Cinématique Analyse de la division cellulaire et Expansion: Quantifier la base cellulaire de la croissance et du développement d'échantillonnage Zones dans<em> Zea mays</em> Feuilles
Summary
Quantifying cell division and expansion is of crucial importance to the understanding of whole-plant growth. Here, we present a protocol to calculate cellular parameters determining maize leaf growth rates and highlight the use of these data for investigating molecular growth regulatory mechanisms by directing developmental stage-specific sampling strategies.
Abstract
Growth analyses are often used in plant science to investigate contrasting genotypes and the effect of environmental conditions. The cellular aspect of these analyses is of crucial importance, because growth is driven by cell division and cell elongation. Kinematic analysis represents a methodology to quantify these two processes. Moreover, this technique is easy to use in non-specialized laboratories. Here, we present a protocol for performing a kinematic analysis in monocotyledonous maize (Zea mays) leaves. Two aspects are presented: (1) the quantification of cell division and expansion parameters, and (2) the determination of the location of the developmental zones. This could serve as a basis for sampling design and/or could be useful for data interpretation of biochemical and molecular measurements with high spatial resolution in the leaf growth zone. The growth zone of maize leaves is harvested during steady-state growth. Individual leaves are used for meristem length determination using a DAPI stain and cell-length profiles using DIC microscopy. The protocol is suited for emerged monocotyledonous leaves harvested during steady-state growth, with growth zones spanning at least several centimeters. To improve the understanding of plant growth regulation, data on growth and molecular studies must be combined. Therefore, an important advantage of kinematic analysis is the possibility to correlate changes at the molecular level to well-defined stages of cellular development. Furthermore, it allows for a more focused sampling of specified developmental stages, which is useful in case of limited budget or time.
Introduction
Analyse de la croissance dépend d'un ensemble d'outils qui sont couramment utilisés par les scientifiques de plantes pour décrire le génotype déterminé les différences de croissance et / ou des réponses phénotypiques à des facteurs environnementaux. Ils comprennent la taille et le poids des mesures de la plante entière ou un organe et les calculs des taux de croissance à explorer les mécanismes sous-jacents de la croissance. Croissance organique est déterminée par la division cellulaire et de l'expansion au niveau cellulaire. Par conséquent, incluant la quantification de ces deux procédés dans des analyses de croissance est essentiel pour comprendre les différences de croissance organe entier 1. Par conséquent, il est crucial d'avoir une méthodologie appropriée pour déterminer les paramètres de croissance cellulaire qui est relativement facile à utiliser par des laboratoires non spécialisés.
L' analyse cinématique a déjà été établie comme une approche fournissant un cadre puissant pour le développement de modèles de croissance de l' organe 2. La technique a été optimisé pour les systèmes linéaires,tels que les racines d' Arabidopsis thaliana et les feuilles monocotylédones, mais également pour les systèmes non-linéaires, tels que les feuilles dicotylédone 3. De nos jours, cette méthodologie est de plus en plus utilisée pour étudier comment génétiques, hormonaux, développementaux et les facteurs environnementaux influencent la division cellulaire et l' expansion dans divers organes (tableau 1). En outre, il fournit également un cadre pour lier les processus cellulaires à leurs règlements biochimiques, moléculaires et physiologiques sous – jacents (tableau 2), bien que les limites peuvent être imposées par la taille des organes et de l' organisation spatiale des techniques qui nécessitent des quantités plus élevées de matières végétales (par exemple, métabolite mesures, de la protéomique, etc.).
Feuilles monocotylédones telles que le maïs (Zea mays) feuille, représentent des systèmes linéaires dans lesquels les cellules se déplacent à partir de la base de la feuille vers la pointe, en passant successivement à travers la zone de méristèmes et de l' allongement pour atteindre la maturitézone. Cela en fait un système de modèle idéal pour les études quantitatives de la configuration spatiale de la croissance 4. De plus, les feuilles de maïs ont de grandes zones de croissance (méristème et zone d'allongement couvrant plusieurs centimètres 5) et offrent des possibilités d'études à d' autres niveaux d' organisation. Cela permet de l'enquête sur les mécanismes de régulation (putatifs) contrôlant la division cellulaire et de l' expansion, quantifiée par analyse cinématique grâce à une gamme de techniques moléculaires, des mesures physiologiques, et des approches de biologie cellulaire (tableau 2).
