RA; dilution factor: 1:5) to the BV buffer. Vortex gently.
NOTE: The volumes and information of the antibody reagents are shown in Table 1. - Add the antibody cocktail into the tube with the whole blood sample. Vortex gently and incubate at room temperature for 15 min in the dark.
- Dilute the 10x lysing solution 1:10 with distilled water to prepare 1x lysing solution. Lyse the blood samples by adding 2 mL of 1x lysing solution. Vortex gently and incubate for 10 min in the dark at room temperature.
- Centrifuge at 300 × g for 5 min and decant the supernatant.
- Resuspend the pellet in 2 mL of 1x PBS, centrifuge at 300 × g for 5 min, and decant the supernatant.
- Resuspend the pellet in 0.5 mL of 1x PBS and mix thoroughly. Store at 2 °C to 8 °C. Send the samples to the two labs for flow cytometric analysis.
NOTE: The negative control sample and cell single-stained control must be processed simultaneously.
2. Preparing compensation beads and single-stained control
- Label V500-anti-CD45 single-stained, APC-H7-anti-CD3 single-stained, FITC-anti-CD4 single-stained, R718-anti-CD8 single-stained, BV605-anti-CD27 single-stained, APC-anti-CD45RA single-stained, PE-anti-CD25 single-stained, BV421-anti-CD127 single-stained, BB700-anti-CD19 single-stained, and PE-CY7-anti-IgD single-stained tubes.
- Add 100 µL of 1x Dulbecco's phosphate-buffered saline (DPBS) buffer containing 1% fetal bovine serum (FBS) to a 5 mL round-bottom polystyrene test tube.
- Add 50 µL of the negative beads and 50 µL of the anti-mouse Ig, κ beads to each tube and vortex.
- Add 2 µL of each labeled antibody (CD45, CD3, CD4, CD8, CD25, CD127, CD45RA, CD27, CD19, IgD) to a single-stained tube. Vortex the mixture gently and incubate for 15 min in the dark at room temperature (20 °C to 25 °C).
- Resuspend the beads in 2 mL of 1x PBS, centrifuge at 300 × g for 5 min, and decant the supernatant.
- Resuspend the beads in 0.5 mL of 1x PBS and mix thoroughly. Store at 2 °C to 8 °C.
3. Using identical configuration names on the different cytometers in two labs
- Create a new configuration in the software of cytometer A. Click the Cytometer button and choose view Configurations to display the configuration window.
- In the configuration list, right-click the base configurations folder, choose New Folder, and rename it.
- Right-click the base configuration and choose copy.
- Right-click the new folder and paste the base configuration to create a new configuration. Rename the new configuration "Standardized Method Transfer".
- Drag the parameter name, such as FITC, from the Parameters list onto the appropriate detector (530/30).
- Edit additional parameters (PE, BB700, PE-CY7, APC, R718, APC-H7, BV421, V500, and BV605) to the new configuration.
- Follow this method to create a new configuration on a different flow cytometer model using the same name "Standardized Method Transfer". Ensure that the configuration includes the same parameters for each detector.
NOTE: To successfully identify the experiment template on the two different flow cytometer models, make sure that the name is consistent. - Under the cytometer configurations, use cytometer setup and tracking (CST) beads to define the cytometer baseline and track cytometer performance. Click on the Cytometer button and choose CST to display the cytometer setup and tracking workspace.
- Add three drops (150 µL) of setup beads to 0.5 mL of 1x PBS in a 5 mL round-bottom polystyrene test tube. Place the beads tube on the cytometer.
- In the Setup Control window, choose Define Baseline, and click Run.
4. Standardizing experiment using cytometer A in the transferring lab
- Run a performance check using the cytometer setup and tracking beads to verify that the cytometer is performing well.
- In the software browser window, right-click the cytometer settings and choose Application Settings to create a worksheet.
