Preparation of Quality Inositol Pyrophosphates
Summary
Inositol pyrophosphates play an important role in human pathologies such cancer, diabetes and obesity; however, the exact mechanism of action is a matter of dispute. The lack of commercially available inositol pyrophosphates renders detailed studies problematic. Here we describe a simple protocol to produce and isolate milligrams of inositol pyrophosphates.
Abstract
Myo-inositol is present in nature either unmodified or in more complex phosphorylated derivates. Of the latest, the two most abundant in eukaryotic cells are inositol pentakisphosphate (IP5) and inositol hexakisphosphate (phytic acid or IP6). IP5 and IP6 are the precursors of inositol pyrophosphate molecules that contain one or more pyrophosphate bonds1. Phosphorylation of IP6 generates diphoshoinositolpentakisphosphate (IP7 or PP-IP5) and bisdiphoshoinositoltetrakisphosphate (IP8 or (PP)2-IP4). Inositol pyrophosphates have been isolated from all eukaryotic organisms so far studied. In addition, the two distinct classes of enzymes responsible for inositol pyrophosphate synthesis are highly conserved throughout evolution2-4.
The IP6 kinases (IP6Ks) posses an enormous catalytic flexibility, converting IP5 and IP6 to PP-IP4 and IP7 respectively and subsequently, by using these products as substrates, promote the generation of more complex molecules5,6. Recently, a second class of pyrophosphate generating enzymes was identified in the form of the yeast protein VIP1 (also referred as PP-IP5K), which is able to convert IP6 to IP7 and IP87,8.
Inositol pyrophosphates regulate many disparate cellular processes such as insulin secretion9, telomere length10,11, chemotaxis12, vesicular trafficking13, phosphate homeostasis14 and HIV-1 gag release15. Two mechanisms of actions have been proposed for this class of molecules. They can affect cellular function by allosterically interacting with specific proteins like AKT16. Alternatively, the pyrophosphate group can donate a phosphate to pre-phosphorylated proteins17. The enormous potential of this research field is hampered by the absence of a commercial source of inositol pyrophosphates, which is preventing many scientists from studying these molecules and this new post-translational modification. The methods currently available to isolate inositol pyrophosphates require sophisticated chromatographic apparatus18,19. These procedures use acidic conditions that might lead to inositol pyrophosphate degradation20 and thus to poor recovery. Furthermore, the cumbersome post-column desalting procedures restrict their use to specialized laboratories.
In this study we describe an undemanding method for the generation, isolation and purification of the products of the IP6-kinase and PP-IP5-kinases reactions. This method was possible by the ability of polyacrylamide gel electrophoresis (PAGE) to resolve highly phosphorylated inositol polyphosphates20. Following IP6K1 and PP-IP5K enzymatic reactions using IP6 as the substrate, PAGE was used to separate the generated inositol pyrophosphates that were subsequently eluted in water.
Protocol
1. Enzymatic Reaction – day 1 (1 hour in the afternoon)
- The first step is to prepare 10-20 independent enzymatic reactions in which IP6K1 or VIP1 convert IP6 to the pyrophosphorylated isoforms.
- We use His-IP6K1 and GST-Vip1 enzymes purified from E. coli according to the protocol previously described17,18.
- Prepare 50 μL reactions containing 1X reaction buffer (30 mM Hepes pH 6.8, 50 mM NaCl, 6 mM MgSO4, 1 mM DTT), 6 mM PhosphoCreatine (PCr), 25 U/mL CreatinePhosphoKinase (CPK), 5 mM ATP (Mg salt), 0.3 mM IP6, 0.05-0.1 μg His-IP6K1 or GST-Vip1. Adjust volume with MiliQ ddH2O.
- Briefly spin the reaction and incubate at 37°C overnight with rotation.
2. Polyacrylamide gel casting and loading – day 2 (4 hours in the afternoon)
- The polyacrylamide gel is prepared using 24 cm long,18 cm wide glass plates and 1.5 mm wide spacers. Usually a 16 lane or a preparative single lane comb is used.
