使用功能性近红外光谱和机器人辅助下肢多关节指向运动的实验
Summary
据估计,全世界每 6 个人中就有 1 个人在其一生中会中风,导致长期残疾,其康复机制仍然知之甚少。本研究提出了一种在下肢机器人治疗期间通过功能性近红外光谱 (fNIRS) 评估大脑激活的方案。
Abstract
中风每年影响全球约1700万人,是导致长期残疾的主要原因。机器人疗法在帮助中风患者恢复失去的运动功能方面显示出希望。增加对运动恢复如何发生的理解的一个潜在途径是研究健康个体治疗所针对的运动期间的大脑激活。功能性近红外光谱 (fNIRS) 已成为一种很有前途的神经成像技术,用于检查运动功能的神经基础。本研究旨在调查健康受试者复杂下肢运动的 fNIRS 神经相关性。参与者被要求使用机器人设备进行 6 分钟的休息和运动循环,以进行运动康复。这项任务需要协调膝关节和踝关节运动,以指向计算机屏幕上显示的目标。探索了机器人提供不同运动辅助水平的两种实验条件。结果表明,fNIRS协议在任务期间有效地检测了与运动控制相关的大脑区域。值得注意的是,与辅助条件相比,在无辅助条件下,所有受试者在对侧前运动区域表现出更大的激活。总之,fNIRS似乎是一种有价值的方法,用于检测与下肢多关节指向运动相关的氧合血红蛋白浓度变化。这项研究可能有助于理解中风运动恢复机制,并可能为改善中风患者的康复治疗铺平道路。然而,需要进一步的研究来充分阐明fNIRS在研究运动功能及其在临床环境中的应用方面的潜力。
Introduction
流行病学数据表明,全世界每年有 ~1700 万新发卒中病例,低收入和中等收入国家的发病率有所增加1.据估计,到 2030 年,新病例数将增加到 7700 万例2.中风引起的运动障碍通常会影响患者的活动能力和参与日常生活活动,导致生活质量低下。传统的运动康复包括手法治疗,但在过去的几十年里,已经开发了用于康复的机器人系统。这些系统可以提供高强度、剂量、可量化、可靠性、可重复性和灵活性的治疗3,并已显示出作为急性和慢性卒中患者有效康复治疗的潜力4,5,6。除了提供治疗外,用于康复的机器人系统还可以用作评估工具,因为它们可以配备可以测量患者运动/动力学数据的传感器7,8。对于上肢运动康复,这些数据不仅被证明可用于评估机器人治疗引起的患者运动恢复水平,并作为传统临床评估的补充工具9,10,而且还有助于促进对中风运动恢复过程的理解11,12 以及健康受试者运动和运动学习的神经控制 3,13,14。因此,这些发现为加强康复治疗奠定了基础15.
在过去的二十年中,已经提出了许多用于下肢神经康复的机器人设备,从在步行过程中支持患者体重的外骨骼(例如,在跑步机上,例如 Lokomat16)到允许患者在不走路的情况下锻炼脚踝、膝盖或脚的固定机器人系统(例如罗格斯大学脚踝17, 高性能踝关节康复机器人18 和光州科学技术研究所 (GIST) 踝/足康复机器人19) 或主动足部矫形器,它们是患者佩戴在地面或跑步机上行走的驱动外骨骼(例如电动步态矫形器20 和 MIT 脚踝机器人21)。参见 22,23,4 关于机器人用于下肢康复的综述。
用于中风患者下肢康复的机器人设备的临床研究结果令人鼓舞,并表明这些系统可以改善关节的运动范围 (ROM)、肌肉力量或步态,具体取决于特定的设备和临床方案(参见 24,25 关于下肢机器人康复功效的综述)。虽然已经假设机器人辅助治疗促进神经可塑性变化,最终导致运动能力的提高26,但中风运动恢复过程究竟是如何发生的,以及哪些机器人训练方案优化了下肢运动能力的恢复过程,目前仍不清楚。事实上,康复机器人的不断发展(无论是学术研究人员还是商业实体)与对运动恢复背后的神经生理机制的有限理解之间存在着显着且日益扩大的差距4.使用嵌入式传感器对运动学或关节扭矩的测量有助于定量描述患者恢复下肢运动能力时发生的运动行为变化 27,28,29,部分填补了这一空白。然而,这种变化背后的神经相关性研究较少。这是由于几个原因。