- Using an unstained sample, adjust the photomultiplier tube (PMT) voltages of FSC, SSC, and all fluorescence parameters. FSC: 260; SSC: 460; FITC: 490; PE: 575; BB700: 620; PE-CY7: 640; APC: 600; R718: 620; APC-H7: 620; BV421: 466; V500: 420; and BV605: 505.
- Right-click the experiment cytometer settings and choose application settings to save it.
- Click on the experiment button and choose compensation setup menu. Then, choose Create compensation controls to automatically add compensation controls.
- Record data for all compensation control beads.
- Click on the experiment and choose compensation setup. Then, calculate the compensation automatically.
- Record data for cell single-stained controls.
- Use CD45RA APC to modify the compensation value of R718 to 15% APC. Use CD19 BB700 to modify the compensation value of APC-H7 to 14% BB700. Use CD8 R718 to modify the compensation value of APC-H7 to 60% R718.
- Record data for the CST beads.
- Create a global worksheet of the target value template for the CST bright beads.
- Using an FSC/SSC plot, draw the polygon gate for the CST bright beads. Obtain histogram plots of 10 fluorescence channels for the CST bright beads: FITC, PE, BB700, PE-Cy7, APC, R718, APC-H7, BV421, V500, and BV605. Create interval gates in the histogram plots. Create the statistics view by showing the median of the interval gates.
- Run the samples on the flow cytometer; collect a total of 25,000 lymphocytes.
- Create a global worksheet of the analysis template for the samples.
- Use the following sample gating strategy:
- Using a CD45/side scatter-area (SSC-A) dot plot, draw the polygon gate to identify the intact lymphocyte population while excluding debris.
- Using a CD3/CD19 dot plot, draw a rectangular gate to select CD3+ T cells and CD19+ B cells.
- Using a CD4/CD8 dot plot, draw a quad gate to select CD4+ or CD8+ T cells (cells with a high fluorescence for these markers, respectively).
- Using a CD45RA/CD27 dot plot, draw a quad gate to subdivide CD8+ or CD4+ T cells into naïve (CD27+CD45RA+), TCM (CD27+ CD45RA–), TEM (CD27–CD45RA–), and TEMRA (CD27–CD45RA+) cells.
- Using a CD25/CD127 dot plot, draw a quad gate to subdivide CD4+ T cells into Treg cells (CD4+CD25++CD127low).
- Using a CD27/IgD dot plot, draw a quad gate to subdivide CD19+ B cells into naïve B cells (CD27–IgD+) and memory B cells (CD27+IgD–).
- Save the experimental template on cytometer A.
- Use the CD-ROM to export the experimental template.
5. Transferring the experimental template to cytometer B in the test method lab
NOTE: The experimental template includes instrument settings, the analysis template, and the target value template of median fluorescence intensity (MFI).
- Conduct a performance check using CST beads to verify that the cytometer is performing well.
- Import the experimental template and create an experiment for cytometer B using the template.
- Use the same lot of CST beads to adjust the fluorescent parameter voltages for each fluorescence channel to match the previous MFI instrument.
NOTE: The MFI values vary within ±5% (the MFI of bright beads before and after transfer are shown in Table 2). - Adjust the voltage for FSC using the unstained sample, if needed.
- Right-click on the experimental cytometer settings and choose application settings to save the application settings.
- Use CD45RA APC to modify the compensation value of R718 to 15% APC. Use CD19 BB700 to modify the compensation value of APC-H7 to 24% BB700. Use CD8 R718 to modify the compensation value of APC-H7 to 60% R718.
- Run the samples on the flow cytometer; collect a total of 25,000 lymphocytes.
6. Consistency between the experimental results obtained on the two cytometers
- Assess the differences in lymphocyte subsets between instruments using one-way ANOVA (p < 0.05).
Representative Results
Figure 1 shows a global worksheet of the target value template for the CST bright beads. Using an FSC/SSC plot, a polygon gate is drawn to select the CST bright beads. Histogram plots of 10 fluorescence channels were obtained for the CST bright beads: FITC, PE, BB700, PE-Cy7, APC, R718, APC-H7, BV421, V500, and BV605. The target value for each parameter is displayed by showing the median within the histogram gates in Table 2. The screenshots of templates and parameter settings in the software of cytometer A and cytometer B are shown in Supplemental Figure S1.