Ici, nous fournissons un protocole pour effectuer une analyse cinématique dans les feuilles monocotylédones. Tout d'abord, nous expliquons comment procéder à une analyse appropriée à la fois la division cellulaire et l'élongation des cellules en fonction de la position le long de l'axe de la feuille et la façon de calculer les paramètres cinématiques. Deuxièmement, nous montrons aussi comment cela peut être utilisé comme base pour la conception d'échantillonnage. Ici, nous discutons deux cas: haute résolution d'échantillonnage d'und axée échantillonnage, permettant une meilleure interprétation des données et le gain de temps / argent, respectivement.
Tableau 1. Aperçu des analyses cinématique des méthodes de quantification de la division cellulaire et de l' expansion dans divers organes.
| organe | référence |
| feuilles monocotylédones | 16, 20, 21, 22 |
| pointes des racines | 2, 23, 24, 25, 26, 27, 28, 29 |
| feuilles dicotylédones | 21, 30, 31 |
| méristème apical | 32 |
Tableau 1. Aperçu des analyses cinématique des méthodes de quantification de la division cellulaire et de l' expansion dans divers organes.
<p class="jove_content" fo:keep-together.within-page = "1">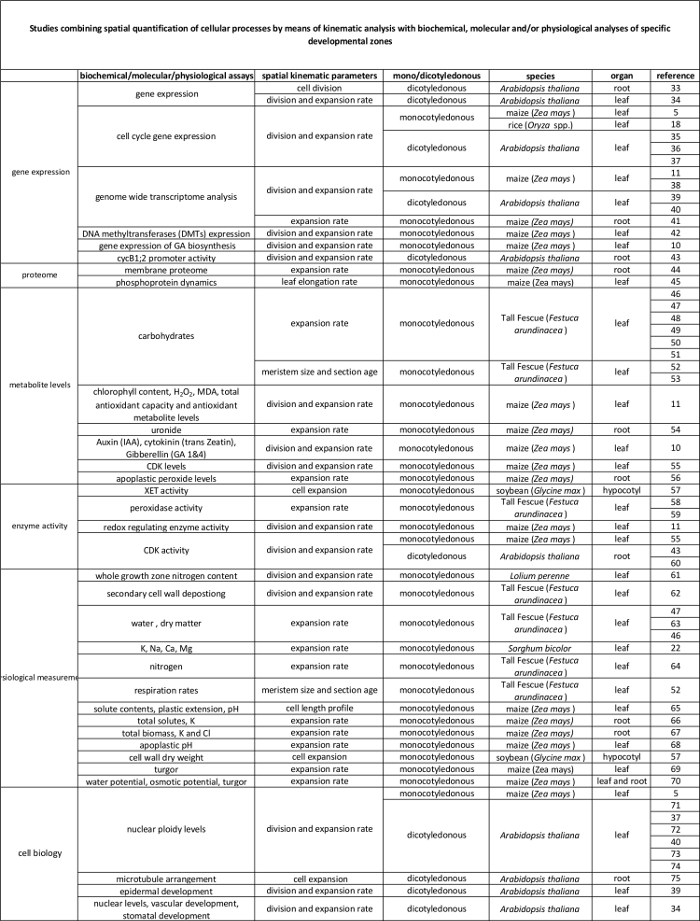
Tableau 2. Lien entre les processus cellulaires quantifiés par l'analyse cinématique à leur régulation au niveau moléculaire. Les références à diverses études reliant la quantification des processus cellulaires aux résultats des tests biochimiques et moléculaires chez diverses espèces et des organes. Endotransglucosylase Xyloglucan (XET), malondialdéhyde (MDA), les kinases cycline-dépendantes (CDK). S'il vous plaît cliquer ici pour voir une version plus grande de cette table.
