体内在肿瘤微环境中 pH 值、 pO2、氧化还原状态、磷酸盐和谷胱甘肽浓度的 EPR 评价
Summary
利用可溶性基团和三苯三苯探针对低场 (L 波段, 1.2 GHz) 电子顺磁共振进行了分析, 以评估乳腺癌小鼠模型中肿瘤微环境中生理上的重要参数。
Abstract
该协议表明, 低场电子顺磁共振 (EPR) 技术的能力, 结合功能顺磁性探针, 提供定量信息的化学肿瘤微环境, 包括pO2, pH 值, 氧化还原状态, 间质无机磷酸盐 (Pi) 和胞内谷胱甘肽 (GSH) 的浓度。特别是, 最近开发的可溶解多功能三苯甲的应用为 pH的体内并行测量提供了无与伦比的机会, pO2和pi 在Extracellular 空间 (希望探针)。用单个探针测量三参数, 可以独立于探针分布和测量时间来进行相关分析。
Introduction
在癌症进展和治疗中的关键作用越来越被赞赏1。在实体肿瘤的重要生理参数中, 组织缺氧2, 酸中毒3,4, 高还原容量5, 细胞内谷胱甘肽6,7,和间质 Pi8有很好的记录。无创性的体内 pO2, pH 值, Pi, GSH 和氧化还原评估提供了独特的洞察力的生物学过程中, 并帮助先进的工具, 临床前筛查抗癌药物和有针对性的治疗策略。通过磁共振成像 (MRI) 和低场强的 EPR 技术, 在组织中合理的射频穿透深度使它们成为无创评估这些参数的最合适方法。MRI 在很大程度上依赖于成像水质子, 并广泛应用于临床设置, 提供解剖分辨率, 但缺乏功能性分辨率。phosphorus-31 核磁共振 (31p-NMR) 测量胞外 Pi 浓度和 pH 值基于来自内源磷酸盐的信号可能有吸引力的特征, 但通常掩盖了几次更高的胞内 Pi 浓度9,10。与此相反, EPR 测量依赖于特殊设计的顺磁探针的光谱学和成像, 以提供功能分辨率。请注意, 由于 epr 的内在敏感性和内生背景 epr 信号的缺乏, 外源 epr 探针比外源核磁共振探针具有更大的优越性。最近开发的双功能 pH 值和氧化还原基团探针11和多功能的三苯基碳探针12为在体内同时测量数个参数提供了无与伦比的机会及其与探针分布和测量时间无关的相关分析。根据我们的知识, 没有其他方法可以同时评估活体中的体内生理上重要的化学参数, 如pO2, pHe, Pi, 氧化还原和 GSH。
探测体内功能测量:
图 1显示用于访问参数的顺磁探头的化学结构, 其中包括微粒和可溶探针。高功能敏感性, 活组织的稳定性和极小的毒性是一些好处, 使微粒探针优于可溶性探针的体内EPR 氧。例如, 微粒探针增加了组织植入部位的保留时间, 与可溶性探针相比, 允许在数周内对组织pO2进行纵向测量。另一方面, 可溶探针通过提供基于 EPR 的成像技术的空间分辨测量, 以及允许多种功能 (pO2、pH 值、Pi、氧化还原和GSH)。
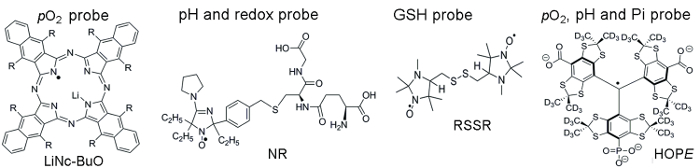
图 1。装配评估试验的顺磁探针的化学结构.这包括微粒pO2探针, 林肯-小波 (R = O (CH2)3CH3) 和可溶性探针: 双重作用 pH 值和氧化还原探针, NR;谷胱甘肽敏感探针, RSSR;和多功能pO2, pH 和 Pi 探针的胞外微环境, 希望探针.在提供的引用11、12中描述了这些探测器的合成。请单击此处查看此图的较大版本.
