An Experiment Using Functional Near-Infrared Spectroscopy and Robot-Assisted Multi-Joint Pointing Movements of the Lower Limb
Summary
It is estimated that 1 in 6 individuals worldwide will have a stroke in their lifetime, causing long-term disability, whose rehabilitation mechanisms are still poorly understood. This study proposes a protocol to evaluate brain activation by functional near-infrared spectroscopy (fNIRS) during a lower limb robotic therapy session.
Abstract
Stroke affects approximately 17 million individuals worldwide each year and is a leading cause of long-term disability. Robotic therapy has shown promise in helping stroke patients regain lost motor functions. One potential avenue for increasing the understanding of how motor recovery occurs is to study brain activation during the movements that are targeted by therapy in healthy individuals. Functional Near-Infrared Spectroscopy (fNIRS) has emerged as a promising neuroimaging technique for examining neural underpinnings of motor function. This study aimed to investigate fNIRS neural correlates of complex lower limb movements in healthy subjects. Participants were asked to perform cycles of rest and movement for 6 min using a robotic device for motor rehabilitation. The task required coordinated knee and ankle joint movements to point to targets displayed on a computer screen. Two experimental conditions with different levels of movement assistance provided by the robot were explored. The results showed that the fNIRS protocol effectively detected brain regions associated with motor control during the task. Notably, all subjects exhibited greater activation in the contralateral premotor area during the no-assistance condition compared to the assisted condition. In conclusion, fNIRS appears to be a valuable approach for detecting changes in oxyhemoglobin concentration associated with multi-joint pointing movements of the lower limb. This research might contribute to the understanding of stroke motor recovery mechanisms and might pave the way for improved rehabilitation treatments for stroke patients. However, further research is needed to fully elucidate the potential of fNIRS in studying motor function and its applications in clinical settings.
Introduction
Epidemiological data indicates that worldwide there are ~17 million new cases of stroke each year, with an increase in incidence in low- and middle-income countries1. The number of new cases is estimated to increase to 77 million by 20302. Motor impairment due to stroke often affects patient's mobility and participation in daily life activities, contributing to a low quality of life. Traditional motor rehabilitation includes manual therapy, but over the past few decades, robotic systems for rehabilitation have been developed. These systems can deliver therapy at high intensity, dose, quantifiability, reliability, repeatability, and flexibility3 and have shown potential as effective rehabilitation treatments for both acute and chronic stroke patients4,5,6. In addition to delivering therapy, robotic systems for rehabilitation can be used as evaluation tools as they can be equipped with sensors that can measure patient movement kinematic/kinetic data7,8. For upper extremity motor rehabilitation, such data has not only been proven to be useful for assessing the level of patient's motor recovery elicited by robotic therapy and served as a supplementary tool to traditional clinical assessments9,10, but it has also contributed to advancing the understanding of the process of motor recovery from stroke11,12 as well as neural control of movement and motor learning in healthy subjects3,13,14. As a result, these findings have provided a foundation for enhancing rehabilitation treatments15.
Over the last two decades, many robotic devices for lower limb neurorehabilitation have been proposed, spanning from exoskeletons that support the patient body weight during walking (e.g., over a treadmill, such as Lokomat16) to stationary robotic systems that allow the patient to exercise the ankle, knee or foot without walking (such as the Rutgers Ankle17, the High-Performance Ankle Rehabilitation Robot18, and the Gwangju Institute of Science and Technology (GIST) ankle/foot rehabilitation robot19) or active foot orthoses that are actuated exoskeletons worn by the patient to walk overground or over a treadmill (such as the Powered Gait Orthosis20 and the MIT Anklebot21). See22,23,4 for a review on robots for lower limb rehabilitation.
Results of clinical studies of robotic devices for lower limb rehabilitation on stroke patients have been encouraging and have shown that these systems may improve joints' Range of Motion (ROM), muscle strength, or gait, depending on the specific device and clinical protocol (see 24,25 for a review on the efficacy of lower limb robots for rehabilitation). While it has been postulated that robot-assisted therapy promotes neuroplastic changes, which ultimately result in improved motor abilities26, how the process of motor recovery from stroke exactly occurs and which robotic training protocols optimize the process of recovery of lower limb motor abilities, remain mostly unclear. In fact, there is a significant, growing disparity between the increasing development of rehabilitation robots (either by academic researchers or commercial entities) and the limited understanding of the neurophysiological mechanisms that underlie motor recovery4. Measurements of movement kinematics or joint torques taken with embedded sensors have contributed to quantitatively describing motor behavioral changes that occur as patients recover lower limb motor abilities27,28,29, partially filling this gap. However, the neural correlates underlying such changes have been less investigated. This is due to several reasons.
Brain functional imaging is time-consuming and sometimes difficult to complete in the context of clinical trials, which often require keeping patient burden minimal to maximize the likelihood of patient adherence to the study. This holds particularly true for individuals who have suffered a stroke, given the fact that post-stroke fatigue and muscle weakness are frequently observed30. Also, imaging modalities that are based on magnetic fields, such as functional Magnetic Resonance Imaging (fMRI), require both patient and robotic hardware to be magnet-safe.
Among non-invasive imaging modalities, functional Near-Infrared Spectroscopy (fNIRS) is an imaging technique particularly suitable for assessing areas of brain activation in subjects undergoing robotic therapy. Similarly to fMRI, fNIRS measures blood oxygenation/deoxygenation in the brain. However, unlike fMRI, fNIRS is fully compatible with robotic hardware, and it is often portable, even being usable at the bedside. Also, fNIRS has low cost and less sensitivity to motion artifacts31,32,33.
Despite its clear advantages and widespread use in many clinical settings since its first introduction in the late 70s34, only a few studies have used fNIRS to quantify brain activation associated with lower limb movements and stroke motor recovery. FNIRS studies aimed at elucidating mechanisms of neural control of movement and/or mechanisms or evaluation of motor recovery from stroke have mostly investigated single-joint movements (e.g., dorsiflexion, plantar flexion or knee extension movements35,36,37), walking38,39,40,41,42,43, or cycling44. See45 for a review. Similarly, fNIRS studies on robot-assisted therapy for the lower limb have mostly focused on robot-assisted gait rehabilitation; see46 for a review. A few studies have focused on using fNIRS as part of a Brain-Computer Interface (BCI) system to derive control signals for robotic devices47,48; while this research area also relies on the processing of fNIRS signals, its goal is different and mainly focused on decoding patient intentions (e.g., patients with severe motor disabilities).