Figure 2 shows the dot plots of one sample between the two instruments after instrument standardization. The data demonstrate consistency between different instruments using an automatic analysis template. The results from six samples were compared across two different instrument models in Table 3. No significant differences in the percentages of 15 lymphoid subsets were measured in the two laboratories. These results show that highly comparable data were obtained following the standardized method. The percentages of 15 lymphoid subsets from different instruments are shown in Supplemental Table S1.
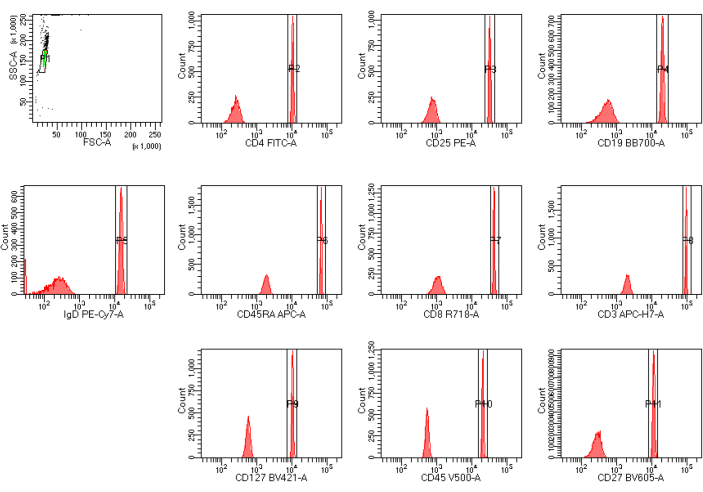
Figure 1: Global worksheet template for the CST bright beads. CST bright beads are gated in the FSC versus SSC plot. Histogram plots of 10 fluorescence channels are presented for the CST bright beads. Interval gates are created to include the bright bead population of CST beads for each fluorescence detector. Abbreviations: FSC-A = forward scattering area; SSC-A = side scattering area. Please click here to view a larger version of this figure.
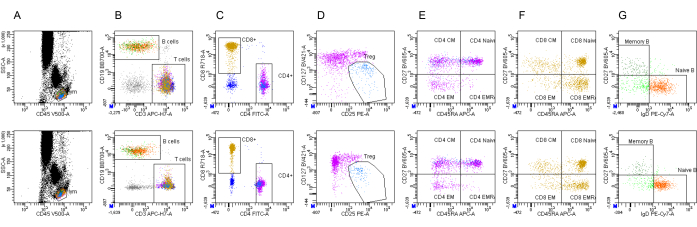
Figure 2: Comparison of dot plots from one sample between the two instruments. (A) CD45/SSC-A dot plot is used to gate lymphocytes. (B) CD3/CD19 dot plot is used to gate CD3+ T cells and CD19+ B cells. (C) CD4/CD8 dot plot is used to identify CD3+ CD8+ T and CD3+ CD4+ T cells. (D) CD25/CD127 dot plot is used to gate Treg cells (CD4+CD25++CD127low). (E) CD45RA/CD27 dot plot for CD4+ T cells is used to gate naïve (CD27+CD45RA+), TCM (CD27+ CD45RA–), TEM (CD27– CD45RA–), and TEMRA (CD27–CD45RA+). (F) CD45RA/CD27 dot plot for CD8+ T cells is used to gate naïve (CD27+CD45RA+), TCM (CD27+ CD45RA–), TEM (CD27– CD45RA–), and TEMRA (CD27–CD45RA+). (G) CD27/IgD dot plot is used to gate naïve B cells (CD27– IgD+) and memory B cells (CD27+ IgD–). The gating strategy is from Zhang et al.7. Abbreviations: SSC-A = side scattering area; TCM = central memory T cells; TEM = effector memory T cells; TEMRA = terminally differential effector memory T cells. Please click here to view a larger version of this figure.
