Bathophenanthroline Sulfonate-Based Colorimetric Assay: A Simple and Rapid Method for Quantitation of Non-Heme Iron in Mouse Liver Tissue
Abstract
Source: Duarte, T. L., Neves, J. V. Measurement of Tissue Non-Heme Iron Content using a Bathophenanthroline-Based Colorimetric Assay. J. Vis. Exp. (2022)
In this video, we describe a colorimetric-based method to determine non-heme iron content in mouse liver tissue. Bathophenanthroline sulfonate is used as the chromogenic substrate in the presence of a reducing agent, which converts ferric iron to chelatable ferrous iron.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board
1. Solution preparation
NOTE: Handle and prepare all reagents and solutions with iron-free glassware or disposable plasticware. Do not allow metallic laboratory materials (e.g., stainless steel spatulas) to come in contact with any reagent or solution, due to the risk of iron contamination. Make sure any reusable glassware is iron-free. Wash the materials with appropriate laboratory detergent for 30-60 min, rinse with deionized water, soak overnight in a 37% nitric acid solution diluted 1:3 with deionized water, rinse again with deionized water, and allow to dry.
- Acid mixture: Add 10 g of trichloroacetic acid to 82.2 mL of 37% hydrochloric acid in a glass bottle, thoroughly dissolve, and adjust the final volume to 100 mL with deionized water. Shake before use. Alternatively, use only 37% hydrochloric acid for tissue digestion.
NOTE: The solution is stable for at least 2 months when stored in dark brown glass reagent bottles.
CAUTION: Hydrochloric acid and trichloroacetic acid are corrosive, and concentrated forms release toxic acidic vapors. Wear protective garments, chemical-resistant gloves, and chemical splash goggles at all times when handling acids. Avoid breathing them in and always handle acids while under a fume hood. - Saturated sodium acetate: Add 228 g of anhydrous sodium acetate to 400 mL of deionized water in a glass bottle and agitate overnight at room temperature. Let the solution rest and precipitate for a day. If no precipitation occurs, continue adding small amounts of sodium acetate. Store the solution in a glass bottle.
- Chromogen Reagent: To prepare 1 mL of chromogen reagent, add 1 mg of 4,7-diphenyl-1,10-phenanthroline disulfonic acid disodium salt to 500 µL of deionized water and 10 µL of concentrated (100%) thioglycolic acid, and thoroughly dissolve. Make up the final volume to 1 mL with deionized water.
NOTE: Prepare as much chromogen reagent as needed. The solution is stable for 1 month when protected from light. - Working Chromogen Reagent (WCR): Add 1 volume of the Chromogen Reagent to 5 volumes of saturated sodium acetate and 5 volumes of deionized water.
NOTE: This solution should be prepared freshly on the day of use. - Stock Iron Standard Solution: To prepare a 20 mM stock iron solution, place 111.5 mg of carbonyl iron powder in a 250 mL volumetric flask containing 5,480 µL of 37% hydrochloric acid. Leave to dissolve overnight at room temperature (or incubate in a boiling water bath). Then, make up the solution to a final volume of 100 mL with deionized water.
NOTE: The standard solution can be kept indefinitely when stored in a tightly sealed vessel. - Working Iron Standard Solution (WISS): Add 13.5 µL of 37% hydrochloric acid to 500 µL of deionized water. Add 10 µL of the Stock Iron Standard Solution and make up the final volume to 1 mL with deionized water (11.169 µg of Fe/mL, 200 µM; measured by AAS).
NOTE: The working solution should be prepared freshly on the day of use.
2. Sample drying
- Cut a sample of tissue weighing 10-100 mg with a scalpel blade. Weigh it accurately in an analytical/precision balance over a small piece of parafilm (Fresh Weight).
- Using plastic tweezers, place the piece of tissue in a 24-well plate (unlidded to allow for water evaporation) and let it dry on a standard incubator at 65 °C for 48 h.
- Alternatively, use a laboratory microwave digestion oven for drying tissue samples. Using plastic tweezers, place the weighed piece of tissue in an iron-free Teflon cup and dry it in the microwave. Set the operating parameters according to the instrument's instructions manual.
NOTE: As a reference, operating parameters for drying liver samples using the specific digestion oven (see Table of Materials) are shown in Table 1. - Using plastic tweezers, place each dried piece of tissue over a small piece of parafilm inside an analytical/precision balance and weigh it accurately (Dry Weight).
3. Sample acidic digestion
- Using plastic tweezers, transfer each dried piece of tissue into a 1.5 mL microcentrifuge tube.
- Add 1 mL of the acid mixture and close the microcentrifuge tube. Prepare an acid blank in the same way, except that the tissue is omitted.
CAUTION: The acid mixture is corrosive and releases toxic vapors. Wear protective garments, chemical-resistant gloves, and chemical splash goggles when handling the acid mixture. Avoid breathing it in and always handle it while under a fume hood. - Digest the tissues by incubating the microcentrifuge tubes in an incubator at 65 °C for 20 h.
- After cooling to room temperature, transfer 500 µL of the clear (yellow) acid extract (supernatant) into a new 1.5 mL microcentrifuge tube using a micropipette fitted with plastic tips. If it is not possible to obtain a clear supernatant, perform a short centrifugation spin.