The pilot study presented herein is part of an initial effort to investigate the effects of a robotic system for lower limb rehabilitation. The robot can deliver target-oriented lower limb rehabilitation that involves training in everyday multi-joint movements as well as deliver therapy to single joints (e.g., knee or ankle) of the lower limb (i.e., implement a bottom-up rehabilitation program).
The study aimed to investigate the feasibility of an experimental protocol that required the acquisition of fNIRS data during the performance of lower limb, multi-joint pointing movements. The duration of the data acquisition period in this study, which was limited to 6 min, is shorter than typical fNIRS protocols. This was a deliberate choice made with the aim of enhancing the practicality and clinical applicability of this research, particularly in patients with limited mobility or strength. Identifying fNIRS correlates of such complex multi-joint movements and gaining insights into how brain activation was modulated by robot assistance were also points of interest. For this purpose, two sessions of experiments were conducted with the same participants: one with no robot assistance and one with robot assistance. Finally, it is important to remark that this study focused on healthy subjects in order to establish a foundation for future research in terms of recording protocol feasibility and evaluation of brain activation during movements targeted by robotic therapy.
Apparatus
A portable robot designed to deliver lower limb rehabilitation (see Figure 1) was used to conduct our experiments. The robot has a 3D reachable workspace and is compact and light, weighing about 35 lb., which makes it easy to transport and install.
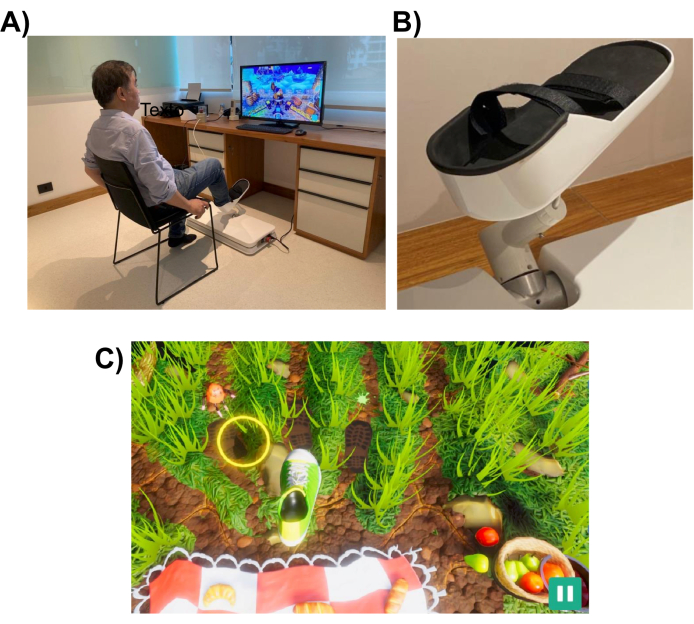
Figure 1: Experimental setup. (A) The robotic system (installed on the floor) designed for the lower limb. A volunteer is shown utilizing the interface with their right foot. (B) Support structure for the subject's foot that enables attachment to the robotic system. (C) A screenshot of the Picnic game. The objective of the game is to move the foot (green and white shoe) to the target (yellow circle). Please click here to view a larger version of this figure.
The robotic system is designed to assist a patient in performing lower limb movements similar to those performed in everyday tasks, such as pointing or kicking. It uses interactive virtual reality games, which are displayed on a computer monitor or a television screen placed in front of the robotic device (see Figure 1). The robot-end effector is attached to the patient's lower limb (e.g., ankle), and its position is mapped to the position of a cursor on the screen. A typical game shows the patient movement targets (e.g., the object to point to or where to kick the ball).
To complete the movement task, the robot may assist the patient with a level of assistance that can range from full assistance to none. The level of robotic assistance is chosen at the beginning of each rehabilitation session based on the patient's level of motor impairment. The movements performed by the subject are used by the game to score the patient's performance and provide them feedback on their performance (e.g., ROM, number of movements, and level of robotic assistance). The games are designed to be interactive and entertaining to sustain patient interest and attention. In this study, participants played the "Picnic game", in which the player had to stop the insects from reaching the towel and stealing the food (see Figure 1, bottom panel, for a screenshot).
Data acquisition was performed with a portable fNIRS acquisition system with two different continuous-wave optodes (760 nm and 850 nm), 8 dual-tip LED sources, and 8 dual-tip active detectors. The signals were acquired using a sampling rate of 10.17 Hz. A laptop was used for the calibration optimization and signal recording using a Wi-Fi network created by the fNIRS system.
A cap was used to hold the optodes in the predetermined locations. The sources and detectors were placed according to the 10-10 international EEG system in a grid spatial distribution. Each fNIRS channel was defined by a source-detector pair with inter-optode distances of approximately 30 mm. The optodes were placed over the supplementary motor, premotor, and motor areas at the locations shown in Figure 2. The total number of channels was 28, where 8 were short-distance channels that were coupled to each source using a fiber optics adapter to a single detector. Given the multiplexing setup of the hardware, it is possible to acquire short-distance information from all sources using only one detector.
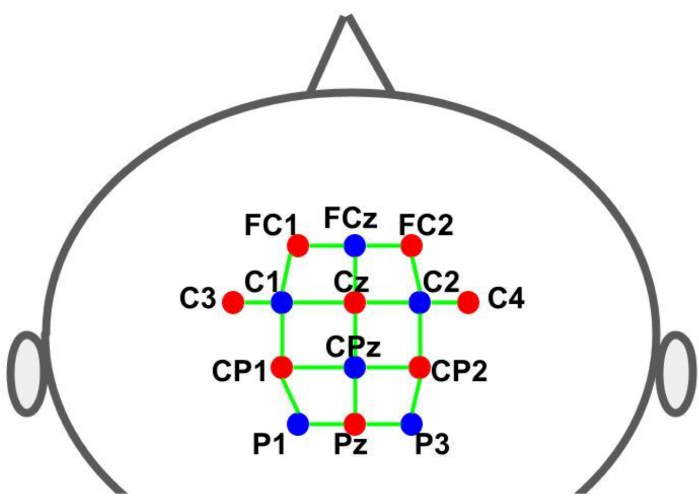
Figure 2: Montage layout using the 10-10 EEG system. The letters and numbers indicate the source/detector locations. The red and blue dots represent the source and detector optodes, respectively. The green lines represent the fNIRS channels which consist of source and detector pairs. Please click here to view a larger version of this figure.
