使用拉姆齐试验来测量液体分泌和离子流率在<em>果蝇</em>马氏管
Summary
这个协议描述了使用的拉姆齐测定来测量流体分泌率从果蝇孤立马氏(肾)细管。此外,使用离子特异性电极来测量分泌的流体钠和钾的浓度,允许跨上皮离子通量的计算,进行说明。
Abstract
肾小管上皮离子转运的调制允许生物体维持离子和渗透压稳态变化的外部条件的面部。 果蝇马氏(肾)肾小管提供研究上皮细胞离子转运的分子机制,因为这种微生物及其肾小管生理研究的辅助强大的遗传学一个无与伦比的机会。在这里,我们描述了使用的拉姆齐测定来测量流体分泌率从分离的飞肾小管,与使用离子特异性电极测量钠和钾的浓度,在分泌的流体。该测定允许研究的跨上皮流体和离子通量〜20小管的时间,而不需要在分泌的流体传送到一个单独的装置来测量离子浓度。遗传上不同的小管可以分析,以评估在运输过程特定基因的作用。另外,将bathing盐水可以被修改来检查添加其化学特性,或药物或激素的作用。综上所述,本技术允许的上皮离子输送这些传输机制中的果蝇细管,以及调控基本机制的分子表征。
Introduction
肾上皮细胞离子转运underlies有机体iono-和渗透压调节。 果蝇马氏(肾)肾小管提供研究上皮细胞离子转运的分子机制提供了无与伦比的机会。这是由于果蝇的强大遗传学,配对的其肾小管生理研究的可访问的组合。拉姆齐检测,谁率先技术1研究者的名字命名,测量液体分泌率从孤立的马氏管,并建立在果蝇于1994年陶氏和他的同事2。这种利用果蝇的遗传工具,如GAL4-UAS系统3,4,确定细胞特异性的信号通路调节液分泌铺平了道路进一步研究。实例包括钙信号响应于肽激素5以及很多其他6,7。
ve_content“>遗传技术和古典生理研究的组合表明,尿代在飞是通过从细管的主要链段的氯化钾富液的分泌。这是通过阳离子的平行跨上皮分泌,主要是K +也钠离子,通过主细胞,和Cl –通过星状细胞分泌8-12分别测量跨上皮K +和Na +通量的能力允许的传输机制比液体分泌的测量更详细的表征孤独。例如,在未刺激的果蝇细管为Na + / K + -ATP酶抑制剂哇巴因对分泌液2没有影响,即使当其摄取到主细胞是由有机阴离子转运抑制剂牛磺胆13抑制。然而,林顿和奥唐纳发现,哇巴因去极化基底外侧膜电位,并增加了钠离子通量9。如图中代表结果,我们重复这些发现,并表明,K +通量是伴随降低14;增大的 Na +通量和降低的 K +通量对液分泌相反的作用,造成在分泌无净的变化。因此,有两项决议的“乌本苷悖论”,即,哇巴因在果蝇小管的分泌液没有效果初步观察:首先,在刺激小管,哇巴因对液体分泌的效果并不明显,由于其吸收的有机阴离子转运13;第二,在未受刺激的小管,哇巴因一直反对对跨上皮Na +和K +流的影响,导致液分泌没有净变化(见代表性的成果和参考文献9)。因此, 钠离子的主要作用/ K + -ATP酶在未刺激的小管是降低细胞内Na +的浓度 ,以产生有利的浓度梯度为Na +的偶联的运输过程横跨基底外侧膜。事实上,通过分别测量Na +和K +通量,我们表明,小管缺乏飞钠-钾-2-氯转运蛋白(NKCC)具有降低的跨上皮的 K +通量,与哇巴因加入后没有进一步降低,并在经上皮无变化Na +的线 14。这些发现支持了我们的结论,即Na +的进入从而NKCC细胞通过的Na + / K + -ATP酶回收。在另一个例子中,Ianowski 等人观察到,降低浴的 K +浓度从10mM至6毫从吸血蝽下降跨上皮K +通量并增加经上皮Na +的磁通在小管,并在流体分泌没有净变化<sup> 15。横跨幼虫小管上的 Na +通量和K +流的不同作用也已在果蝇小管中观察到响应于不同的盐饮食16并在两个蚊种响应于饲养盐度17。在拉姆齐测定制备跨上皮离子通量的测定的最大挑战是分泌的流体中的离子浓度的测定。这个挑战已经取得了不同的解决方案,其中包括火焰photometery 18,使用放射性离子19和电子探针波谱仪20。