- Prepare a mix (50 mL/gel) containing the following: 35.5% (w/v) Acrylamide:Bis-Acrylamide 19:1, 1X Tris/Borate/EDTA (TBE), 0.05% (w/v) ammonium persulfate (APS), 0.05% (w/v) Temed. Pour the mix between the pre-casted glass plates, insert the comb and let polymerize for 30-60 minutes at RT.
- Once the gel has polymerized, transfer the apparatus to the cold room and pre-run in 1X TBE for about 30-60 minutes at 200-300 Volts.
- Add 1X of OrangeG dye (10 mM Tris-HCl pH7.0, 1 mM EDTA, 30% glycerol, 0.1% OrangeG) to each reaction. Prepare a sample containing 2 nmol of IP6 to load as a standard control.
- Wash each well thoroughly with running buffer using a syringe and a 21G needle to remove any precipitate, then load the gel. Avoid loading on the side wells.
- Run the gel overnight at 450-550 Volts (7 mAmp/gel), until the OrangeG dye band is within the last 10 cm from the bottom of the gel.
3. Isolation of IP7 – day 3 (4 hours) and day 4 (6-7 hour SpeedVac drying process)
- Disassemble the gel apparatus and carefully remove one glass plate leaving the gel on the other one. Cut a small portion of the gel from just above the OrangeG dye band to the bottom containing the IP6 standard and one sample lane, as shown in Figure 1.
- Stain the cut portion of the gel with Toluidine Blue (0.1% (w/v) toluidine blue, 20% (w/v) methanol, 2% (w/v) glycerol) for a few minutes (1-3 min) or until the inositol pyrophosphate band appears. Put the glass plate previously removed back on top of the gel to prevent the unstained gel from drying.
The IP7 band should be visible since it runs slightly slower than the IP6 standard. ATP, which runs faster than IP6, should also be visible (Figure 1). Transfer the stained portion of the gel in a de-staining solution (20% (w/v) methanol) for a few minutes, wash away any excess of Toluidine Blue and reposition the gel with the unstained gel.
If visualization of higher pyrophosphorylated inositol isoforms (IP8 and IP9) is required, stain the gel with Toluidine Blue staining solution for 20 minutes at room temperature. Subsequently, wash away the Toluidine Blue with the de-staining solution for about 15 minutes.
- With a razor blade cut the IP7 band on the unstained portion of the gel using as reference the IP7 migrating position determined with the stained gel (Figure 1).
- Put the IP7 band that was cut from the gel on a 15 mL tube and add 10 mL of MilliQ ddH2O. Put tubes in rotation for 10 minutes at room temperature. Discard the liquid to remove excess of TBE and microscopic acrylamide particles.
- Subsequently, perform two dehydration-hydration cycles. Add 5 mL of 50% (w/v) methanol to the tube with the gel containing IP7 and rotate at room temperature for 2 hours. Transfer the gel slice to a new 15 mL tube containing 5 mL of MilliQ ddH2O and rotate at room temperature for 2 hours. Do not discard the methanol and MilliQ H2O from the tubes. Repeat the dehydration-hydration cycle once more by re-transferring the polyacrylamide gel in the 15 mL tubes previously used. One of the washes can be performed over night.
- To concentrate the eluted IP7, dry the 10 mL together (5 mL MilliQddH2O and 5 mL 50% (w/v) methanol) using a SpeedVac heated at 60°C.
- Once the samples are nearly dry, transfer the remaining liquid (300-600 μl) to a1.5 mL centrifuge tube and spin for 2 minutes at 5000 rpm.
- Collect supernatant and transfer into a fresh 1.5 mL centrifuge tube; leave the bottom 20-30 μL since it may contain acrylamide particles.
- If necessary continue the drying process using an unheated SpeedVac. The recovery of IP7 is dramatically reduced if the samples dry completely, therefore terminate the drying process when the samples reach the volume of 100-300 μL.