Experimental design
The experiment was conducted under two distinct experimental conditions, differing in the level of assistance provided by the robot for the subject's movements. In the first condition, the robot was programmed not to provide any assistance to the subject's movements, while in the second condition, the robot controlled the subject's foot and leg movements (robot-assisted movement).
Each experiment followed a block design paradigm involving alternating cycles of a motor task (playing the game – 30 s) and resting (30 s), as illustrated in Figure 3. The starting and conclusion of each phase (play/game or rest) were visually signaled to the subject through the computer screen. During the rest phase, a message indicating a pause was displayed. Each cycle (play/game + rest) had a duration of 60 s and was repeated six times, resulting in a total runtime of 360 s (6 min).
The participants played the "Picnic game", wherein the objective was to prevent insects from reaching the towel and stealing food. This game involved a sequence of lower limb movements, starting from a designated home target (initial position) and extending towards one of three outer targets before returning to the home target. On the screen, the outer targets were visually represented as animated moving insects, which the participants had to reach and step onto. There were three outer-reaching targets, each randomly presented an equal number of times, alongside a common home target for every movement. The distance that the foot needed to travel from the home target to the position of the outer targets formed an arc, approximately measuring 26 cm. The motor task required the execution of multi-joint movements, demanding coordination between knee flexion/extension, plantar flexion, and dorsiflexion movements.
The fNIRS data recordings were synchronized with the visual stimuli presented by the game to the subject through a transistor-transistor-logic (TTL) pulse generated by the robot. Pulses were generated at the outset of each phase (play/game and rest). Thus, all timing control was performed by the game, which provided visual cues (targets) to the participant to start each movement, sent TTL signals to the fNIRS system to mark the brain activity recordings, and, if required by the experiment, sent signals to the robot control system to initiate movement assistance.
Protocol
This study was approved by the local Ethics Review Board of the UNICEP (Centro Universitario Paulista). All participants provided informed consent following all institutional guidelines and federal norms regarding scientific research involving humans. They received no financial compensation, as required by Brazilian federal regulations.
1. fNIRS system
- Prepare the cap using 16 optodes: 8 light sources (760 nm and 850 nm) and 8 light detectors (see Figure 2; location of sources: FC1, FC2, C3, Cz, C4, CP1, CP2 and Pz; location of detectors (FCz, C1, C2, CPz, P1 and P3). Attach the eighth detector to the short-distance adaptor, which is connected to each of the source optodes.
- Open the fNIRS acquisition software and load the montage with the placement of each optode.
- Set fNIRS signal temporal sampling rate at 10.17 Hz.
2. Participants
- Briefly explain the research relevance and the experimental protocol to the participant.
- If the participant agrees to volunteer, ensure that the volunteer provides informed consent following all institutional guidelines and federal norms regarding scientific research involving humans.
- Instruct the volunteer to sit on an armchair in front of the robot, positioned approximately 150 cm away from the computer monitor.
- After removing the shoes, comfortably attach the participant's foot to the robot arm with Velcro (hook-and-loop fastener) straps (see Figure 1, top left panel).
- Place the fNIRS cap with the optodes in the participant's head and securely fasten it with the hook-and-loop fastener below the chin.
- Place the overcap onto the optode cap to reduce the interference of ambient light.
3. Data acquisition
- Instruct each participant not to make sudden head movements to minimize the risk of movement artifacts.
- Calibrate the fNIRS system for optimal signal quality.
- Certify that all channels are of good quality, as shown by the interface. If not, try to remove the hair between the scalp and the optode tip.
- Explain to the participant how to play the game by moving the foot attached to the robot to reach the targets indicated on the screen.
- Explain to the participants that there will be two conditions: without and with robotic assistance.
4. Robotic system
- Power up the system by connecting the monitor and the robot to the electric network source. After the operational system is initialized, the first game interface appears.
- Position the robotic arm in the standard location and wait to receive the robotic arm positioned correctly feedback message.
- Enter descriptive data about the participant and experimenter into the system to log in to the robot gaming interface.
- In the game selection interface, configure the session time to 6 min with 30 s rest intervals.
- Select the appropriate leg (right or left) to be used by the participant.
- Select the Picnic Game and click on Go to play the game.
5. Data analysis
- Convert the raw signals to hemodynamic states (oxyhemoglobin (HbO) and deoxyhemoglobin (HbR)) using the modified Beer-Lambert equation49.
- Create individual activation maps using the General Linear Model (GLM) with robust estimation and autocorrelation estimation, which is less affected by motion artifacts50. Use the experimental design (rest = 0; game/play = 1) convoluted by the canonical Hemodynamic Response Function (HRF) as the independent variable and the observed HbO or HbR signal as the dependent variable.
- For each channel, extract the beta coefficient (which reflects the strength of activation of the channel) during the game/play compared to the rest.
- Merge channels in three Regions of Interest (ROI) and average the activation coefficients (GLM betas) across the ROI channels. Define the ROIs as follows (see Figure 2): (1) premotor area for the left hemisphere (channels [FC1-FCz, C1-FC1, C1-Cz, C3-C1]); (2) somatomotor area for the left hemisphere (channels [C3-CP1, CP1-P1, CP1-CPz, P1-Pz]); and (3) supplementary motor area (SMA) (channels [Cz-FCz, CPz-Cz]).
NOTE: Based on previous literature on motor tasks similar to the one investigated herein51,52,53 and given that all participants executed the motor task with their right leg, it was hypothesized that the left (contralateral) premotor and somatomotor, as well as SMA regions, would display activation during game/play performance in both conditions, with stronger activation in the unassisted condition. - Apply the non-parametric Wilcoxon test to these betas. Set the Type I Error at 5% (one-tailed analysis).
Representative Results
All six subjects completed both experiments. In the no-assistance condition, an average of 76.67 trials (std. 10.73) was completed by each subject (note, for each subject, the number of trials depended on the number of successful reaches since a new target was only shown if the previous one was reached). In the assistance condition, where the subject's movement was fully aided by the robot, all subjects completed 70 trials. fNIRS data was successfully recorded from all subjects.