在临床试验的背景下,脑功能成像非常耗时,有时难以完成,这通常需要将患者负担降至最低,以最大限度地提高患者依从性的可能性。这对于中风患者尤其如此,因为中风后疲劳和肌肉无力经常被观察到30.此外,基于磁场的成像模式,如功能性磁共振成像(fMRI),要求患者和机器人硬件都是磁铁安全的。
在非侵入性成像方式中,功能性近红外光谱 (fNIRS) 是一种成像技术,特别适用于评估接受机器人治疗的受试者的大脑激活区域。与功能磁共振成像类似,fNIRS测量大脑中的血氧/脱氧。然而,与fMRI不同的是,fNIRS与机器人硬件完全兼容,而且它通常是便携式的,甚至可以在床边使用。此外,fNIRS具有低成本和对运动伪影的敏感性较低31,32,33。
尽管自 70 年代末首次引入以来,fNIRS 具有明显的优势并在许多临床环境中得到广泛使用34,但只有少数研究使用 fNIRS 来量化与下肢运动和中风运动恢复相关的大脑激活。FNIRS 研究旨在阐明运动神经控制机制和/或中风运动恢复机制或评估运动恢复,主要研究单关节运动(例如,背屈、跖屈或膝关节伸展运动 35,36,37)、步行38、39、40、41、42、43 或骑自行车44。有关评论,请参见45。同样,关于机器人辅助治疗下肢的fNIRS研究主要集中在机器人辅助步态康复上;有关评论,请参见46。一些研究的重点是使用fNIRS作为脑机接口(BCI)系统的一部分来获取机器人设备的控制信号47,48;虽然该研究领域也依赖于fNIRS信号的处理,但其目标不同,主要集中在解码患者意图(例如,患有严重运动障碍的患者)。
本文介绍的试点研究是研究机器人系统对下肢康复影响的初步努力的一部分。该机器人可以提供以目标为导向的下肢康复,包括日常多关节运动的训练,以及对下肢的单个关节(例如膝盖或脚踝)进行治疗(即实施自下而上的康复计划)。
该研究旨在调查一种实验方案的可行性,该实验方案要求在执行下肢多关节指向运动期间获取 fNIRS 数据。本研究中数据采集期的持续时间限制为 6 分钟,比典型的 fNIRS 协议短。这是一个深思熟虑的选择,旨在提高这项研究的实用性和临床适用性,特别是在行动不便或力量有限的患者中。识别这种复杂的多关节运动的fNIRS相关性,并深入了解机器人辅助如何调节大脑激活也是感兴趣的点。为此,对相同的参与者进行了两次实验:一次没有机器人辅助,另一次有机器人辅助。最后,需要指出的是,本研究的重点是健康受试者,以便为未来在记录协议可行性和评估机器人治疗靶向运动期间大脑激活方面的研究奠定基础。
装置
一个设计用于提供下肢康复的便携式机器人(见 图1)用于进行我们的实验。该机器人具有 3D 可触及的工作空间,结构紧凑,重量轻,重约 35 磅,便于运输和安装。
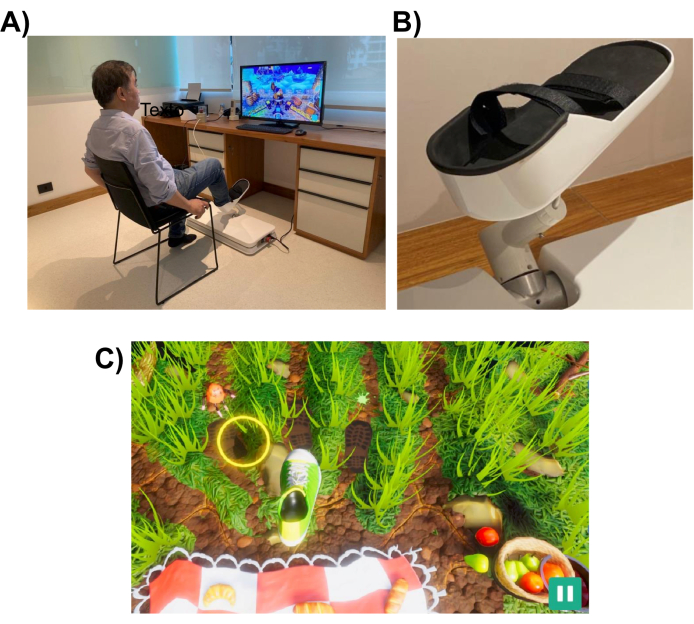
图 1:实验设置。 (A) 专为下肢设计的机器人系统(安装在地板上)。图中显示一名志愿者用右脚使用界面。(B) 受试者脚部的支撑结构,能够连接到机器人系统。(C) 野餐游戏的屏幕截图。游戏的目标是将脚(绿色和白色的鞋子)移动到目标(黄色圆圈)。 请点击这里查看此图的较大版本.