这些技术需要分泌的液体滴转移到仪器的离子浓度的测量。因为流体的由未刺激的果蝇细管分泌的体积是小的,典型地〜0.5标升/分钟,这会带来一个技术挑战,也引入了错误,如果一些分泌的流体是在传输丢失。与此相反,使用离子特异性电极允许原位离子活性(从该离子浓度可以计算)的测量。目前的协议改编自所使用的Maddrell和同事使用缬氨霉素作为第 K +离子载体21测量通过吸血肾小管跨上皮的 K +通量,并且还描述了使用4- 叔 -butylcalix [4]芳烃-四乙酸的四乙酯基钠离子的特征在于Messerli 等特异性离子特异性电极。人。22。离子特异性电极也被用于测量离子浓度在流体中的拉姆齐测定在成人9,23和幼虫16 果蝇由马氏管分泌,新西兰阿尔派恩威塔(Hemideina毛利 )24和在蚊子17。
在这里,我们详细描述了使用的拉姆齐作为说来测量流体分泌率在从果蝇马氏管,以及使用离子特异性电极以所分泌的流体中确定的 K + 和 Na +的浓度,从而跨上皮离子通量的计算。该试验的概述在图1中提供 。
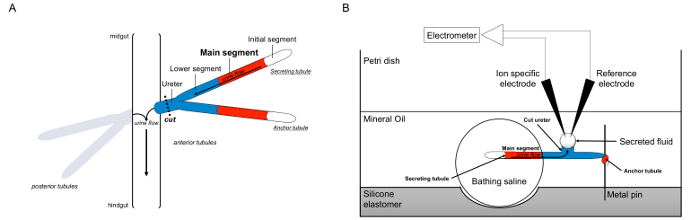
图1.原理马氏管和拉姆齐分析与使用离子专用电极来测量离子浓度 。这个数字说明了该设置为拉姆齐检测。 (A)中的每个飞有四个小管,一对前管和一对后小管的,即浮在腹腔内通过血淋巴包围。在每一对中,两个小管联接在输尿管,然后在肠和hindgu交界清空尿吨。该管的盲端。尿被流体分泌主段(显示为红色)中产生,并朝向输尿管和流出到肠道内流动。解剖后,细管对被从肠道通过横断输尿管解离。 (B)的一对细管然后被转移到洗澡盐水的液滴内测定皿的孔中。其中的两个小管的,这里所指的“锚小管,”所缠绕的金属销和是惰性的。其他小管的分泌小管。起始段(其不分泌液)和分泌小管主体段保持洗澡盐水的液滴中。离子和水移动从洗澡盐水到主段的小管内腔,然后移向输尿管,正如在体内发生。下段(蓝色)是沐浴盐水,因此惰性外部。由于输尿管被切断时,分泌的流体出现从输尿管的切断端的液滴。 Ť他分泌流体液滴放大随时间而分泌的继续,其直径是使用目镜测微尺测定。矿物油的层防止分泌流体的蒸发。参考和离子专用电极测量分泌液的离子浓度。 请点击此处查看该图的放大版本。
Protocol
Representative Results
Discussion
使用该拉姆齐检测,加上离子特定电极,允许液体分泌率和离子流的隔离昆虫马氏(肾)管的测量。二十个或更多小管可以一次进行测定,从而允许更高的吞吐量相比,个别的体外 microperfused小管的测定法。此外,离子特定电极允许在原位分泌液中的离子浓度的测定,从而限制了可以在小体积的流体至第二装置的转移而引入的误差。在协议中的关键步骤包括确保小管切开而不租金或?…
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors wish to thank Drs. Sung-wan An and Mike O’Donnell for practical advice on establishing this assay, Dr. Chih-Jen Cheng for helpful discussions on the use of ion-specific electrodes, and Dr. Chou-Long Huang for his mentorship and support. This work was supported by the National Institutes of Health (K08DK091316 to ARR) and the American Society of Nephrology Gottschalk Award to ARR.