Figure 4 shows the single-subject brain activation maps comparing the play/game vs rest periods for HbO and HbR. For each subject, the maps associated with HbO changes showed statistically significant activation (p < 0.01) in the experimental condition with no robot assistance, while only two subjects showed statistically significant activation in the experimental condition with robot assistance. Regarding HbR, four subjects presented statistically significant activation (p < 0.01) in both conditions, albeit in fewer channels as compared to HbO.
Figure 5 summarizes the individual subjects' results of the ROI-based hypothesis-driven analyses. Among all ROIs examined, only the left premotor cortex displayed a statistically significant increase in HbO activation during the game/play relative to rest comparison (p = 0.046) and only in the condition without robotic assistance. In addition, this ROI presented a greater activation in the condition without robotic assistance in comparison to the condition with robotic assistance (p = 0.016). There were no statistically significant differences for the other ROIs or in HbR-based analyses, either in play/game vs. rest analyses or in comparisons between conditions (p>0.05). Thus, at the group level, no statistically significant differences were found at the left somatomotor cortex, SMA, or in HbR-based analyses.
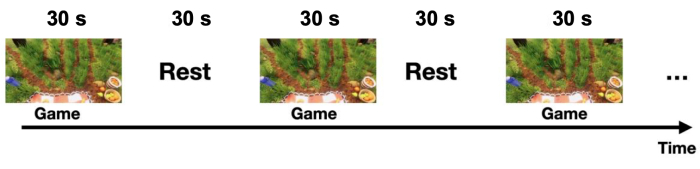
Figure 3: Block design experiment with alternated blocks of game and resting state conditions. The subject played the Picnic game continuously for 30 s (game period) and remained still for the following 30 s (rest period). This cycle was repeated 6 times, resulting in a session duration of 6 min (note, only 150 s are shown in the figure). Please click here to view a larger version of this figure.
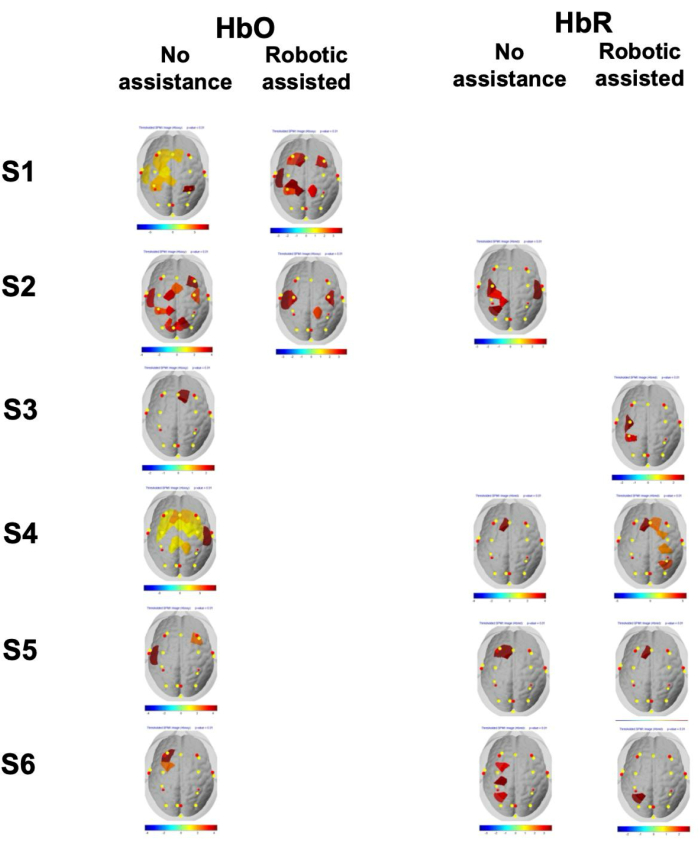
Figure 4: Single-subject brain thresholded statistical activation maps. Single-subject brain thresholded (channel p-value < 0.01) statistical activation maps of the six subjects (S1-S6) for HbO and HbR in both experimental conditions (movements with and without robotic assistance). Note that only maps displaying a statistically significant activation are presented (i.e., any subject and condition with a blank slot indicates that no significant activation was observed for that particular combination). The color bar represents the t-statistics amplitude of the activation coefficient (GLM beta) of each fNIRS channel. Please click here to view a larger version of this figure.
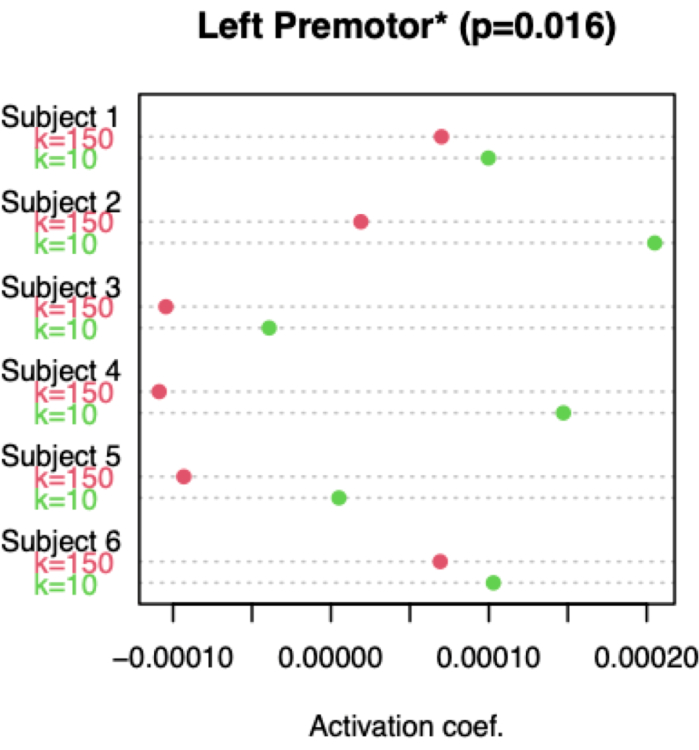
Figure 5: Activation of left premotor cortex based on HbO signals. As detailed in step 5.2, the beta coefficients were estimated using a GLM, and the amplitude of activation was quantified for each subject, experimental condition (unassisted and assisted movements), and specific channels (note, the beta values for the ROI were obtained by averaging the channels within the left premotor cortex). The dot chart visually represents the ROI average beta coefficients for each subject and condition, with the red and green dots indicating the assisted and unassisted movements, respectively. For each subject, the ROI beta coefficient of the unassisted condition is greater than that of the assisted condition, signifying greater activation in the former condition. This result is consistent across all subjects. Please click here to view a larger version of this figure.