4. Determination of IP7 concentration and purity.
- Use 2-5 μL of the recovered IP7 sample to run on a PAGE gel, similarly to Sections 2.2-2.5. Load several dilutions of IP6 (i.e. 0.5, 1, 2, 4 nmol) as concentration standard and 4 nmol of Poly-P marker. After running the gel, visualize the inositol pyrophosphate isoforms by staining and de-staining the entire gel with Toluidine Blue solution, following the procedure described in Section 3.2 (Figure 2A).
- After Toluidine staining, the concentrations can be determined by scanning the gel and comparing the differences in intensity between IP6 and IP7, using imaging software such as Image-J, as shown in Figure 2B.
5. Representative Results:
The preparative enzymatic conversion of IP6 to IP7 using IP6K1 and VIP1 enzymes can be easily resolved using PAGE analysis (Figure 1). The loading of IP6 as a size control together with Toluidine Blue gel staining allows the identification of the pyrophosphorylated derivates, since they run slower depending on the number of phosphate groups present on the inositol ring. The procedure described above allows the easy purification of IP7. The analysis of the purified inositol pyrophosphate by PAGE revealed the purity of our IP7 (Figure 2A). Interestingly, the 1/3PP-IP5 isomer of IP7 product of VIP1 migrates slightly slower than the 5PP-IP5 isomer of IP7 that is generated by the IP6K1. Use of IP6 standards permit an easy quantification of the concentration of the purified IP7 (Figure 2B). Before using IP7 for further experiments, its biological activity can be assessed
(Figure 3). 5PP-IP5 is incubated with VIP1 and with the IP7 phosphatase DDP1 (diphosphoinositol polyphosphate phosphohydrolase). Routinely, the purified IP7 is converted to IP8 by VIP1 and to IP6 by DDP1 (Figure 3).
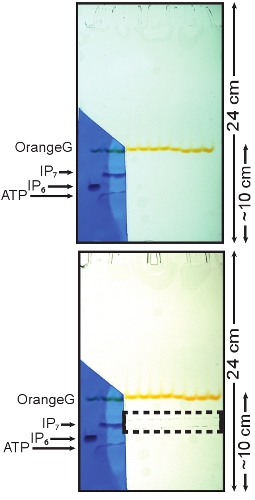
Figure 1: Toluidine staining of PAGE and isolation of the IP7 band. The portion of the gel containing the standard (IP6) was cut and stained using a Toluidine Blue solution. The three bands represent (top to bottom) IP7, IP6 and ATP. The stained portion of the gel was then aligned with the remaining of the gel. This allows the localization of the portion of the gel containing IP7, which can then be cut and purified (dashed box).
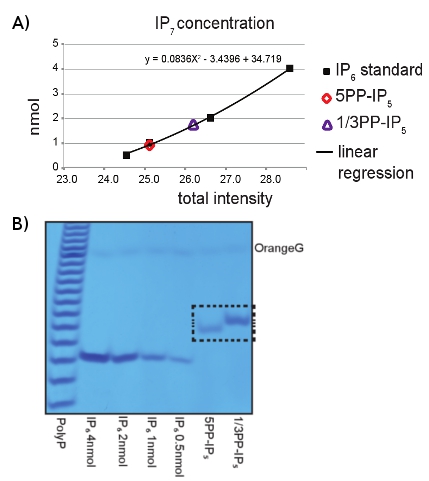
Figure 2: PAGE analysis of IP6K1 and VIP1 reaction products. A) Analysis of IP6 (4, 2, 1, 0.5 nmol) by Toluidine Blue staining was used in order to determine the IP7 concentration purified from both IP6K1 (5PP-IP5) and VIP1 (1/3PP-IP5) reactions. B) Scatter plot analysis to determine the concentration of the purified IP7. Concentrations were determined according to band intensity, calculated using imageJ software, compared to pre-determined amounts of IP6. The X-axis represents intensities; the Y-axis represents concentrations expressed in nmol.