Protocol
Representative Results
Discussion
所提出的方法允许对化学药品的关键参数进行无创的体内评估, 即pO2, pH 值, 氧化还原状态, 以及间质 Pi 和胞内谷胱甘肽的浓度。磁共振技术, 如 MRI 和低场强 EPR, 是在这些参数的无创体内分析的选择方法。MRI 能直观地解剖结构, 但缺乏功能敏感性。与 MRI 相比, EPR 技术在与功能旋转探针结合使用时, 对微环境的局部参数提供了功能敏感性。根据我们的知识, 没有其他方法?…
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
这项工作得到了 NIH 赠款 CA194013、CA192064 和 U54GM104942 的部分支持。WVCTSI 被确认为 VVK、AB 和 TDE 的启动。作者感谢 m. Gencheva 博士和 k. Steinberger 的帮助与说明性实验。内容完全是作者的责任, 不一定代表 NIH 的官方观点。
Materials
| L-band EPR spectrometer | Magnettech, Germany | L-band (1.2 GHz) electron paramagnetic resonance (EPR) spectrometer for collection in vitro and in vivo spectra of paramagnetic molecules | |
| Temperature & Gas Controller | Noxygen, Germany | Temperature & Gas Controller designed to control and adjust the temperature and gas composition | |
| Sonicator | Fisher Scientific | ||
| GSH (L-Glutathione reduced) | Sigma-Aldrich | G4251 | |
| MMTV-PyMT mice | In house | ||
| DMEM | Thermo Fisher Scientific | 11995065 | |
| Met-1 murine breast cancer cells | In house | ||
| C57Bl/6 wild type mice | Jackson Laboratory | ||
| Trypsin | Thermo Fisher Scientific | 25200056 | |
| Trypan Blue Exclusion Dye | Thermo Fisher Scientific | T10282 | |
| Ohmeda Fluotec 3 | |||
| Isoflurane (IsoFlo) | Abbott Laboratories | ||
| Sodium phosphate dibasic | Sigma-Aldrich | S9763 | |
| Sodium phosphate monobasic | sigma-Aldrich | S07051 | |
| Sodium Chloride | sigma-Aldrich | S7653 | |
| Hydrochloric acid | sigma-Aldrich | 320331 | |
| Sodium Hydroxide | sigma-Aldrich | S8045 | |
| Glucose | sigma-Aldrich | ||
| Glucose oxydase | sigma-Aldrich | ||
| Lauda Circulator E100 | Lauda-Brikmann | ||
| pH meter Orion | Thermo Scientific | ||
| LiNc-BuO probe | In house | The Octa-n-Butoxy-Naphthalocyanine probe was synthesizided according to ref 13 | |
| NR probe | In house | The Nitroxide probe was synthesizided according to ref 11 | |
| RSSR probe | In house | The di-Nitroxide probe was synthesizided according to ref 15 | |
| HOPE probe | In house | The monophoshonated Triarylmethyl probe was synthesizided according to ref 12 |
Riferimenti
- Siemann, D. W. . Tumor Microenvironment. , (2011).
- Tatum, J. L., et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 82 (10), 699-757 (2006).
- Brahimi-Horn, M. C., Chiche, J., Pouyssegur, J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol. 19 (2), 223-229 (2007).
- Haulica, A., Ababei, L. Comparative study of glycolytic activity in the erythrocytes of animals with chronic experimental hypoxia and with tumours. Neoplasma. 21 (1), 29-35 (1974).
- Matsumoto, K., et al. High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin Cancer Res. 12 (8), 2455-2462 (2006).
- Estrela, J. M., Ortega, A., Obrador, E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 43 (2), 143-181 (2006).
- Voegtlin, C., Thompson, J. W. Glutathione content of tumor animals. J. Biol. Chem. 70, 801-806 (1926).
- Bobko, A. A., et al. Interstitial Inorganic Phosphate as a Tumor Microenvironment Marker for Tumor Progression. Sci Rep. 7, 41233 (2017).
- Gillies, R. J., Raghunand, N., Garcia-Martin, M. L., Gatenby, R. A. pH imaging. A review of pH measurement methods and applications in cancers. IEEE Eng Med Biol Mag. 23 (5), 57-64 (2004).