机器人系统旨在帮助患者执行类似于日常任务(例如指向或踢腿)的下肢运动。它使用交互式虚拟现实游戏,这些游戏显示在计算机显示器或放置在机器人设备前面的电视屏幕上(见 图1)。机器人末端执行器连接到患者的下肢(例如脚踝),其位置映射到屏幕上光标的位置。典型的游戏显示患者的运动目标(例如,要指向的物体或踢球的位置)。
为了完成运动任务,机器人可以协助患者提供一定程度的帮助,范围从完全协助到无协助。机器人辅助的水平是在每次康复课程开始时根据患者的运动障碍程度来选择的。游戏使用受试者执行的动作来对患者的表现进行评分,并为他们提供有关其表现的反馈(例如,ROM、动作次数和机器人辅助水平)。这些游戏旨在具有互动性和娱乐性,以保持患者的兴趣和注意力。在这项研究中,参与者玩了“野餐游戏”,玩家必须阻止昆虫到达毛巾并偷走食物(屏幕截图见 图1底部面板)。
使用便携式fNIRS采集系统进行数据采集,该系统具有两个不同的连续波光电极(760 nm和850 nm),8个双尖LED光源和8个双尖有源探测器。信号是使用 10.17 Hz 的采样率采集的。使用笔记本电脑使用fNIRS系统创建的Wi-Fi网络进行校准优化和信号记录。
使用盖子将视光管固定在预定位置。源和探测器根据 10-10 国际脑电图系统放置在网格空间分布中。每个fNIRS通道由光极间距离约为30 mm的源-检测器对定义。光电极放置在辅助电机、前电机和电机区域的 图 2 所示位置。通道总数为 28 个,其中 8 个是短距离通道,使用光纤适配器耦合到单个探测器的每个源。考虑到硬件的多路复用设置,只需使用一个探测器就可以从所有来源获取短距离信息。
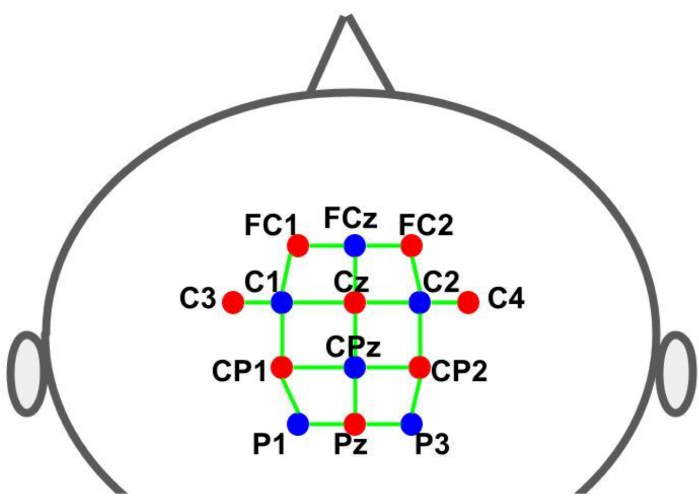
图 2:使用 10-10 EEG 系统的蒙太奇布局。 字母和数字表示源/探测器位置。红点和蓝点分别代表光源和检测器光电极。绿线表示fNIRS通道,由源和检测器对组成。 请点击这里查看此图的较大版本.