Materials
| Sylgard 184 Silicone Elastomer Kit | Ellsworth Adhesives | http://www.ellsworth.com/dow-corning-sylgard-184-silicone-encapsulant-0-5kg-kit-clear/ | May be purchased from multiple distributors |
| Petri dish, polystyrene, 100 mm x 15 mm | Fisher | FB0875712 | Specific brand is not important |
| Petri dish, polystyrene, 35 mm x 10 mm | Corning Life Sciences | Fisher 08-757-100A | Specific brand is not important |
| Scalpel Handle #3 | Fine Science Tools | 10003-12 | Specific brand is not important |
| Scalpel Blades #1 | Fine Science Tools | 10011-00 | Specific brand is not important; use appropriate sharps precautions |
| Needle, 30G x 1/2 | Becton Dickinson | 305106 | Use appropriate sharps precautions |
| Minutien pins, black anodized, 0.15 mm | Fine Science Tools | 26002-15 | |
| Stereomicroscope with ocular micrometer | Nikon | SMZ800 | Specific brand is not important; this is given as an example |
| Sheet of black stained glass, 3 mm (1/8 inch) thick | Hobby shop | Example includes Spectrum Black Opal by Spectrum Glass (http://www.delphiglass.com/spectrum-glass/opalescent/spectrum-black-opal) | |
| Glass cutting tools (glass cutter, glass cutting pliers) | Hobby shop | Examples include the Studio Pro Lightweight Running Pliers by Diamond Tech (http://www.delphiglass.com/glass-cutters-tools/pliers-nippers/studio-pro-lightweight-running-pliers) and the Studio Pro Brass Glass Cutter by Diamond Tech (http://www.delphiglass.com/glass-cutters-tools/glass-cutters/studio-pro-brass-glass-cutter). Use appropriate safety precautions when cutting glass | |
| Borosilicate glass capillary tube, unfilamented, GC120-10, OD 1.2 mm, ID 0.69 mm, length 10 cm | Warner Instruments | 30-0042 | |
| Borosilicate glass capillary tube, filamented, GC120F-10, OD 1.2 mm, ID 0.69 mm, length 10 cm | Warner Instruments | 30-0044 | |
| Nitric acid, 70% | Sigma | 438073 | CAUTION: see Material Data Safety Sheet for appropriate storage and handling guidelines. Specific brand is not important |
| Cimarec 7 in x 7 in hotplate | Fisher | 11675911Q | Specific brand is not important; caution when heated |
| Selectophore dichlorodimethylsilane | Sigma | 40136-1ML | CAUTION: see Material Data Safety Sheet for appropriate storage and handling guidelines |
| Two-step vertical pipet puller | Narishige | PC-10 | Other pipet pullers can be used; this is given as an example |
| Glass petri dish, 150 mm diameter x 15 mm height | Fisher | 08-748E | Specific brand is not important; only one dish needed |
| World Precision Instruments E210 1 mm micropipette storage jar | Fisher | 50-821-852 | May be available from other distributors. Useful to have two jars. Note that although this jar is specified for 1 mm pipets, and the pipets used here are 1.2 mm, in our experience the 1 mm jar works best for the 1.2 mm pipets. |
| Silica Gel, Tel-Tale Desiccant, indicating, 10-18 mesh | Fisher | S161-500 | Indicating silica useful for determining whether silica gel retains desiccating ability |
| World Precision Instruments MicroFil, 34G | Fisher | 50-821-914 | May be available from other distributors. |
| 1 ml syringe with luer lock | Becton Dickinson | 309659 | May be available from other distributors. |
| 3 ml syringe with luer lock | Becton Dickinson | 309657 | May be available from other distributors. |
| D300 3-way stopcock with female luer lock inlet port, male luer outlet port with rotating collar and guard | Cole-Parmer | UX-30600-02 | Specific brand is not important |
| Female Luer Locking Connector | 4 Medical Solutions | ADC 9873-10 | Specific brand is not important; barbed end is ~4 mm at narrowest point and ~7 mm at widest point. |
| Silicone Tubing I.D. x O.D. x Wall: 1/16 x 1/8 x 1/32 in. (1.59 x 3.18 x 0.79 mm) | Fisher | 14-179-110 | Specific brand is not important |