Protocol
Representative Results
Discussion
Une analyse cinématique complète sur les feuilles de maïs permet la détermination de la base cellulaire de la croissance des feuilles et permet la conception de stratégies d'échantillonnage efficaces. Bien que le protocole est relativement simple, une certaine prudence est recommandée dans les étapes essentielles suivantes: (1) Il est important de détacher les feuilles plus jeunes fermés (étape 2.3) sans endommager le méristème, puisque la longueur du méristème détermination (étape 3) exige la compl…
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Ce travail a été soutenu par une bourse de doctorat de l'Université d'Anvers à VA; une bourse de doctorat de la Fondation flamande Science (FWO, 11ZI916N) à KS; subventions de projet du FWO (G0D0514N); une activité de recherche concertée (GOA) subvention de recherche, "Biologie Approche A Systèmes de Leaf morphogenèse" du conseil de recherche de l'Université d'Anvers; et l'activité Interuniversitaire Polonais (IUAP VII / 29, MARS), "Le maïs et Arabidopsis racines et des pousses de croissance" de l'Office belge de la Politique scientifique fédérale (BELSPO) à CSGT Han Asard, Bulelani L. Sizani et Hamada Abdelgawad ont tous contribué à la vidéo .
Materials
| Pots | Any | Any | We use pots with the following measueres, but can be different depending on the treatment/study : bottom diameter: 11cm, opening diameter: 15 cm, height: 12 cm. We grow one maize plant per pot. |
| Planting substrate | Any | Any | We use potting medium (Jiffy, The Netherlands), but other substrates can be used, depending on treatment/study. |
| Ruler | Any | Any | An extension ruler that covers at least 1,5 meters is needed to measure the final leaf length of the plants. |
| Seeds | Any | NA | Seeds can be ordered from a breeder. |
| Scalpel | Any | Any | The scalpel is used during leaf harvesting to detach the leaf of interest from its surrounding leaves and right after harvesting to cut a proper sample for cell length and meristem length measurements. |
| 15 ml falcon tubes | Any | Any | The 15 ml falcon tubes are used for storing samples used for cell length measurements during sample clearing with absolute ethanol and lactic acid. |
| Eppendorf tubes | Any | Any | The eppendorf tubes are used for storing samples used for meristem length measurements in ethanol:acetic acid 3:1 (v:v) solution. |
| Gloves | Any | Any | Latex gloves, which protect against corrosive reagents. |
| Acetic acid | Any | Any | CAUTION: Corrosive to metals, category 1 Skin corrosion, categories 1A,1B,1C Serious eye damage, category 1; Flammable liquids, categories 1,2,3 |
| Absolute ethanol | Any | Any | CAUTION: Hazardous in case of skin contact (irritant), of eye contact (irritant), of inhalation. Slightly hazardous in case of skin contact (permeator), of ingestion |
| Lactic acid >98% | Any | Any | CAUTION: Corrosive to metals, category 1 Skin corrosion, categories 1A,1B,1C Serious eye damage, category 1 |
| Sodium chloride (NaCl) | Any | Any | |
| Ethylenediaminetetraacetic acid (EDTA) | Any | Any | CAUTION: Acute toxicity (oral, dermal, inhalation), category 4 Skin irritation, category 2 Eye irritation, category 2 Skin sensitisation, category 1 Specific Target Organ Toxicity – Single exposure, category 3 |
| Tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl) | Any | Any | This material can be an irritant, contact with eyes and skin should be avoided. Inhalation of dust may be irritating to the respiratory tract. |
| 4′,6-Diamidine-2′-phenylindole dihydrochloride (DAPI) | Any | Any | Cell permeable fluorescent minor groove-binding probe for DNA. Causes skin irritation. May cause an allergic skin reaction. May cause respiratory irritation. |
| Ice | Any | NA | The DAPI solution has to be kept on ice. |
| Fluorescent microscope | AxioScope A1, Axiocam ICm1 from Zeiss or other | Any fluorescent microscope can be used for determining meristem length. | |
| Microscopic slide | Any | Any | |
| Cover glass | Any | Any | |
| Tweezers | Any | Any | Tweezers are needed for unfolding the rolled maize leaf right after harvesting in order to cut a proper sample for cell length and meristem length measurements. |
| Image-analysis software | Axiovision (Release 4.8) from Zeiss | NA | The software can be downloaded at: http://www.zeiss.com/microscopy/en_de/downloads/axiovision.html. Other softwares such as ImageJ (https://imagej.nih.gov/ij/) could be used as well. |
| Microscope equipped with DIC | AxioScope A1, Axiocam ICm1 from Zeiss or other | Any microscope, equipped with differential interference contrast (DIC) can be used to measure cell lengths. | |
| R statistical analysis software | R Foundation for Statistical Computing | NA | Open source; Could be downloaded at https://www.r-project.org/ |
| R script | NA | NA | We use the kernel smoothing function locpoly of the Kern Smooth package (Wand MP, Jones MC. Kernel Smoothing: Chapman & Hall/CRC (1995)). The script is available for Mac and Windows upon inquire with the corresponding author. We have versions for Mac and Windows. |
Riferimenti
- Fiorani, F., Beemster, G. T. S. Quantitative analyses of cell division in plants. Plant Mol. Biol. 60, 963-979 (2006).