试验设计
该实验是在两种不同的实验条件下进行的,机器人为受试者的运动提供的帮助水平不同。在第一种情况下,机器人被编程为不为受试者的运动提供任何帮助,而在第二种情况下,机器人控制受试者的脚和腿运动(机器人辅助运动)。
每个实验都遵循一个模块设计范式,包括运动任务(玩游戏 – 30 秒)和休息(30 秒)的交替循环,如 图 3 所示。每个阶段(游戏/游戏或休息)的开始和结束都通过计算机屏幕向受试者发出视觉信号。在休息阶段,显示一条指示暂停的消息。每个循环(游戏/游戏 + 休息)的持续时间为 60 秒,重复 6 次,总运行时间为 360 秒(6 分钟)。
参与者玩了“野餐游戏”,目的是防止昆虫到达毛巾并偷走食物。该游戏涉及一系列下肢运动,从指定的本垒目标(初始位置)开始,延伸到三个外围目标之一,然后返回本垒目标。在屏幕上,外部目标在视觉上表现为动画移动的昆虫,参与者必须伸手踩到它们。有三个向外延伸的目标,每个目标随机出现的次数相等,每个动作都有一个共同的本垒目标。脚从本目标到外部目标位置所需的距离形成一个弧形,大约为 26 厘米。运动任务需要执行多关节运动,要求膝关节屈曲/伸展、跖屈和背屈运动之间的协调。
fNIRS数据记录与游戏通过机器人产生的晶体管-晶体管-逻辑(TTL)脉冲呈现给受试者的视觉刺激同步。脉冲在每个阶段(游戏/游戏和休息)开始时产生。因此,所有计时控制均由游戏执行,游戏向参与者提供视觉提示(目标)以开始每个动作,向fNIRS系统发送TTL信号以标记大脑活动记录,并在实验需要时向机器人控制系统发送信号以启动运动辅助。
Protocol
Representative Results
Discussion
在这项概念验证研究中,研究了使用健康受试者的 fNIRS 数据对大脑激活映射进行推断的可行性,同时他们使用机器人进行不同类型的运动进行下肢康复。成人的典型 fNIRS 记录会话超过 6 分钟54。然而,为了使记录在康复环境中可行,必须最大限度地减少实验的总持续时间,以避免受试者不必要的疲劳和努力。本研究还测试了具有 6 个任务重复块(会话持续时间为 6 分钟)的块设…
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
本研究的观点、假设、结论和建议是作者的观点、假设、结论和建议,并不一定代表资助机构的意见。JRS 感谢圣保罗研究基金会(FAPESP,资助号 2021/05332-8、2018/04654-9、2018/21934-5 和 2023/02538-0)和 Jackson Cionek 的技术支持。AMM 和 Vivax Ltda 感谢 FAPESP(圣保罗研究基金会)和 FIEP(巴西创新机构)。该项目由FAPESP(资助号2018/09559-4)和FINEP(资助号2019/09933-6)资助。
Materials
| 32 inch Smart TV | Samsung | N/A | TV connected to robot via HDMI cable |
| 8-detector silicon photodiode (SiPD) optodes for optical detection with dual tip | NIRx Medical Technologies (Glen Head, NY, USA) | https://nirx.net/nirsport | |
| 8-source optodes bundle for optical illumination with dual tip | NIRx Medical Technologies (Glen Head, NY, USA) | https://nirx.net/nirsport | |
| Aurora acquisition software | NIRx Medical Technologies (Glen Head, NY, USA) | https://nirx.net/nirsport | |
| Laptop Precision XPS 13 | Dell Technologies (Round Rock, TX, USA) | ||
| nirsLAB fNIRS Analysis software | NIRx Medical Technologies (Glen Head, NY, USA) | https://nirx.net/nirsport | |
| NIRSports2 fNIRS acquisition system | NIRx Medical Technologies (Glen Head, NY, USA) | https://nirx.net/nirsport | It has two different continuous wave optics (760 and 850 nm), 8 dual-ended LED sources and 8 dual-ended active detectors. |
| R | R-project.org (open source software) | https://www.r-project.org/ | |
| Standard cut cap, black color for up to 128 holders. | Easycap GmbH (Wörthsee, Germany) | https://www.easycap.de/ | |
| Vivax Assistive Rehabilitation Machine (ARM) | Vivax Ltda (São Paulo, Brazil) | https://vivaxbr.com/home/ | It is a portable robot designed to deliver lower limb rehabilitation. It has a 3D reachable workspace and is compact and light, weighing about 35 lb., which makes it easy to transport and to install. |
Riferimenti
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. Neurology. 18 (5), 439-458 (2019).