| Antibody Target | Fluorochrome | Clone | Catalog No | Volume Per Test (μL) |
| CD4 | FITC | SK3 | 566320 | 5 |
| CD25 | PE | M-A251 | 555432 | 20 |
| CD19 | BB700 | HIB19 | 745907 | 5 |
| IgD | PE-CY7 | IA6-2 | 561314 | 5 |
| CD45RA | APC | HI100 | 550855 | 20 |
| CD8 | R718 | G42-8 | 751953 | 5 |
| CD3 | APC-H7 | SK7 | 560176 | 5 |
| CD127 | BV421 | HIL-7R-M21 | 562436 | 5 |
| CD45 | V500 | HI30 | 560777 | 5 |
| CD27 | BV605 | L128 | 562656 | 5 |
Table 1: Antibody volumes and conjugated fluorophore information for flow cytometric analyses. Abbreviations: BV = brilliant violet; BB = brilliant blue; FITC = fluorescein isothiocyanate; PE = phycoerythrin; APC = allophycocyanin.
| MFI of bright beads | |||
| Channel | Cytometer A | Cytometer B | %Diff in MFI |
| FSC | 22,133 | 22,689 | 2.51% |
| SSC | 1,51,361 | 1,51,261 | -0.07% |
| FITC | 9,956 | 9,964 | 0.08% |
| PE | 30,877 | 31,264 | 1.25% |
| BB700 | 19,265 | 19,440 | 0.91% |
| PE-CY7 | 14,406 | 14,375 | -0.22% |
| APC | 64,494 | 64,340 | -0.24% |
| R718 | 41,271 | 41,456 | 0.45% |
| APC-H7 | 90,666 | 90,837 | 0.19% |
| BV421 | 9,882 | 9,776 | -1.07% |
| V500 | 19,680 | 19,425 | -1.30% |
| BV605 | 10,794 | 10,356 | -4.06% |
Table 2: MFI of bright beads before and after transfer. Abbreviations: MFI = mean fluorescence intensity; BV = brilliant violet; BB = brilliant blue; FITC = fluorescein isothiocyanate; PE = phycoerythrin; APC = allophycocyanin.
| Average of Percentages | Average of Percentages | ||
| Cytometer A (n=6) | Cytometer B (n=6) | P value | |
| T cells | 71.30 | 74.73 | 0.19 |
| CD4+ T cells | 48.63 | 47.75 | 0.82 |
| CD4+ TCM | 30.68 | 28.20 | 0.06 |
| CD4+ naïve | 57.60 | 58.25 | 0.90 |
| CD4+ TEM | 8.98 | 10.52 | 0.51 |
| CD4+ TEMRA | 2.73 | 3.07 | 0.81 |
| Treg | 9.07 | 8.57 | 0.74 |
| CD8+ T cells | 40.63 | 40.45 | 0.98 |
| CD8+ TCM | 16.83 | 14.78 | 0.75 |
| CD8+ naïve | 49.08 | 47.70 | 0.80 |
| CD8+ TEM | 6.35 | 8.20 | 0.51 |
| D4+ TEMRA | 27.75 | 29.30 | 0.77 |
| B cells | 13.88 | 13.20 | 0.84 |
| naïve B | 67.88 | 70.38 | 0.84 |
| Memory B | 11.88 | 11.80 | 0.95 |
Table 3: Assessment of differences in lymphocyte subsets between instruments. p > 0.05 indicates no significant difference. Abbreviations: TCM = central memory T cells; TEM = effector memory T cells; TEMRA = terminally differentiated effector memory T cells.
Supplemental Table S1: Percentages oflymphocyte subsets in six samplesfrom different instruments. Please click here to download this File.
Supplemental Figure S1: The screenshots of templates and parameter settings in the software of cytometer A and cytometer B. (A) The XML file of templates. (B) The screenshots of templates. (C) The parameter settings in cytometer A. (D) The parameter settings in cytometer B. Please click here to download this File.