| E-3603 tubing, I.D. x O.D.: 1/32 x 3/32 in | Fisher | 14171208 | Specific brand is not important |
| Modeling clay | Specific brand is not important | ||
| Selectophore potassium ionophore I, cocktail B | Sigma | 99373 | CAUTION: see Material Data Safety Sheet for appropriate storage and handling guidelines |
| Selectophore sodium ionophore X | Sigma | 71747 | Sodium ionphore X = 4-tert-butylcalix[4]arene-tetraacetic acid tetraethylester |
| Selectophore 2-nitrophenyl octyl ether | Sigma | 73732 | |
| Selectophore sodium tetraphenylborate | Sigma | 72018 | |
| Schneider's Drosophila medium | Life Technologies | 21720024 | |
| High impedance electrometer | World Precision Instruments | FD223a | |
| Microelectrode holder 1 mm with 45° body, vented, with handle | Warner Instruments | 64-1051 | |
| Microelectrode holder 1 mm with straight body, vented | Warner Instruments | 64-1007 | |
| Silver wire | Warner Instruments | 64-1318 | |
| Micromanipulators, pair | Leitz | Various brands/models will work; this is an example | |
| Faraday cage | Technical Manufacturing Corporation | 81-334-03 | This is an example; any Faraday cage will work |
| Single gooseneck fiberoptic light | Nikon | Specific brand is not important | |
| mineral oil | Fisher | BP-2629 | Specific brand is not important |
| forceps, Dumont #5 with Biologie tip | Fine Science Tool | 11295-10 | May be available from other distributors. |
Riferimenti
- Ramsay, J. A. Active Transport of Water by the Malpighian Tubules of the Stick Insect, Dixippus-Morosus (Orthoptera, Phasmidae). J Exp Biol. 31, 104-113 (1954).
- Dow, J. A., et al. The malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J Exp Biol. 197, 421-428 (1994).
- Brand, A. H., Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118, 401-415 (1993).
- Sozen, M. A., Armstrong, J. D., Yang, M., Kaiser, K., Dow, J. A. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc Natl Acad Sci U S A. 94, 5207-5212 (1997).
- Rosay, P., et al. Cell-type specific calcium signalling in a Drosophila epithelium. J Cell Sci. 110 (15), 1683-1692 (1997).
- Dow, J. T., Davies, S. A. Integrative physiology and functional genomics of epithelial function in a genetic model organism. Physiol Rev. 83, 687-729 (2003).
- Beyenbach, K. W., Skaer, H., Dow, J. A. The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol. 55, 351-374 (2010).
- Donnell, M. J., et al. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol. 274, 1039-1049 (1998).
- Linton, S. M., O’Donnell, M. J. Contributions of K+:Cl- cotransport and Na+/K+-ATPase to basolateral ion transport in malpighian tubules of Drosophila melanogaster. J Exp Biol. 202, 1561-1570 (1999).
- Rheault, M. R., O’Donnell, M. J. Analysis of epithelial K(+) transport in Malpighian tubules of Drosophila melanogaster: evidence for spatial and temporal heterogeneity. J Exp Biol. 204, 2289-2299 (2001).
- Donnell, M. J., Dow, J. A., Huesmann, G. R., Tublitz, N. J., Maddrell, S. H. Separate control of anion and cation transport in malpighian tubules of Drosophila Melanogaster. J Exp Biol. 199, 1163-1175 (1996).
- Cabrero, P., et al. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc Natl Acad Sci U S A. 111, 14301-14306 (2014).
- Torrie, L. S., et al. Resolution of the insect ouabain paradox. Proc Natl Acad Sci U S A. 101, 13689-13693 (2004).
- Rodan, A. R., Baum, M., Huang, C. L. The Drosophila NKCC Ncc69 is required for normal renal tubule function. Am J Physiol Cell Physiol. 303, 883-894 (2012).
- Ianowski, J. P., Christensen, R. J., O’Donnell, M. J. Na+ competes with K+ in bumetanide-sensitive transport by Malpighian tubules of Rhodnius prolixus. J Exp Biol. 207, 3707-3716 (2004).
- Naikkhwah, W., O’Donnell, M. J. Salt stress alters fluid and ion transport by Malpighian tubules of Drosophila melanogaster: evidence for phenotypic plasticity. J Exp Biol. 214, 3443-3454 (2011).