- Gade, T. P., et al. Imaging intratumoral convection: pressure-dependent enhancement in chemotherapeutic delivery to solid tumors. Clin Cancer Res. 15 (1), 247-255 (2009).
- Bobko, A. A., et al. In vivo monitoring of pH, redox status, and glutathione using L-band EPR for assessment of therapeutic effectiveness in solid tumors. Magn Reson Med. 67, 1827-1836 (2012).
- Dhimitruka, I., Bobko, A. A., Eubank, T. D., Komarov, D. A., Khramtsov, V. V. Phosphonated Trityl Probe for Concurrent In Vivo Tissue Oxygen and pH Monitoring Using EPR-based Techniques. JACS. 135, 5904-5910 (2013).
- Pandian, R. P., Parinandi, N. L., Ilangovan, G., Zweier, J. L., Kuppusamy, P. Novel particulate spin probe for targeted determination of oxygen in cells and tissues. Free Radic Biol Med. 35 (9), 1138-1148 (2003).
- Bobko, A. A., Evans, J., Denko, N. C., Khramtsov, V. V. Concurrent Longitudinal EPR Monitoring of Tissue Oxygenation, Acidosis, and Reducing Capacity in Mouse Xenograft Tumor Models. Cell Biochem Biophys. 75, 247-253 (2017).
- Khramtsov, V. V., Yelinova, V. I., Glazachev Yu, I., Reznikov, V. A., Zimmer, G. Quantitative determination and reversible modification of thiols using imidazolidine biradical disulfide label. J Biochem Biophys Methods. 35 (2), 115-128 (1997).
- Roshchupkina, G. I., et al. In vivo EPR measurement of glutathione in tumor-bearing mice using improved disulfide biradical probe. Free Rad. Biol. Med. 45, 312-320 (2008).
- Khramtsov, V. V., Zweier, J. L., Hicks, R. . Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds. , 537-566 (2010).
- Bobko, A. A., Dhimitruka, I., Zweier, J. L., Khramtsov, V. V. Fourier Transform EPR of Trityl Radicals for Multifunctional Assessment of Chemical Microenvironment). Angew. Chem. Int. Edit. 53, 2735-2738 (2014).
- Martin, M. L., Martin, G. J., Delpuech, J. J. . Practical NMR spectroscopy. , (1980).
- Lin, E. Y., et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 163 (5), 2113-2126 (2003).
- Eubank, T. D., et al. Granulocyte macrophage colony-stimulating factor inhibits breast cancer growth and metastasis by invoking an anti-angiogenic program in tumor-educated macrophages. Cancer Res. 69 (5), 2133-2140 (2009).
- Khramtsov, V. V., et al. Quantitative determination of SH groups in low- and high-molecular-weight compounds by an electron spin resonance method. Anal Biochem. 182 (1), 58-63 (1989).
- Komarov, D. A., et al. Electron paramagnetic resonance monitoring of ischemia-induced myocardial oxygen depletion and acidosis in isolated rat hearts using soluble paramagnetic probes. Magnetic Resonance in Medicine. 68 (2), 649-655 (2012).
- Song, Y. G., Liu, Y. P., Liu, W. B., Villamena, F. A., Zweier, J. L. Characterization of the binding of the Finland trityl radical with bovine serum albumin. Rsc Advances. 4 (88), 47649-47656 (2014).
- Khramtsov, V. V., Bobko, A. A., Tseytlin, M., Driesschaert, B. Exchange Phenomena in the Electron Paramagnetic Resonance Spectra of the Nitroxyl and Trityl Radicals: Multifunctional Spectroscopy and Imaging of Local Chemical Microenvironment. Analyt. Chem. 89 (9), 4758-4771 (2017).
- Samouilov, A., et al. In Vivo Proton-Electron Double-Resonance Imaging of Extracellular Tumor pH Using an Advanced Nitroxide Probe. Analyt. Chem. 86 (2), 1045-1052 (2014).
- Goodwin, J., et al. In vivo tumour extracellular pH monitoring using electron paramagnetic resonance: the effect of X-ray irradiation. NMR Biomed. 27 (4), 453-458 (2014).