CAUTION: The supernatant is highly acidic. Wear protective garments, chemical-resistant gloves, and chemical splash goggles when handling the supernatants. Always handle them while under a fume hood.
NOTE: At this point, the acid extracts can be immediately used for the colorimetric assay or frozen at -20 °C for later use. Completely thaw frozen samples to room temperature and vortex them prior to use.
4. Color development
- Prepare chromogen reactions as indicated in Table 2 in 1.5 mL microcentrifuge tubes or, for higher throughput, directly into the flat bottom, 96-well, clear, untreated polystyrene microplates. Prepare all reactions (acid blank, standard, and sample) at least in duplicate.
- Incubate at room temperature for 15 min.
5. Absorbance reading
- Measure sample absorbance in a spectrophotometer or plate reader at a wavelength of 535 nm against a deionized water reference. Plates can be read unlidded or lidded. In the lidded case, remove any condensation formed due to the release of acid vapors from the lid just prior to the measurement to avoid possible interference with the absorbance reading.
NOTE: The optical absorbance of the acid blank read against deionized water (reference) should be less than 0.015; the optical absorbance of the standard and samples should be between 0.100 and 1.000. For samples with very high or very low iron content, the volumes of acid extract (supernatant) and diH2O may need to be adjusted (Table 2): if absorbance is greater than 1.0, use a smaller sample (supernatant) volume; when absorbance is lower than 0.1, use a higher volume of the supernatant. Sample volume (Vsmp) is taken into account when calculating each sample's tissue iron content (see step 6).
6. Calculation of tissue iron content
- Calculate non-heme tissue iron content with the following equation:
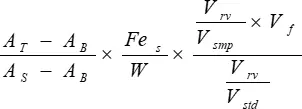
Tissue iron (µg/g dry tissue) = Equation 1
AT = absorbance of test sample
AB = absorbance of acid blank
AS = absorbance of standard
Fes = iron concentration of WISS (µg Fe/mL)
W = weight of dry tissue (g)
Vsmp = sample volume (variable volume of Supernatant in Table 2 converted to mL)
Vf = final volume of acid mixture after overnight incubation at 65 °C (corresponding to acid volume plus dry tissue volume in mL; if sample weights do not differ significantly, assume a constant volume ≈ 1 mL)
Vstd = iron standard volume (volume of WISS in Table 2 converted to mL)
Vrv = final reaction volume (Total volume in Table 2 converted to mL)
Table 1: Operating parameters for liver drying in a microwave digestion oven used here
| Power 650W (%): | 10 | 15 | 20 | 25 | 30 |
| Pressure (PSI): | 0 | 0 | 0 | 0 | 0 |
| Time (min): | 10 | 15 | 30 | 30 | 40 |
| Time at pressure (TAP): | 0 | 0 | 0 | 0 | 0 |
| FAN: | 50 | 50 | 50 | 50 | 50 |
Table 2: Preparation of chromogen reactions in either 1.5 mL tubes or 96-well plates.
| WCR (µL) | Acid mixture (µL) | Supernatant (µL) | WISS (µL) | diH2O (µL) | Total volume (µL) | ||
| 1.5 mL tubes | Acid Blank | 1000 | 150 | 150 | 1300 | ||
| Standard | 1000 | 150 | 150 | 1300 | |||
| Sample | 1000 | Variable | Variable | 1300 | |||
| 96-well microplate | Acid Blank | 150 | 22.5 | 22.5 | 195 | ||
| Standard | 150 | 22.5 | 22.5 | 195 | |||
| Sample | 150 | Variable | Variable | 195 |
Divulgazioni
The authors have nothing to disclose.
Materials
| 96 well UV transparent plate | Sarstedt | 82.1581.001 | |
| Analytical balance | Kern | ABJ 220-4M | |
| Anhydrous sodium acetate | Merck | 106268 | |
| Bathophenanthroline sulfonate (4,7-Diphenyl-1,10-phenantroline dissulfonic acid) | Sigma-Aldrich | B1375 | |
| C57BL/6 mice (Mus musculus) | Charles River Laboratories | ||
| Carbonyl iron powder, ≥99.5% | Sigma-Aldrich | 44890 | |
| Disposable cuvettes in polymethyl methacrylate (PMMA) | VWR | 634-0678P | |
| Double distilled, sterile water | B. Braun | 0082479E | |
| Fluorescence microplate reader | BioTek Instruments | FLx800 | |
| Hydrochloric acid, 37% | Sigma-Aldrich | 258148 | |
| Microwave digestion oven and white teflon cups | CEM | MDS-2000 | |
| Nitric acid | Fisher Scientific | 15687290 | |
| Oven | Binder | ED115 | |
| Rodent chow | Harlan Laboratories | 2014S | Teklad Global 14% Protein Rodent Maintenance Diet containing 175 mg/kg iron |
| Sea bass (Dicentrarchus labrax) | Sonrionansa | ||
| Sea bass feed | Skretting | L-2 Alterna 1P | |
| Single beam UV-Vis spectrophotometer | Shimadzu | UV mini 1240 | |
| Thioglycolic acid | Merck | 100700 | |
| Trichloroacetic acid | Merck | 100807 |