Discussion
In this proof-of-concept study, the feasibility of making inferences on brain activation mapping using fNIRS data from healthy subjects while they exercised with different types of movements using a robot for lower limb rehabilitation was investigated. Typical fNIRS recording sessions in adults are longer than 6 min54. However, to make recordings feasible in the context of a rehabilitation setting, the overall duration of the experiment must be minimized to avoid unnecessary fatigue and effort for the subject. This study also tested whether a block design experiment with 6 task repetition blocks (with a session duration of 6 min) was enough to assess hemodynamic response and to detect the difference between the assistance and the no-assistance condition. The findings suggest that these recordings are informative and fNIRS might be indeed a useful method to detect changes in HbO concentration associated with exercise regimes with different levels of robot assistance to lower limbs. Even with a small sample size, a significant contralateral premotor activation as measured by HbO when comparing task vs rest blocks was found; all subjects presented greater activation in this region in the no-assistance condition when compared to the robot-assisted one.
fNIRS data was recorded during two different conditions, corresponding to two different levels of subject's effort needed to complete the motor task. In the first set of experiments, the robot K was set to 10, which corresponded to no robot assistance. In the second set of experiments, K was set to 150; with this value of K the subject's lower limb was almost entirely moved by the robot. As depicted in Figure 4, all subjects displayed statistically significant brain activation in the unassisted condition; when K was set to 150, only two subjects presented significant activation.
As summarized in Figure 5, the results also showed that the changes in HbO concentration associated with unassisted exercises (higher level of effort) were overall higher than the changes associated with assisted exercises. However, a significant difference was only found in the contralateral Premotor Cortex (PMC). This finding is consistent with the results of several previous neuroimaging studies on humans that found increasing neuronal activation with increasing levels of force55,56. Most previous studies, however, investigated the upper limb; the majority focused on static, isometric tasks, while only a few have evaluated dynamic movements57,58,59.
Relatively fewer studies have focused on the lower limb. A few of them have focused on static motor tasks. Alexandre et al.51 investigated fNIRS correlates of maximal and submaximal voluntary contractions (MVC) of knee extensors in healthy and chronic obstructive pulmonary disease subjects. Using fMRI, Yoon et al.52 investigated cortical activation changes during isometric contractions with the ankle dorsiflexor muscles at different MVC levels. Other studies have focused on gait60,61,62. Using fNIRS, Harada et al.60 investigated cortical activation patterns during walking at three intensities in healthy subjects and found that increased locomotor speed and cadence were associated with a higher degree of cortical activation. Bonnal et al.62 investigated cortical activation in healthy subjects during exoskeleton-mediated gait at different levels of physical assistance and also found the greatest cortical activation during the two unassisted conditions (50% and 100%) but only slight differences in cortical activation between the assisted conditions. The results of this study are consistent with the results of these previous studies overall.
The role of cortical areas associated with neural control of lower limb movements is still little understood and less studied compared to upper limb neural control. In the context of rehabilitation especially, such understanding is key for establishing a foundation for identifying neuroplasticity of these areas, which can occur, e.g., as a result of motor disorders of neurological origin, motor exercise, or aging. As shown in Figure 4, all subjects showed activation in the PMC or SMA areas during the unassisted condition. The channel-specific results presented in the figure indicate that although activation was bilateral, it was more prominent in the contralateral hemisphere. Overall, these findings are consistent with the study of Alexandre et al.51 that showed activation of contralateral primary motor (M1) and primary somatosensory (S1), PMC, and prefrontal (PFC) cortical areas during knee extension. In addition, these findings are consistent with the results of the study of Yoon et al.52 that showed activation in the contralateral M1, SMA, putamen, pallidum cingulate cortex, and ipsilateral cerebellum during ankle dorsi-flexion, and with the results of the study of Ciccarelli et al.53 that found activation of contralateral M1 and sensory cortices, as well as PMC and subcortical regions during ankle movements.
Hemibody movements are considered to be controlled by the contralateral hemisphere of the cerebral cortex. However, ipsilateral cortical involvement has been reported for unimanual movements56,63,64, as well as unilateral foot movements (see also65 for a review). Differently from these studies, the analysis did not reveal consistent activation in the somatomotor cortex across all subjects, as illustrated in Figure 4; additionally, the outcomes of the ROI analysis did not indicate significant activation. This finding could be attributed to the relatively small size and deeper location of the somatomotor cortex for lower limbs, as compared to upper limbs, which is situated at the longitudinal sulcus66. Additionally, the fNIRS signal is less sensitive to non-superficial brain areas67.
When comparing the no assistance with the assistance condition, only the contralateral PMC displayed a significantly different activation between the two conditions. This is not surprising given that the motor task was visually guided, and thus likely requiring more attention and motor effort in the no assistance condition. PMC is considered to have a role in executive function, including motor preparation and planning68. Furthermore, recent studies have suggested a PMC role in spatial attention and working memory69. The result of this study is in line with the previous ones that highlighted the role of this area in executive function68 and motor preparation of upper limb70,71,72 and lower limb movements73. This result is also consistent with the results of gait studies involving dual tasks that have shown the correlation between attention required to perform the task and PMC activation74,75.
This study has several limitations. First, the sample size was small. Future investigations should expand upon the current research by increasing the sample size, including a more diverse demographic, such as females and other age groups, to establish normative values for both HbO and HbR (this study included 5 males and 1 female, between 40 and 62 years old). Second, the cap montage was based on the 10-10 EEG system, and ROI analysis used a standard MNI152 brain model. Future studies could use individual structural MRIs, brain navigation, or high-density fNIRS. In addition, recordings were performed in a single session, and thus, the intra-subject variability of fNIRS recordings was not evaluated. Furthermore, the results of the HbO and HbR analyses were different, although both these measurements are associated with the same neurophysiological phenomena (namely, the hemodynamic response function). However, the observed difference aligns with prior literature indicating unequal sensitivity of these two chromophores to other systemic confounders and the lack of agreement on its interpretation34. Finally, since the speed of playing the game in the no assistance condition was different for each subject (because the next trial only started after the current target was successfully achieved), this might have increased the variance of subjects' brain activation.