- Silk, W. K., Erickson, R. O. Kinematics of Plant-Growth. J. Theor. Biol. 76, 481-501 (1979).
- Rymen, B., Coppens, F., Dhondt, S., Fiorani, F., Beemster, G. T. S., Hennig, L., Köhler, C. Kinematic Analysis of Cell Division and Expansion. Plant Developmental Biology. , (2010).
- Avramova, V., Sprangers, K., Beemster, G. T. S. The Maize Leaf: Another Perspective on Growth Regulation. Trends Plant Sci. 20, 787-797 (2015).
- Rymen, B., et al. Cold nights impair leaf growth and cell cycle progression in maize through transcriptional changes of cell cycle genes. Plant Physiol. 143, 1429-1438 (2007).
- Muller, B., Reymond, M., Tardieu, F. The elongation rate at the base of a maize leaf shows an invariant pattern during both the steady-state elongation and the establishment of the elongation zone. J. Exp. Bot. 52, 1259-1268 (2001).
- Beemster, G. T. S., Masle, J., Williamson, R. E., Farquhar, G. D. Effects of soil resistance to root penetration on leaf expansion in wheat (Triticum aestivum L): Kinematic analysis of leaf elongation. J. Exp. Bot. 47, 1663-1678 (1996).
- Bernstein, N., Silk, W. K., Lauchli, A. Growth and Development of Sorghum Leaves under Conditions of Nacl Stress – Spatial and Temporal Aspects of Leaf Growth-Inhibition. Planta. 191, 433-439 (1993).
- Sylvester, A. W., Smith, L. G., Bennetzen, J. L., Hake, S. C. Cell Biology of Maize Leaf Development. Handbook of maize: It’s Biology. , (2009).
- Nelissen, H., et al. A Local Maximum in Gibberellin Levels Regulates Maize Leaf Growth by Spatial Control of Cell Division. Curr. Biol. 22, 1183-1187 (2012).
- Avramova, V., et al. Drought Induces Distinct Growth Response, Protection, and Recovery Mechanisms in the Maize Leaf Growth Zone. Plant Physiol. 169, 1382-1396 (2015).
- Picaud, J. C., et al. Total malondialdehyde (MDA) concentrations as a marker of lipid peroxidation in all-in-one parenteral nutrition admixtures (APA) used in newborn infants. Pediatr. Res. 53, 406 (2003).
- Basu, P., Pal, A., Lynch, J. P., Brown, K. M. A novel image-analysis technique for kinematic study of growth and curvature. Plant Physiol. 145, 305-316 (2007).
- Vander Weele, C. M., et al. A new algorithm for computational image analysis of deformable motion at high spatial and temporal resolution applied to root growth. Roughly uniform elongation in the meristem and also, after an abrupt acceleration, in the elongation zone. Plant Physiol. 132, 1138-1148 (2003).
- Nelissen, H., Rymen, B., Coppens, F., Dhondt, S., Fiorani, F., Beemster, G. T. S., DeSmet, I. . Plant Organogenesis. , (2013).
- Ben-Haj-Salah, H., Tardieu, F. Temperature Affects Expansion Rate of Maize Leaves without Change in Spatial-Distribution of Cell Length – Analysis of the Coordination between Cell-Division and Cell Expansion. Plant Physiol. 109, 861-870 (1995).