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. Neurology. 20 (10), 795-820 (2021).
- Huang, V. S., Krakauer, J. W. Robotic neurorehabilitation: a computational motor learning perspective. Journal of NeuroEngineering and Rehabilitation. 6, 5 (2009).
- Hobbs, B., Artemiadis, P. A Review of Robot-Assisted Lower-Limb Stroke Therapy: Unexplored Paths and Future Directions in Gait Rehabilitation. Frontiers in neurorobotics. 14, 19 (2020).
- Bertani, R., Melegari, C., De Cola, M. C., Bramanti, A., Bramanti, P., Calabrò, R. S. Effects of robot-assisted upper limb rehabilitation in stroke patients: a systematic review with meta-analysis. Neurological Sciences. 38 (9), 1561-1569 (2017).
- Warutkar, V., Dadgal, R., Mangulkar, U. R. Use of robotics in gait rehabilitation following stroke: A review. Cureus. 14 (11), e31075 (2022).
- Dipietro, L., et al. Changing motor synergies in chronic stroke. Journal of Neurophysiology. 98 (2), 757-768 (2007).
- Dipietro, L., et al. Learning, not adaptation, characterizes stroke motor recovery: evidence from kinematic changes induced by robot-assisted therapy in trained and untrained task in the same workspace. IEEE Trans Neural Syst Rehabil Eng. 20 (1), 48-57 (2012).
- Bosecker, C., Dipietro, L., Volpe, B., Krebs, H. I. Kinematic robot-based evaluation scales and clinical counterparts to measure upper limb motor performance in patients with chronic stroke. Neurorehabilitation and Neural Repair. 24 (1), 62-69 (2010).
- Krebs, H. I., et al. Robotic measurement of arm movements after stroke establishes biomarkers of motor recovery. Stroke. 45 (1), 200-204 (2014).
- Volpe, B. T., et al. Robotic devices as therapeutic and diagnostic tools for stroke recovery. Archives of Neurology. 66 (9), 1086-1090 (2009).
- Hogan, N., et al. Motions or muscles? Some behavioral factors underlying robotic assistance of motor recovery. Journal of Rehabilitation Research and Development. 43 (5), 605-618 (2006).
- Shadmehr, R., Wise, S. P. . The Computational Neurobiology of Reaching and Pointing: A Foundation for Motor Learning. , (2005).
- Dipietro, L., Poizner, H., Krebs, H. I. Spatiotemporal dynamics of online motor correction processing revealed by high-density electroencephalography. J Cogn Neurosci. 26 (9), 1966-1980 (2014).
- Krebs, H., et al. Rehabilitation robotics: Performance-based progressive robot-assisted therapy. Autonomous Robots. 15, 7-20 (2003).
- Colombo, G., Joerg, M., Schreier, R., Dietz, V. Treadmill training of paraplegic patients using a robotic orthosis. Journal of Rehabilitation Research and Development. 37 (6), 693-700 (2000).
- Girone, M., et al. A Stewart platform-based system for ankle telerehabilitation. Autonomous Robots. 10, 203-212 (2001).
- Saglia, J. A., Tsagarakis, N. G., Dai, J. S., Caldwell, D. G. A high-performance redundantly actuated parallel mechanism for ankle rehabilitation. The International Journal of Robotics Research. 28 (9), 1216-1227 (2009).
- Yoon, J., Ryu, J. A novel reconfigurable ankle/foot rehabilitation robot. , 2290-2295 (2005).