Based on the results of this feasibility study, we are planning to use a fNIRS experimental protocol similar to the one presented herein in a pilot clinical study currently underway at the Federal University of Sao Carlos, Brazil, which aims at assessing the therapeutic effects of the robot on lower limb motor abilities of chronic stroke patients. The brain activation maps presented herein may potentially offer a reference for brain activation in healthy individuals that could be helpful in studying the effects of the robotic therapy on the lower limb.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The opinions, hypotheses, conclusions, and recommendations of this study are those of the authors and do not necessarily represent the opinions of the funding agency. JRS is grateful to Sao Paulo Research Foundation (FAPESP, grant numbers 2021/05332-8, 2018/04654-9, 2018/21934-5 and 2023/02538-0) and Jackson Cionek for technological support. AMM and Vivax Ltda are grateful to FAPESP (São Paulo Research Foundation) and FINEP (Brazilian Innovation Agency). This project was funded with grants from FAPESP (grant number 2018/09559-4) and FINEP (grant number 2019/09933-6).
Materials
| 32 inch Smart TV | Samsung | N/A | TV connected to robot via HDMI cable |
| 8-detector silicon photodiode (SiPD) optodes for optical detection with dual tip | NIRx Medical Technologies (Glen Head, NY, USA) | https://nirx.net/nirsport | |
| 8-source optodes bundle for optical illumination with dual tip | NIRx Medical Technologies (Glen Head, NY, USA) | https://nirx.net/nirsport | |
| Aurora acquisition software | NIRx Medical Technologies (Glen Head, NY, USA) | https://nirx.net/nirsport | |
| Laptop Precision XPS 13 | Dell Technologies (Round Rock, TX, USA) | ||
| nirsLAB fNIRS Analysis software | NIRx Medical Technologies (Glen Head, NY, USA) | https://nirx.net/nirsport | |
| NIRSports2 fNIRS acquisition system | NIRx Medical Technologies (Glen Head, NY, USA) | https://nirx.net/nirsport | It has two different continuous wave optics (760 and 850 nm), 8 dual-ended LED sources and 8 dual-ended active detectors. |
| R | R-project.org (open source software) | https://www.r-project.org/ | |
| Standard cut cap, black color for up to 128 holders. | Easycap GmbH (Wörthsee, Germany) | https://www.easycap.de/ | |
| Vivax Assistive Rehabilitation Machine (ARM) | Vivax Ltda (São Paulo, Brazil) | https://vivaxbr.com/home/ | It is a portable robot designed to deliver lower limb rehabilitation. It has a 3D reachable workspace and is compact and light, weighing about 35 lb., which makes it easy to transport and to install. |
Riferimenti
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. Neurology. 18 (5), 439-458 (2019).
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. Neurology. 20 (10), 795-820 (2021).
- Huang, V. S., Krakauer, J. W. Robotic neurorehabilitation: a computational motor learning perspective. Journal of NeuroEngineering and Rehabilitation. 6, 5 (2009).
- Hobbs, B., Artemiadis, P. A Review of Robot-Assisted Lower-Limb Stroke Therapy: Unexplored Paths and Future Directions in Gait Rehabilitation. Frontiers in neurorobotics. 14, 19 (2020).
- Bertani, R., Melegari, C., De Cola, M. C., Bramanti, A., Bramanti, P., Calabrò, R. S. Effects of robot-assisted upper limb rehabilitation in stroke patients: a systematic review with meta-analysis. Neurological Sciences. 38 (9), 1561-1569 (2017).
- Warutkar, V., Dadgal, R., Mangulkar, U. R. Use of robotics in gait rehabilitation following stroke: A review. Cureus. 14 (11), e31075 (2022).
- Dipietro, L., et al. Changing motor synergies in chronic stroke. Journal of Neurophysiology. 98 (2), 757-768 (2007).
- Dipietro, L., et al. Learning, not adaptation, characterizes stroke motor recovery: evidence from kinematic changes induced by robot-assisted therapy in trained and untrained task in the same workspace. IEEE Trans Neural Syst Rehabil Eng. 20 (1), 48-57 (2012).
- Bosecker, C., Dipietro, L., Volpe, B., Krebs, H. I. Kinematic robot-based evaluation scales and clinical counterparts to measure upper limb motor performance in patients with chronic stroke. Neurorehabilitation and Neural Repair. 24 (1), 62-69 (2010).
- Krebs, H. I., et al. Robotic measurement of arm movements after stroke establishes biomarkers of motor recovery. Stroke. 45 (1), 200-204 (2014).
- Volpe, B. T., et al. Robotic devices as therapeutic and diagnostic tools for stroke recovery. Archives of Neurology. 66 (9), 1086-1090 (2009).
- Hogan, N., et al. Motions or muscles? Some behavioral factors underlying robotic assistance of motor recovery. Journal of Rehabilitation Research and Development. 43 (5), 605-618 (2006).
- Shadmehr, R., Wise, S. P. . The Computational Neurobiology of Reaching and Pointing: A Foundation for Motor Learning. , (2005).
- Dipietro, L., Poizner, H., Krebs, H. I. Spatiotemporal dynamics of online motor correction processing revealed by high-density electroencephalography. J Cogn Neurosci. 26 (9), 1966-1980 (2014).
- Krebs, H., et al. Rehabilitation robotics: Performance-based progressive robot-assisted therapy. Autonomous Robots. 15, 7-20 (2003).
- Colombo, G., Joerg, M., Schreier, R., Dietz, V. Treadmill training of paraplegic patients using a robotic orthosis. Journal of Rehabilitation Research and Development. 37 (6), 693-700 (2000).
- Girone, M., et al. A Stewart platform-based system for ankle telerehabilitation. Autonomous Robots. 10, 203-212 (2001).
- Saglia, J. A., Tsagarakis, N. G., Dai, J. S., Caldwell, D. G. A high-performance redundantly actuated parallel mechanism for ankle rehabilitation. The International Journal of Robotics Research. 28 (9), 1216-1227 (2009).