- Fiorani, F., Beemster, G. T. S., Bultynck, L., Lambers, H. Can meristematic activity determine variation in leaf size and elongation rate among four Poa species? A kinematic study. Plant Physiol. 124, 845-855 (2000).
- Pettko-Szandtner, A., et al. Core cell cycle regulatory genes in rice and their expression profiles across the growth zone of the leaf. J. Plant Res. 128, 953-974 (2015).
- Poorter, H., Remkes, C. Leaf-Area Ratio and Net Assimilation Rate of 24 Wild-Species Differing in Relative Growth-Rate. Oecologia. 83, 553-559 (1990).
- Macadam, J. W., Volenec, J. J., Nelson, C. J. Effects of Nitrogen on Mesophyll Cell-Division and Epidermal-Cell Elongation in Tall Fescue Leaf Blades. Plant Physiol. 89, 549-556 (1989).
- Tardieu, F., Granier, C. Quantitative analysis of cell division in leaves: methods, developmental patterns and effects of environmental conditions. Plant Mol. Biol. 43, 555-567 (2000).
- Bernstein, N., Silk, W. K., Lauchli, A. Growth and Development of Sorghum Leaves under Conditions of Nacl Stress – Possible Role of Some Mineral Elements in Growth-Inhibition. Planta. 196, 699-705 (1995).
- Erickson, R. O., Sax, K. B. Rates of Cell-Division and Cell Elongation in the Growth of the Primary Root of Zea-Mays. P. Am. Philos. Soc. 100, 499-514 (1956).
- Beemster, G. T. S., Baskin, T. I. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol. 116, 1515-1526 (1998).
- Goodwin, R. H., Stepka, W. Growth and differentiation in the root tip of Phleum pratense. Am. J. Bot. 32, 36-46 (1945).
- Hejnowicz, Z. Growth and Cell Division in the Apical Meristem of Wheat Roots. Physiologia Plantarum. 12, 124-138 (1959).
- Gandar, P. W. Growth in Root Apices .1. The Kinematic Description of Growth. Bot. Gaz. 144, 1-10 (1983).
- Baskin, T. I., Cork, A., Williamson, R. E., Gorst, J. R. Stunted-Plant-1, a Gene Required for Expansion in Rapidly Elongating but Not in Dividing Cells and Mediating Root-Growth Responses to Applied Cytokinin. Plant Physiol. 107, 233-243 (1995).
- Sacks, M. M., Silk, W. K., Burman, P. Effect of water stress on cortical cell division rates within the apical meristem of primary roots of maize. Plant Physiol. 114, 519-527 (1997).
- Granier, C., Tardieu, F. Spatial and temporal analyses of expansion and cell cycle in sunflower leaves – A common pattern of development for all zones of a leaf and different leaves of a plant. Plant Physiol. 116, 991-1001 (1998).
- De Veylder, L., et al. Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell. 13, 1653-1667 (2001).
- Kwiatkowska, D. Surface growth at the reproductive shoot apex of Arabidopsis thaliana pin-formed 1 and wild type. J. Exp. Bot. 55, 1021-1032 (2004).
- Kutschmar, A., et al. PSK-alpha promotes root growth in Arabidopsis. New Phytol. 181, 820-831 (2009).
- Vanneste, S., et al. Plant CYCA2s are G2/M regulators that are transcriptionally repressed during differentiation. Embo J. 30, 3430-3441 (2011).
- Eloy, N. B., et al. Functional Analysis of the anaphase-Promoting Complex Subunit 10. Plant J. 68, 553-563 (2011).
- Eloy, N. B., et al. SAMBA, a plant-specific anaphase-promoting complex/cyclosome regulator is involved in early development and A-type cyclin stabilization. P. Natl. Acad. Sci. USA. 109, 13853-13858 (2012).
- Dhondt, S., et al. SHORT-ROOT and SCARECROW Regulate Leaf Growth in Arabidopsis by Stimulating S-Phase Progression of the Cell Cycle. Plant Physiol. 154, 1183-1195 (2010).
- Baute, J., et al. Correlation analysis of the transcriptome of growing leaves with mature leaf parameters in a maize RIL population. Genome Biol. 16, (2015).