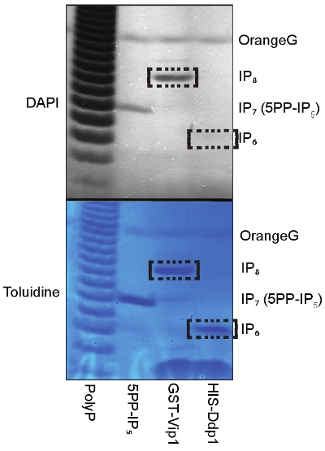
Figure 3: Analysis of IP7 biological activity. To determine the quality of the purified IP7 we incubated 5PP-IP5 (IP6K1 generated IP7) with VIP1 or with the IP7 phosphatase DDP1 and then resolved the reaction on PAGE. The DAPI and Toluidine staining revealed the expected production of IP8 by VIP1 and the conversion of IP7 to IP6 by DDP1.
Discussion
The use of inositol pyrophosphate in biochemistry is severely limited by the commercial unavailability of such compounds and the poor sensitivity of the existing detection methods. The combination of PAGE, which enables the separation of molecules possessing different number of phosphate groups, and Toluidine Blue (Figure 1), a metachromatic dye which binds to phosphate groups, enables the easy detection of inositol pyrophoshate isoforms opening new avenues of research20.
The described use of PAGE technology to purify inositol pyrophosphate products of the enzymatic reaction carried outby either IP6K1 or VIP1 is a simple, economic and reliable method that allows for the production of large amounts of high quality IP7. The method described above is not limited to the simple purification of IP7 but minor modifications of the described protocol may allow the purification of a different range of inositol pyrophosphates. Higher phosphorylated inositol pyrophosphate isoforms, containing more than eight phosphate groups can be detected using IP7 or different amounts of IP6 as a substrate20,6. These inositol pyrophosphates can be detected by increasing the length of the staining procedure and subsequently purified (section 3.2). Moreover, the use of IP5 as substrate for the enzymatic reaction would allow the purification of PP-IP5 and other inositol pyrophosphates containing a hydroxyl group on the inositol ring.
In conclusion, this undemanding method allows for the reliable purification of milligram quantities of inositol pyrophosphates with widely available instruments, thus opening new avenues for this exciting research field.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank A. Riccio for helpful comments and to read the manuscript. This work was supported by the Medical Research Council (MRC) funding to the Cell Biology Unit and by a Human Frontier Science Program Grant (RGP0048/2009-C).
Materials
| Name of the reagent | Company | Catalogue number |
|---|---|---|
| Phytic Acid (IP6) | Sigma-Aldrich | P8810 |
| Poly-P (sodium hexametaphosphate) | Sigma-Aldrich | P8510 |
| ATP-Mg2+ salt | Sigma-Aldrich | A9187 |
| OrangeG | Sigma-Aldrich | O3756 |
| PhosphoCreatine (PCr) | Sigma-Aldrich | P7936 |
| CreatinePhospho Kinase (CPK) | Sigma-Aldrich | C3755 |
| GST-Vip1 | 17 | 17 |
| His-IP6K1 | 18 | 18 |
| His-Ddp1 | Available in lab | Available in lab |
| Acrylamide:Bis-Acrylamide 19:1 (40%) | Flowgen | H16972 |
| Ammonium Persulfate (APS) | Sigma-Aldrich | A9164 |
| Tris/Borate/EDTA (TBE) | Sigma-Aldrich | T 9060 |
| Temed | BDH | 43083G |
| Toluidine Blue | Sigma-Aldrich | 198161 |
| SpeedVac | Christ | 100218 |
| Gel apparatus | Hoefer | SE600 |
| Vacuum manifold | Christ | Alpha 2-4 |
| Vacuum pump | ABM Greiffenberger | 4EKF63CX |
Riferimenti
- Bennett, M., Onnebo, S. M., Azevedo, C., Saiardi, A. Inositol pyrophosphates: metabolism and signaling. Cell Mol Life Sci. 63, 552-564 (2006).