- Yoon, J., Ryu, J. A novel reconfigurable ankle/foot rehabilitation robot. , 2290-2295 (2005).
- Ruthenberg, B. J., Wasylewski, N. A., Beard, J. E. An experimental device for investigating the force and power requirements of a powered gait orthosis. Journal of Rehabilitation Research and Development. 34 (2), 203-213 (1997).
- Forrester, L. W., et al. Clinical application of a modular ankle robot for stroke rehabilitation. NeuroRehabilitation. 33 (1), 85-97 (2013).
- Díaz, I., Gil, J. J., Sánchez, E. Lower-limb robotic rehabilitation: Literature review and challenges. Journal of Robotics. 2011, 759764 (2011).
- Zhang, X., Yue, Z., Wang, J. Robotics in lower-limb rehabilitation after stroke. Behavioural Neurology. 2017, 3731802 (2017).
- Zhang, M., Davies, T. C., Xie, S. Effectiveness of robot-assisted therapy on ankle rehabilitation – a systematic review. Journal of NeuroEngineering and Rehabilitation. 10, 30 (2013).
- Lo, K., Stephenson, M., Lockwood, C. Effectiveness of robotic assisted rehabilitation for mobility and functional ability in adult stroke patients: a systematic review protocol. JBI Database of Systematic Reviews and Implementation Reports. 15 (1), 39-48 (2017).
- Belda-Lois, J. M., et al. Rehabilitation of gait after stroke: a review towards a top-down approach. Journal of NeuroEngineering and Rehabilitation. 8, 66 (2011).
- Bortole, M., et al. The H2 robotic exoskeleton for gait rehabilitation after stroke: early findings from a clinical study. Journal of NeuroEngineering and Rehabilitation. 12, 54 (2015).
- Banala, S. K., Kim, S. H., Agrawal, S. K., Scholz, J. P. Robot assisted gait training with active leg exoskeleton (ALEX). IEEE Transactions on Neural Systems and Rehabilitation Engineering. 17 (1), 2-8 (2009).
- Bartenbach, V., Wyss, D., Seuret, D., Riener, R. A lower limb exoskeleton research platform to investigate human-robot interaction. 2015 IEEE International Conference on Rehabilitation Robotics (ICORR). 2015, 600-605 (2015).
- Hinkle, J. L., et al. Poststroke fatigue: Emerging evidence and approaches to management: A scientific statement for healthcare professionals from the American heart association. Stroke. 48 (7), e159-e170 (2017).
- Balardin, J. B., Zimeo Morais, G. A., Furucho, R. A., Trambaiolli, L. R., Sato, J. R. Impact of communicative head movements on the quality of functional near-infrared spectroscopy signals: negligible effects for affirmative and negative gestures and consistent artifacts related to raising eyebrows. Journal of Biomedical Optics. 22 (4), 4601 (2017).
- Nazeer, H., Naseer, N., Mehboob, A., Khan, M. J., Khan, R. A., Khan, U. S., Ayaz, Y. Enhancing classification performance of fNIRS-BCI by identifying cortically active channels using the z-score method. Sensors. 20 (23), 6995 (2020).
- Ayaz, H., et al. Optical imaging and spectroscopy for the study of the human brain: status report. Neurophotonics. 9, S24001 (2022).
- Chen, W. L., et al. Functional near-infrared spectroscopy and its clinical application in the field of neuroscience: Advances and future directions. Frontiers in Neuroscience. 14, 724 (2020).
- Yamamoto, K., Miyata, T., Onozuka, A., Koyama, H., Ohtsu, H., Nagawa, H. Plantar flexion as an alternative to treadmill exercise for evaluating patients with intermittent claudication. European Journal of Vascular and Endovascular Surgery. 33 (3), 325-329 (2007).
- Formenti, D., et al. Effects of knee extension with different speeds of movement on muscle and cerebral oxygenation. PeerJ. 6, 5704 (2018).
- Miyai, I., et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. NeuroImage. 14 (5), 1186-1192 (2001).
- Miyai, I., et al. Premotor cortex is involved in restoration of gait in stroke. Annals of Neurology. 52 (2), 188-194 (2002).
- Mihara, M., et al. Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke?. NeuroImage. 37 (4), 1338-1345 (2007).
- Rea, M., et al. Lower limb movement preparation in chronic stroke: A pilot study toward an fNIRS-BCI for gait rehabilitation. Neurorehabilitation and Neural Repair. 28 (6), 564-575 (2014).
- Holtzer, R., Verghese, J., Allali, G., Izzetoglu, M., Wang, C., Mahoney, J. R. Neurological gait abnormalities moderate the functional brain signature of the posture first hypothesis. Brain Topography. 29 (2), 334-343 (2016).
- Kim, H. Y., Yang, S. P., Park, G. L., Kim, E. J., You, J. S. Best facilitated cortical activation during different stepping, treadmill, and robot-assisted walking training paradigms and speeds: A functional near-infrared spectroscopy neuroimaging study. NeuroRehabilitation. 38 (2), 171-178 (2016).
- Khan, H., Nazeer, H., Engell, H., Naseer, N., Korostynska, O., Mirtaheri, P. Prefrontal cortex activation measured during different footwear and ground conditions using fNIRS-A case study. 2021 IEEE International Conference on Artificial Intelligence and Mechatronics Systems (AIMS). , 1-6 (2021).
- Lin, P. Y., Chen, J. J., Lin, S. I. The cortical control of cycling exercise in stroke patients: an fNIRS study). Human Brain Mapping. 34 (10), 2381-2390 (2013).
- Yang, M., Yang, Z., Yuan, T., Feng, W., Wang, P. A systemic review of functional near-infrared spectroscopy for stroke: Current application and future directions. Frontiers in Neurology. 10, 58 (2019).
- Berger, A., Horst, F., Müller, S., Steinberg, F., Doppelmayr, M. Current state and future prospects of EEG and fNIRS in robot-assisted gait rehabilitation: A brief review. Frontiers in Human Neuroscience. 13, 172 (2019).
- Khan, R. A., Naseer, N., Qureshi, N. K., et al. fNIRS-based Neurorobotic Interface for gait rehabilitation. J NeuroEngineering Rehabil. 15 (1), 7 (2018).