Discussion
Immunophenotyping of peripheral blood lymphocyte subsets can help understand the changes in cell-mediated adaptive immunity after vaccination in children. In clinical applications, unexpected situations occur, such as a failure to detect samples in a timely manner or the replacement of a flow cytometer; therefore, rapid standardized methods that facilitate transfers between flow cytometers in different labs are needed9,10,11. Here, we describe a rapid standardized method to ensure consistent results between different instruments. The standardized method was used to monitor immune cells in the peripheral blood of children after vaccination, and consistent results were obtained in two laboratories. The standardized method was established between BD Canto and LSRFortessa. The protocol is also suitable for FACSCelesta, FACSymphony A5, and FACSAria II. Compared with other standardization methods2,3, this standard method does not require the use of third-party software to obtain the consistency of data. It only uses the characteristics of Diva software to create a template module containing target value position and automatic analysis to realize the method of transfer between different instruments, and reduces the requirement of data analysis. The method saves time, is easy to operate, and does not require the development of an automated gating tool.
Standardization of a flow cytometry protocol in this study is achieved by creating an experimental template on one instrument that includes cytometry settings, automatic data analysis, and target position worksheet. Then, the other instruments use CST beads to adjust the parameter voltages to reach the target value. Several important features should be considered to complete successful standardization across different flow cytometer instruments. First, the standardized method requires the instruments to use the same software system, so that the XML file of the experiment template can be shared between the software of the instruments. Second, the two instruments should have the same name for the configuration, and the parameters should also be named identically. Third, the CST beads should be collected under the specified voltage conditions without compensation as target values. Given the different laser powers and PMT sensitivities of different instruments, it is necessary to use CST beads from the same lot number to adjust the voltage parameters, according to the target values on other instruments, and ensure that the MFI value is within ±5%. Fourth, daily quality control assessments of instruments are also very important. If one instrument fails the assessment, the flow cell must be cleaned for bubbles or subjected to laser calibration. Lastly, to reduce differences caused by preparation procedures, the samples should be prepared in an identical manner when such large multi-center studies are conducted, in addition to transferring settings to be able to analyze the samples in different labs. However, there is still room for panel optimization for better identification of memory B cells in this protocol. The frequency of memory B cells is relatively low in 2-year-old children, and CD27 expression on the surface of these cells is diffuse. This may better identify memory B cells if the marker is replaced with a brighter fluorescein than BV605.
However, some limitations should be noted. First, two flow cytometers were used for standardization in this experiment; additional instrument models should be included in subsequent experiments. Second, the cell surface staining method was used to distinguish and determine lymphocyte subsets and did not involve the detection of more intracellular cytokines. Third, we did not compare the experimental results with and without the standardization process of two flow cytometers.
The advantage of this standardized method is that different instruments can share the XML file of the experiment template if the software is the same. The experimental template contains the fixed target values template, and data can be obtained only after adjusting PMT voltages to the target values between instruments. In addition, the experimental template also contains automatic gate strategy analysis. Once the data are obtained, the automatic analysis report is generated. This procedure is easy to operate and reduces the differences in results caused by manual analysis. This standardized method can be used to assess changes in lymphocyte subsets in the whole blood of JE-vaccinated children using different instruments across labs, achieving the goal to ensure that the study project is performed consistently across multiple centers. In this study, we aim to test the convertibility of the standardized method by surface staining, and its success will lay a foundation for the practice of later intracellular staining.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
RW was supported by Beijing Natural Science Foundation, China (No. 7222059), National Natural Science Foundation of China (No. 82002130), XZ was supported by the CAMS Innovation Fund for Medical Sciences (No. 2019-I2M-5-026).