- Andriankaja, M., et al. Exit from Proliferation during Leaf Development in Arabidopsis thaliana: A Not-So-Gradual Process. Dev. Cell. 22, 64-78 (2012).
- Beemster, G. T. S., et al. Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis. Plant Physiol. 138, 734-743 (2005).
- Spollen, W. G., et al. Spatial distribution of transcript changes in the maize primary root elongation zone at low water potential. Bmc Plant Biol. 8, (2008).
- Candaele, J., et al. Differential Methylation during Maize Leaf Growth Targets Developmentally Regulated Genes. Plant Physiol. 164, 1350-1364 (2014).
- West, G., Inze, D., Beemster, G. T. S. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 135, 1050-1058 (2004).
- Zhang, Z., Voothuluru, P., Yamaguchi, M., Sharp, R. E., Peck, S. C. Developmental distribution of the plasma membrane-enriched proteome in the maize primary root growth zone. Front. Plant Sci. 4, (2013).
- Bonhomme, L., Valot, B., Tardieu, F., Zivy, M. Phosphoproteome Dynamics Upon Changes in Plant Water Status Reveal Early Events Associated With Rapid Growth Adjustment in Maize Leaves. Mol. Cell Proteomics. 11, 957-972 (2012).
- Schnyder, H., Nelson, C. J. Growth-Rates and Assimilate Partitioning in the Elongation Zone of Tall Fescue Leaf Blades at High and Low Irradiance. Plant Physiol. 90, 1201-1206 (1989).
- Schnyder, H., Nelson, C. J., Spollen, W. G. Diurnal Growth of Tall Fescue Leaf Blades .2. Dry-Matter Partitioning and Carbohydrate-Metabolism in the Elongation Zone and Adjacent Expanded Tissue. Plant Physiol. 86, 1077-1083 (1988).
- Schnyder, H., Nelson, C. J. Growth-Rates and Carbohydrate Fluxes within the Elongation Zone of Tall Fescue Leaf Blades. Plant Physiol. 85, 548-553 (1987).
- Vassey, T. L., Shnyder, H. S., Spollen, W. G., Nelson, C. J. Cellular Characterisation and Fructan Profiles in Expanding Tall Fescue. Curr. T. Pl. B. 4, 227-229 (1985).
- Allard, G., Nelson, C. J. Photosynthate Partitioning in Basal Zones of Tall Fescue Leaf Blades. Plant Physiol. 95, 663-668 (1991).
- Spollen, W. G., Nelson, C. J. Response of Fructan to Water-Deficit in Growing Leaves of Tall Fescue. Plant Physiol. 106, 329-336 (1994).
- Volenec, J. J., Nelson, C. J. Carbohydrate-Metabolism in Leaf Meristems of Tall Fescue .1. Relationship to Genetically Altered Leaf Elongation Rates. Plant Physiol. 74, 590-594 (1984).
- Volenec, J. J., Nelson, C. J. Carbohydrate-Metabolism in Leaf Meristems of Tall Fescue .2. Relationship to Leaf Elongation Rates Modified by Nitrogen-Fertilization. Plant Physiol. 74, 595-600 (1984).
- Silk, W. K., Walker, R. C., Labavitch, J. Uronide Deposition Rates in the Primary Root of Zea-Mays. Plant Physiol. 74, 721-726 (1984).
- Granier, C., Inze, D., Tardieu, F. Spatial distribution of cell division rate can be deduced from that of p34(cdc2) kinase activity in maize leaves grown at contrasting temperatures and soil water conditions. Plant Physiol. 124, 1393-1402 (2000).
- Voothuluru, P., Sharp, R. E. Apoplastic hydrogen peroxide in the growth zone of the maize primary root under water stress.1. Increased levels are specific to the apical region of growth maintenance. J. Exp. Bot. 64, 1223-1233 (2012).
- Wu, Y. J., Jeong, B. R., Fry, S. C., Boyer, J. S. Change in XET activities, cell wall extensibility and hypocotyl elongation of soybean seedlings at low water potential. Planta. 220, 593-601 (2005).