- Ruthenberg, B. J., Wasylewski, N. A., Beard, J. E. An experimental device for investigating the force and power requirements of a powered gait orthosis. Journal of Rehabilitation Research and Development. 34 (2), 203-213 (1997).
- Forrester, L. W., et al. Clinical application of a modular ankle robot for stroke rehabilitation. NeuroRehabilitation. 33 (1), 85-97 (2013).
- Díaz, I., Gil, J. J., Sánchez, E. Lower-limb robotic rehabilitation: Literature review and challenges. Journal of Robotics. 2011, 759764 (2011).
- Zhang, X., Yue, Z., Wang, J. Robotics in lower-limb rehabilitation after stroke. Behavioural Neurology. 2017, 3731802 (2017).
- Zhang, M., Davies, T. C., Xie, S. Effectiveness of robot-assisted therapy on ankle rehabilitation – a systematic review. Journal of NeuroEngineering and Rehabilitation. 10, 30 (2013).
- Lo, K., Stephenson, M., Lockwood, C. Effectiveness of robotic assisted rehabilitation for mobility and functional ability in adult stroke patients: a systematic review protocol. JBI Database of Systematic Reviews and Implementation Reports. 15 (1), 39-48 (2017).
- Belda-Lois, J. M., et al. Rehabilitation of gait after stroke: a review towards a top-down approach. Journal of NeuroEngineering and Rehabilitation. 8, 66 (2011).
- Bortole, M., et al. The H2 robotic exoskeleton for gait rehabilitation after stroke: early findings from a clinical study. Journal of NeuroEngineering and Rehabilitation. 12, 54 (2015).
- Banala, S. K., Kim, S. H., Agrawal, S. K., Scholz, J. P. Robot assisted gait training with active leg exoskeleton (ALEX). IEEE Transactions on Neural Systems and Rehabilitation Engineering. 17 (1), 2-8 (2009).
- Bartenbach, V., Wyss, D., Seuret, D., Riener, R. A lower limb exoskeleton research platform to investigate human-robot interaction. 2015 IEEE International Conference on Rehabilitation Robotics (ICORR). 2015, 600-605 (2015).
- Hinkle, J. L., et al. Poststroke fatigue: Emerging evidence and approaches to management: A scientific statement for healthcare professionals from the American heart association. Stroke. 48 (7), e159-e170 (2017).
- Balardin, J. B., Zimeo Morais, G. A., Furucho, R. A., Trambaiolli, L. R., Sato, J. R. Impact of communicative head movements on the quality of functional near-infrared spectroscopy signals: negligible effects for affirmative and negative gestures and consistent artifacts related to raising eyebrows. Journal of Biomedical Optics. 22 (4), 4601 (2017).
- Nazeer, H., Naseer, N., Mehboob, A., Khan, M. J., Khan, R. A., Khan, U. S., Ayaz, Y. Enhancing classification performance of fNIRS-BCI by identifying cortically active channels using the z-score method. Sensors. 20 (23), 6995 (2020).
- Ayaz, H., et al. Optical imaging and spectroscopy for the study of the human brain: status report. Neurophotonics. 9, S24001 (2022).
- Chen, W. L., et al. Functional near-infrared spectroscopy and its clinical application in the field of neuroscience: Advances and future directions. Frontiers in Neuroscience. 14, 724 (2020).
- Yamamoto, K., Miyata, T., Onozuka, A., Koyama, H., Ohtsu, H., Nagawa, H. Plantar flexion as an alternative to treadmill exercise for evaluating patients with intermittent claudication. European Journal of Vascular and Endovascular Surgery. 33 (3), 325-329 (2007).
- Formenti, D., et al. Effects of knee extension with different speeds of movement on muscle and cerebral oxygenation. PeerJ. 6, 5704 (2018).
- Miyai, I., et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. NeuroImage. 14 (5), 1186-1192 (2001).
- Miyai, I., et al. Premotor cortex is involved in restoration of gait in stroke. Annals of Neurology. 52 (2), 188-194 (2002).
- Mihara, M., et al. Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke?. NeuroImage. 37 (4), 1338-1345 (2007).