- Donini, A., et al. Secretion of water and ions by malpighian tubules of larval mosquitoes: effects of diuretic factors, second messengers, and salinity. Physiol Biochem Zool. 79, 645-655 (2006).
- Maddrell, S. H. Secretion by Malpighian Tubules of Rhodnius movements of Ions and Water. J Exp Biol. 51, 71-97 (1969).
- Maddrell, S. H., Overton, J. A. Stimulation of sodium transport and fluid secretion by ouabain in an insect malpighian tubule. J Exp Biol. 137, 265-276 (1988).
- Williams, J. C., Beyenbach, K. W. Differential effects of secretagogues on Na and K secretion in the Malpighian tubules of Aedes Aegypti (L). J Comp Physiol. 149, 511-517 (1983).
- Maddrell, S. H., O’Donnell, M. J., Caffrey, R. The regulation of haemolymph potassium activity during initiation and maintenance of diuresis in fed Rhodnius prolixus. J Exp Biol. 177, 273-285 (1993).
- Messerli, M. A., Kurtz, I., Smith, P. J. Characterization of optimized Na+ and Cl- liquid membranes for use with extracellular, self-referencing microelectrodes. Anal Bioanal Chem. 390, 1355-1359 (2008).
- Ianowski, J. P., O’Donnell, M. J. Basolateral ion transport mechanisms during fluid secretion by Drosophila Malpighian tubules: Na+ recycling, Na+:K+:2Cl- cotransport and Cl- conductance. J Exp Biol. 207, 2599-2609 (2004).
- Neufeld, D. S., Leader, J. P. Electrochemical characteristics of ion secretion in malpighian tubules of the New Zealand alpine weta (Hemideina maori). J Insect Physiol. 44, 39-48 (1997).
- Greenspan, R. J. . Fly Pushing: The Theory and Practice of Drosophila Genetics. , (1997).
- Jayakannan, M., Babourina, O., Rengel, Z. Improved measurements of Na+ fluxes in plants using calixarene-based microelectrodes. J Plant Physiol. 168, 1045-1051 (2011).
- Wu, Y., Schellinger, J. N., Huang, C. L., Rodan, A. R. Hypotonicity Stimulates Potassium Flux through the WNK-SPAK/OSR1 Kinase Cascade and the Ncc69 Sodium-Potassium-2-Chloride Cotransporter in the Drosophila Renal Tubule. J Biol Chem. 289, 26131-26142 (2014).
- Blumenthal, E. M. Modulation of tyramine signaling by osmolality in an insect secretory epithelium. Am J Physiol Cell Physiol. 289, 1261-1267 (2005).
- Dow, J. A., Maddrell, S. H., Davies, S. A., Skaer, N. J., Kaiser, K. A novel role for the nitric oxide-cGMP signaling pathway: the control of epithelial function in Drosophila. Am J Physiol. 266, 1716-1719 (1994).
- Dube, K., McDonald, D. G., O’Donnell, M. J. Calcium transport by isolated anterior and posterior Malpighian tubules of Drosophila melanogaster: roles of sequestration and secretion. J Insect Physiol. 46, 1449-1460 (2000).
- Efetova, M., et al. Separate roles of PKA and EPAC in renal function unraveled by the optogenetic control of cAMP levels in vivo. J Cell Sci. 126, 778-788 (2013).
- Rheault, M. R., O’Donnell, M. J. Organic cation transport by Malpighian tubules of Drosophila melanogaster: application of two novel electrophysiological methods. J Exp Biol. 207, 2173-2184 (2004).
- Donnell, M. J. Too much of a good thing: how insects cope with excess ions or toxins in the diet. J Exp Biol. 212, 363-372 (2009).
- Cheng, C. J., Truong, T., Baum, M., Huang, C. L. Kidney-specific WNK1 inhibits sodium reabsorption in the cortical thick ascending limb. Am J Physiol Renal Physiol. 303, 667-673 (2012).
- Cheng, C. J., Yoon, J., Baum, M., Huang, C. L. STE20/SPS1-related Proline/alanine-rich Kinase (SPAK) is Critical for Sodium Reabsorption in Isolated Perfused Thick Ascending Limb. Am J Physiol Renal Physiol. , (2014).