- Burton, A., Hu, X., Saiardi, A. Are inositol pyrophosphates signalling molecules?. J Cell Physiol. 220, 8-15 (2009).
- Barker, C. J., Illies, C., Gaboardi, G. C., Berggren, P. O. Inositol pyrophosphates: structure, enzymology and function. Cell Mol Life Sci. 66, 3851-3871 (2009).
- Shears, S. B. Diphosphoinositol polyphosphates: metabolic messengers. Mol Pharmacol. 76, 236-252 (2009).
- Saiardi, A., Erdjument-Bromage, H., Snowman, A. M., Tempst, P., Snyder, S. H. Synthesis of diphosphoinositol pentakisphosphate by a newly identified family of higher inositol polyphosphate kinases. Curr Biol. 9, 1323-1326 (1999).
- Draskovic, P. Inositol hexakisphosphate kinase products contain diphosphate and triphosphate groups. Chem Biol. 15, 274-286 (2008).
- Mulugu, S. A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science. 316, 106-109 (2007).
- Fridy, P. C., Otto, J. C., Dollins, D. E., York, J. D. Cloning and characterization of two human VIP1-like inositol hexakisphosphate and diphosphoinositol pentakisphosphate kinases. J Biol Chem. 282, 30754-30762 (2007).
- Illies, C. Requirement of inositol pyrophosphates for full exocytotic capacity in pancreatic beta cells. Science. 318, 1299-1302 (2007).
- Saiardi, A., Resnick, A. C., Snowman, A. M., Wendland, B., Snyder, S. H. Inositol pyrophosphates regulate cell death and telomere length through phosphoinositide 3-kinase-related protein kinases. Proc Natl Acad Sci U S A. 102, 1911-1914 (2005).
- York, S. J., Armbruster, B. N., Greenwell, P., Petes, T. D., York, J. D. Inositol diphosphate signaling regulates telomere length. J Biol Chem. 280, 4264-4269 (2005).
- Luo, H. R. Inositol pyrophosphates mediate chemotaxis in Dictyostelium via pleckstrin homology domain-PtdIns(3,4,5)P3 interactions. Cell. 114, 559-572 (2003).
- Saiardi, A., Sciambi, C., McCaffery, J. M., Wendland, B., Snyder, S. H. Inositol pyrophosphates regulate endocytic trafficking. Proc Natl Acad Sci U S A. 99, 14206-14211 (2002).
- Auesukaree, C., Tochio, H., Shirakawa, M., Kaneko, Y., Harashima, S. Plc1p, Arg82p, and Kcs1p, enzymes involved in inositol pyrophosphate synthesis, are essential for phosphate regulation and polyphosphate accumulation in Saccharomyces cerevisiae. J Biol Chem. 280, 25127-25133 (2005).
- Azevedo, C., Burton, A., Ruiz-Mateos, E., Marsh, M., Saiardi, A. Inositol pyrophosphate mediated pyrophosphorylation of AP3B1 regulates HIV-1 Gag release. Proc Natl Acad Sci U S A. 106, 21161-21166 (2009).
- Bhandari, R. Protein pyrophosphorylation by inositol pyrophosphates is a posttranslational event. Proc Natl Acad Sci U S A. 104, 15305-15310 (2007).
- Azevedo, C., Burton, A., Bennett, M., Onnebo, S. M., Saiardi, A. Synthesis of InsP7 by the Inositol Hexakisphosphate Kinase 1 (IP6K1). Methods Mol Biol. 645, 73-85 (2010).
- Otto, J. C. Biochemical analysis of inositol phosphate kinases. Methods Enzymol. 434, 171-185 (2007).
- Losito, O., Szijgyarto, Z., Resnick, A. C., Saiardi, A. Inositol pyrophosphates and their unique metabolic complexity: analysis by gel electrophoresis. PLoS One. 4, e5580-e5580 (2009).