- Rea, M., et al. Lower limb movement preparation in chronic stroke: A pilot study toward an fNIRS-BCI for gait rehabilitation. Neurorehabilitation and Neural Repair. 28 (6), 564-575 (2014).
- Holtzer, R., Verghese, J., Allali, G., Izzetoglu, M., Wang, C., Mahoney, J. R. Neurological gait abnormalities moderate the functional brain signature of the posture first hypothesis. Brain Topography. 29 (2), 334-343 (2016).
- Kim, H. Y., Yang, S. P., Park, G. L., Kim, E. J., You, J. S. Best facilitated cortical activation during different stepping, treadmill, and robot-assisted walking training paradigms and speeds: A functional near-infrared spectroscopy neuroimaging study. NeuroRehabilitation. 38 (2), 171-178 (2016).
- Khan, H., Nazeer, H., Engell, H., Naseer, N., Korostynska, O., Mirtaheri, P. Prefrontal cortex activation measured during different footwear and ground conditions using fNIRS-A case study. 2021 IEEE International Conference on Artificial Intelligence and Mechatronics Systems (AIMS). , 1-6 (2021).
- Lin, P. Y., Chen, J. J., Lin, S. I. The cortical control of cycling exercise in stroke patients: an fNIRS study). Human Brain Mapping. 34 (10), 2381-2390 (2013).
- Yang, M., Yang, Z., Yuan, T., Feng, W., Wang, P. A systemic review of functional near-infrared spectroscopy for stroke: Current application and future directions. Frontiers in Neurology. 10, 58 (2019).
- Berger, A., Horst, F., Müller, S., Steinberg, F., Doppelmayr, M. Current state and future prospects of EEG and fNIRS in robot-assisted gait rehabilitation: A brief review. Frontiers in Human Neuroscience. 13, 172 (2019).
- Khan, R. A., Naseer, N., Qureshi, N. K., et al. fNIRS-based Neurorobotic Interface for gait rehabilitation. J NeuroEngineering Rehabil. 15 (1), 7 (2018).
- Khan, H., Naseer, N., Yazidi, A., Eide, P. K., Hassan, H. W., Mirtaheri, P. Analysis of Human Gait Using Hybrid EEG-fNIRS-Based BCI System: A Review. Front. Hum. Neurosci. 14, (2020).
- Delpy, D. T., Cope, M. Quantification in tissue near-infrared spectroscopy. Philosophical Transactions of the Royal Society B: Biological Sciences. 352 (1354), 649-659 (1997).
- Huppert, T. J. Commentary on the statistical properties of noise and its implication on general linear models in functional near-infrared spectroscopy. Neurophotonics. 3, 010401 (2016).
- Alexandre, F., Heraud, N., Oliver, N., Varray, A. Cortical implication in lower voluntary muscle force production in non-hypoxemic COPD patients. PLoS One. 9 (6), 100961 (2014).
- Yoon, T., Vanden Noven, M. L., Nielson, K. A., Hunter, S. K. Brain areas associated with force steadiness and intensity during isometric ankle dorsiflexion in men and women. Experimental Brain Research. 232 (10), 3133-3145 (2014).
- Ciccarelli, O., et al. Identifying brain regions for integrative sensorimotor processing with ankle movements. Experimental Brain Research. 166 (1), 31-42 (2005).
- Udina, C., et al. Functional near-infrared spectroscopy to study cerebral hemodynamics in older adults during cognitive and motor tasks: A review. Frontiers in Aging Neuroscience. 11, 367 (2020).
- Thickbroom, G. W., Phillips, B. A., Morris, I., Byrnes, M. L., Mastaglia, F. L. Isometric force-related activity in sensorimotor cortex measured with functional MRI. Experimental Brain Research. 121 (1), 59-64 (1998).
- Derosière, G., Alexandre, F., Bourdillon, N., Mandrick, K., Ward, T. E., Perrey, S. Similar scaling of contralateral and ipsilateral cortical responses during graded unimanual force generation. NeuroImage. 85 (1), 471-477 (2014).
- Shi, P., Li, A., Yu, H. Response of the cerebral cortex to resistance and non-resistance exercise under different trajectories: A functional near-infrared spectroscopy study. Frontiers in Neuroscience. 15, 685920 (2021).