- Macadam, J. W., Nelson, C. J., Sharp, R. E. Peroxidase-Activity in the Leaf Elongation Zone of Tall Fescue .1. Spatial-Distribution of Ionically Bound Peroxidase-Activity in Genotypes Differing in Length of the Elongation Zone. Plant Physiol. 99, 872-878 (1992).
- Macadam, J. W., Sharp, R. E., Nelson, C. J. Peroxidase-Activity in the Leaf Elongation Zone of Tall Fescue .2. Spatial-Distribution of Apoplastic Peroxidase-Activity in Genotypes Differing in Length of the Elongation Zone. Plant Physiol. 99, 879-885 (1992).
- Beemster, G. T. S., De Vusser, K., De Tavernier, E., De Bock, K., Inze, D. Variation in growth rate between Arabidopsis ecotypes is correlated with cell division and A-type cyclin-dependent kinase activity. Plant Physiol. 129, 854-864 (2002).
- Kavanova, M., Lattanzi, F. A., Schnyder, H. Nitrogen deficiency inhibits leaf blade growth in Lolium perenne by increasing cell cycle duration and decreasing mitotic and post-mitotic growth rates. Plant Cell Environ. 31, 727-737 (2008).
- Macadam, J. W., Nelson, C. J. Secondary cell wall deposition causes radial growth of fibre cells in the maturation zone of elongating tall fescue leaf blades. Ann. Bot-London. 89, 89-96 (2002).
- Schnyder, H., Nelson, C. J. Diurnal Growth of Tall Fescue Leaf Blades .1. Spatial-Distribution of Growth, Deposition of Water, and Assimilate Import in the Elongation Zone. Plant Physiol. 86, 1070-1076 (1988).
- Gastal, F., Nelson, C. J. Nitrogen Use within the Growing Leaf Blade of Tall Fescue. Plant Physiol. 105, 191-197 (1994).
- Vanvolkenburgh, E., Boyer, J. S. Inhibitory Effects of Water Deficit on Maize Leaf Elongation. Plant Physiol. 77, 190-194 (1985).
- Silk, W. K., Hsiao, T. C., Diedenhofen, U., Matson, C. Spatial Distributions of Potassium, Solutes, and Their Deposition Rates in the Growth Zone of the Primary Corn Root. Plant Physiol. 82, 853-858 (1986).
- Meiri, A., Silk, W. K., Lauchli, A. Growth and Deposition of Inorganic Nutrient Elements in Developing Leaves of Zea-Mays L. Plant Physiol. 99, 972-978 (1992).
- Neves-Piestun, B. G., Bernstein, N. Salinity-induced inhibition of leaf elongation in maize is not mediated by changes in cell wall acidification capacity. Plant Physiol. 125, 1419-1428 (2001).
- Bouchabke, O., Tardieu, F., Simonneau, T. Leaf growth and turgor in growing cells of maize (Zea mays L.) respond to evaporative demand under moderate irrigation but not in water-saturated soil. Plant Cell Environ. 29, 1138-1148 (2006).
- Westgate, M. E., Boyer, J. S. Transpiration-Induced and Growth-Induced Water Potentials in Maize. Plant Physiol. 74, 882-889 (1984).
- Horiguchi, G., Gonzalez, N., Beemster, G. T. S., Inze, D., Tsukaya, H. Impact of segmental chromosomal duplications on leaf size in the grandifolia-D mutants of Arabidopsis thaliana. Plant J. 60, 122-133 (2009).
- Fleury, D., et al. The Arabidopsis thaliana homolog of yeast BRE1 has a function in cell cycle regulation during early leaf and root growth. Plant Cell. 19, 417-432 (2007).
- Vlieghe, K., et al. The DP-E2F-like gene DEL1 controls the endocycle in Arabidopsis thaliana. Curr. Biol. 15, 59-63 (2005).
- Boudolf, V., et al. The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis. Plant Cell. 16, 2683-2692 (2004).
- Baskin, T. I., Beemster, G. T. S., Judy-March, J. E., Marga, F. Disorganization of cortical microtubules stimulates tangential expansion and reduces the uniformity of cellulose microfibril alignment among cells in the root of Arabidopsis. Plant Physiol. 135, 2279-2290 (2004).