Materials
| BD CompBeads Anti-Mouse Ig, κ/Negative Control Compensation Particles Set | BD | 552843 | compensation |
| BD FACSCanto | BD | FACSCanto | flow Cytometry A in the transferring lab |
| BD FACSDiva CS&T Research Beads | BD | 655051 | define flow cytometer baseline and track cytometer performance |
| BD Horizon BV421 Mouse Anti-Human CD127 | BD | 562436 | Fluorescent antibody |
| BD Horizon BV605 Mouse Anti-Human CD27 | BD | 562656 | Fluorescent antibody |
| BD Horizon V500 Mouse Anti-Human CD45 | BD | 560777 | Fluorescent antibody |
| BD LSRFortessa | BD | LSRFortessa | flow Cytometry B in the test method lab |
| BD OptiBuild BB700 Mouse Anti-Human CD19 | BD | 745907 | Fluorescent antibody |
| BD OptiBuild R718 Mouse Anti-Human CD8 | BD | 751953 | Fluorescent antibody |
| BD Pharmingen APC Mouse Anti-Human CD45RA | BD | 550855 | Fluorescent antibody |
| BD Pharmingen APC-H7 Mouse Anti-Human CD3 | BD | 560176 | Fluorescent antibody |
| BD Pharmingen FITC Mouse Anti-Human CD4 | BD | 566320 | Fluorescent antibody |
| BD Pharmingen PE Mouse Anti-Human CD25 | BD | 555432 | Fluorescent antibody |
| BD Pharmingen PE-Cy7 Mouse Anti-Human IgD | BD | 561314 | Fluorescent antibody |
| Brilliant Staining Buffer Plus | BD | 566385 | Staining Buffer |
| Centrifuge | Eppendorf | 5810 | Cell centrifugation |
| Centrifuge Tube | BD Falcon | BD-35209715 | 15 mL centrifuge tube |
| CS&T IVD Beads | BD | 662414 | standard beads to setup cytometer settings in different flow cytometer |
| Lysing Solution 10x Concentrate | BD | 349202 | lysing red blood cells |
| Phosphate-buffered Saline (PBS) | Gibco | 10010-023 | PBS |
| Round-bottom test tube | BD Falcon | 352235 | 5 mL test tube |
Riferimenti
- Cabanski, M., et al. Flow cytometric method transfer: Recommendations for best practice. Cytometry Part B. Clinical Cytometry. 100 (1), 52-62 (2021).
- Le Lann, L., et al. Standardization procedure for flow cytometry data harmonization in prospective multicenter studies. Scientific Reports. 10 (1), 11567 (2020).
- Linskens, E., et al. Improved standardization of flow cytometry diagnostic screening of primary immunodeficiency by software-based automated gating. Frontiers in Immunology. 11, 584646 (2020).
- Glier, H., et al. Standardization of 8-color flow cytometry across different flow cytometer instruments: A feasibility study in clinical laboratories in Switzerland. Journal of Immunological Methods. 475, 112348 (2019).
- Wang, R., et al. The epidemiology and disease burden of children hospitalized for viral infections within the family Flaviviridae in China: A national cross-sectional study. PLOS Neglected Tropical Diseases. 16 (7), e0010562 (2022).
- Ding, Y., et al. Reference values for peripheral blood lymphocyte subsets of healthy children in China. The Journal of Allergy and Clinical Immunology. 142 (3), 970-973 (2018).
- Zhang, L., et al. Detection of polyfunctional T cells in children vaccinated with Japanese encephalitis vaccine via the flow cytometry technique. Journal of Visualized Experiments. (187), e64671 (2022).
- Yu, N., et al. CD4(+) CD25 (+) CD127 (low/-) T cells: a more specific Treg population in human peripheral blood. Inflammation. 35 (6), 1773-1180 (2012).
- Kalina, T. Reproducibility of flow cytometry through standardization: opportunities and challenges. Cytometry Part A. 97 (2), 137-147 (2020).
- Sommer, U., et al. Implementation of highly sophisticated flow cytometry assays in multicenter clinical studies: considerations and guidance. Bioanalysis. 7 (10), 1299-1311 (2015).
- Glier, H., et al. Comments on EuroFlow standard operating procedures for instrument setup and compensation for BD FACS Canto II, Navios and BD FACS Lyric instruments. Journal of Immunological Methods. 475, 112680 (2019).