- Dettmers, C., et al. Relation between cerebral activity and force in the motor areas of the human brain. Journal of Neurophysiology. 74 (2), 802-815 (1995).
- Keisker, B., Hepp-Reymond, M. C., Blickenstorfer, A., Kollias, S. S. Differential representation of dynamic and static power grip force in the sensorimotor network. The European Journal of Neuroscience. 31 (8), 1483-1491 (2010).
- Harada, T., Miyai, I., Suzuki, M., Kubota, K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Experimental Brain Research. 193 (3), 445-454 (2009).
- Saleh, S., et al. The role of premotor areas in dual tasking in healthy controls and persons with multiple sclerosis: An fNIRS imaging study. Frontiers in Behavioral Neuroscience. 12, 296 (2018).
- Bonnal, J., et al. Relation between cortical activation and effort during robot-mediated walking in healthy people: A functional near-infrared spectroscopy neuroimaging study (fNIRS). Sensors. 22 (15), 5542 (2022).
- Shibuya, K., Sadamoto, T., Sato, K., Moriyama, M., Iwadate, M. Quantification of delayed oxygenation in ipsilateral primary motor cortex compared with contralateral side during a unimanual dominant-hand motor task using near-infrared spectroscopy. Brain Research. 1210, 142-147 (2008).
- Dai, T. H., Liu, J. Z., Sahgal, V., Brown, R. W., Yue, G. W. Relationship between muscle output and functional MRI-measured brain activation. Experimental brain research. 140 (3), 290-300 (2001).
- Cabibel, V., Hordacre, B., Perrey, S. Implication of the ipsilateral motor network in unilateral voluntary muscle contraction: the cross-activation phenomenon. Journal of Neurophysiology. 123 (5), 2090-2098 (2020).
- Akselrod, M., Martuzzi, R., Serino, A., vander Zwaag, W., Gassert, R., Blanke, O. Anatomical and functional properties of the foot and leg representation in areas 3b, 1 and 2 of primary somatosensory cortex in humans: A 7T fMRI study. NeuroImage. 159, 473-487 (2017).
- Brigadoi, S., Cooper, R. J. How short is short? Optimum source-detector distance for short-separation channels in functional near-infrared spectroscopy. Neurophotonics. 2 (2), 025005 (2015).
- Funahashi, S. Prefrontal contribution to decision-making under free-choice conditions. Frontiers in Neuroscience. 11, 431 (2017).
- Simon, S. R., Meunier, M., Piettre, L., Berardi, A. M., Segebarth, C. M., Boussaoud, D. Spatial attention and memory versus motor preparation: premotor cortex involvement as revealed by fMRI. Journal of Neurophysiology. 88 (4), 2047-2057 (2002).
- Desmurget, M., Sirigu, A. A parietal-premotor network for movement intention and motor awareness. Trends in Cognitive Sciences. 13 (10), 411-419 (2009).
- Nachev, P., Kennard, C., Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nature reviews. Neuroscience. 9 (11), 856-869 (2008).
- Thoenissen, D., Zilles, K., Toni, I. Differential involvement of parietal and precentral regions in movement preparation and motor intention. The Journal of neuroscience: the official journal of the Society for Neuroscience. 22 (20), 9024-9034 (2002).
- Al-Quraishi, M. S., Elamvazuthi, I., Tang, T. B., Al-Qurishi, M., Adil, S. H., Ebrahim, M. Bimodal data fusion of simultaneous measurements of EEG and fNIRS during lower limb movements. Brain Sciences. 11 (6), 713 (2021).
- Bishnoi, A., Holtzer, R., Hernandez, M. E. Brain Activation Changes While Walking in Adults with and without Neurological Disease: Systematic Review and Meta-Analysis of Functional Near-Infrared Spectroscopy Studies. Brain sciences. 11 (3), 291 (2021).
- Oh, S., Song, M., Kim, J. Validating attentive locomotion training using interactive treadmill: an fNIRS study. Journal of NeuroEngineering and Rehabilitation. 15 (1), 122 (2018).