- Khan, H., Naseer, N., Yazidi, A., Eide, P. K., Hassan, H. W., Mirtaheri, P. Analysis of Human Gait Using Hybrid EEG-fNIRS-Based BCI System: A Review. Front. Hum. Neurosci. 14, (2020).
- Delpy, D. T., Cope, M. Quantification in tissue near-infrared spectroscopy. Philosophical Transactions of the Royal Society B: Biological Sciences. 352 (1354), 649-659 (1997).
- Huppert, T. J. Commentary on the statistical properties of noise and its implication on general linear models in functional near-infrared spectroscopy. Neurophotonics. 3, 010401 (2016).
- Alexandre, F., Heraud, N., Oliver, N., Varray, A. Cortical implication in lower voluntary muscle force production in non-hypoxemic COPD patients. PLoS One. 9 (6), 100961 (2014).
- Yoon, T., Vanden Noven, M. L., Nielson, K. A., Hunter, S. K. Brain areas associated with force steadiness and intensity during isometric ankle dorsiflexion in men and women. Experimental Brain Research. 232 (10), 3133-3145 (2014).
- Ciccarelli, O., et al. Identifying brain regions for integrative sensorimotor processing with ankle movements. Experimental Brain Research. 166 (1), 31-42 (2005).
- Udina, C., et al. Functional near-infrared spectroscopy to study cerebral hemodynamics in older adults during cognitive and motor tasks: A review. Frontiers in Aging Neuroscience. 11, 367 (2020).
- Thickbroom, G. W., Phillips, B. A., Morris, I., Byrnes, M. L., Mastaglia, F. L. Isometric force-related activity in sensorimotor cortex measured with functional MRI. Experimental Brain Research. 121 (1), 59-64 (1998).
- Derosière, G., Alexandre, F., Bourdillon, N., Mandrick, K., Ward, T. E., Perrey, S. Similar scaling of contralateral and ipsilateral cortical responses during graded unimanual force generation. NeuroImage. 85 (1), 471-477 (2014).
- Shi, P., Li, A., Yu, H. Response of the cerebral cortex to resistance and non-resistance exercise under different trajectories: A functional near-infrared spectroscopy study. Frontiers in Neuroscience. 15, 685920 (2021).
- Dettmers, C., et al. Relation between cerebral activity and force in the motor areas of the human brain. Journal of Neurophysiology. 74 (2), 802-815 (1995).
- Keisker, B., Hepp-Reymond, M. C., Blickenstorfer, A., Kollias, S. S. Differential representation of dynamic and static power grip force in the sensorimotor network. The European Journal of Neuroscience. 31 (8), 1483-1491 (2010).
- Harada, T., Miyai, I., Suzuki, M., Kubota, K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Experimental Brain Research. 193 (3), 445-454 (2009).
- Saleh, S., et al. The role of premotor areas in dual tasking in healthy controls and persons with multiple sclerosis: An fNIRS imaging study. Frontiers in Behavioral Neuroscience. 12, 296 (2018).
- Bonnal, J., et al. Relation between cortical activation and effort during robot-mediated walking in healthy people: A functional near-infrared spectroscopy neuroimaging study (fNIRS). Sensors. 22 (15), 5542 (2022).
- Shibuya, K., Sadamoto, T., Sato, K., Moriyama, M., Iwadate, M. Quantification of delayed oxygenation in ipsilateral primary motor cortex compared with contralateral side during a unimanual dominant-hand motor task using near-infrared spectroscopy. Brain Research. 1210, 142-147 (2008).
- Dai, T. H., Liu, J. Z., Sahgal, V., Brown, R. W., Yue, G. W. Relationship between muscle output and functional MRI-measured brain activation. Experimental brain research. 140 (3), 290-300 (2001).
- Cabibel, V., Hordacre, B., Perrey, S. Implication of the ipsilateral motor network in unilateral voluntary muscle contraction: the cross-activation phenomenon. Journal of Neurophysiology. 123 (5), 2090-2098 (2020).
- Akselrod, M., Martuzzi, R., Serino, A., vander Zwaag, W., Gassert, R., Blanke, O. Anatomical and functional properties of the foot and leg representation in areas 3b, 1 and 2 of primary somatosensory cortex in humans: A 7T fMRI study. NeuroImage. 159, 473-487 (2017).
- Brigadoi, S., Cooper, R. J. How short is short? Optimum source-detector distance for short-separation channels in functional near-infrared spectroscopy. Neurophotonics. 2 (2), 025005 (2015).
- Funahashi, S. Prefrontal contribution to decision-making under free-choice conditions. Frontiers in Neuroscience. 11, 431 (2017).
- Simon, S. R., Meunier, M., Piettre, L., Berardi, A. M., Segebarth, C. M., Boussaoud, D. Spatial attention and memory versus motor preparation: premotor cortex involvement as revealed by fMRI. Journal of Neurophysiology. 88 (4), 2047-2057 (2002).
- Desmurget, M., Sirigu, A. A parietal-premotor network for movement intention and motor awareness. Trends in Cognitive Sciences. 13 (10), 411-419 (2009).
- Nachev, P., Kennard, C., Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nature reviews. Neuroscience. 9 (11), 856-869 (2008).
- Thoenissen, D., Zilles, K., Toni, I. Differential involvement of parietal and precentral regions in movement preparation and motor intention. The Journal of neuroscience: the official journal of the Society for Neuroscience. 22 (20), 9024-9034 (2002).
- Al-Quraishi, M. S., Elamvazuthi, I., Tang, T. B., Al-Qurishi, M., Adil, S. H., Ebrahim, M. Bimodal data fusion of simultaneous measurements of EEG and fNIRS during lower limb movements. Brain Sciences. 11 (6), 713 (2021).
- Bishnoi, A., Holtzer, R., Hernandez, M. E. Brain Activation Changes While Walking in Adults with and without Neurological Disease: Systematic Review and Meta-Analysis of Functional Near-Infrared Spectroscopy Studies. Brain sciences. 11 (3), 291 (2021).
- Oh, S., Song, M., Kim, J. Validating attentive locomotion training using interactive treadmill: an fNIRS study. Journal of NeuroEngineering and Rehabilitation. 15 (1), 122 (2018).

