Measuring Active and Passive Tameness Separately in Mice
Summary
Tameness in animals includes a reduction of avoidance responses to humans (passive tameness) and an increase in active approaches to humans (active tameness). Here, we describe detailed protocols for three behavioral tests (active tameness, passive tameness, and stay-on-hand tests) to separately measure the active and passive tameness of mice.
Abstract
Domesticated animals such as dogs and laboratory mice show a high level of tameness, which is important for humans to handle them easily. Tameness has two behavioral components: a reluctance to avoid humans (passive tameness) and a motivation to approach humans (active tameness). To quantify these components in mice, we previously developed behavioral tests for active tameness, passive tameness, and the willingness to stay on a human hand, each designed to be completed within 3 min. The data obtained were used for selective breeding, with a large number of mice analyzed per generation. The active tameness test measures the movement of the mouse toward a human hand and the contact it engages in. The passive tameness test measures the duration of time that a mouse tolerates human touch. In the stay-on-hand test, a mouse is placed on a human hand and touched slowly using the thumb of that hand; the duration of time that the animal remains on the hand is measured. Here, we describe the test set-up and apparatus, explain the procedures, and discuss the appropriate data analysis. Finally, we explain how to interpret the results.
Introduction
Selective breeding during domestication has changed the characteristics of domesticated animals to make them better adapted for living with humans1,2,3. "Tameness" is a common behavioral trait observed in domesticated animals. The studies of selective breeding for tameness in animals reveal how their phenotypes have changed in the course of domestication. In a famous study, foxes were selectively bred for tameness and a group of foxes that exhibited a high level of tameness was established2. The foxes also showed other phenotype changes involving morphology, reproduction, coat color, and physiological traits. Selective breeding for tameness in mice, a popular laboratory animal, can help reveal the mechanism underlying the change in a variety of phenotypes during domestication.
Tameness has two behavioral components: active approaches to humans (active tameness) and a reduction in the avoidance of humans (passive tameness)4. In a previous study, we established three behavioral tests to characterize the tameness levels in mice: active tameness, passive tameness, and stay-on-hand tests5. The first test was designed to quantify a mouse's active tameness by measuring the latency of its spontaneous approach toward a human and the time it spent in contact with a human hand. The second test was designed to quantify passive tameness by measuring how long a mouse tolerated being touched by a human hand. The stay-on-hand test was intended to quantify behavioral avoidance of being stroked mildly by a human hand. These three assessments require only an open-field apparatus, a digital camera, and a large pair of tweezers. They can be completed sequentially within 3 min, enabling a large quantity of data to be collected per day.
In the previous study, we characterized and compared tameness behaviors using a series of inbred mouse strains: 10 wild strains, one Japanese fancy mouse-derived strain, and six laboratory strains5. Most domesticated strains showed a significantly higher passive tameness than wild strains, but the active tameness did not differ between the two groups. From these results, we concluded that the domesticated mice that are the founders of laboratory strains were predominantly selected for their passive tameness, but not for active tameness, in their breeding history.
We next conducted selective breeding with eight wild mouse strains to generate a genetically heterogeneous population6. We chose the animals that scored high on the active tameness test and successfully increased their active tameness in successive generations, compared with a control group. This outcome indicated that the tests reliably measure the tameness in mice.
Here, we describe the comprehensive method for measuring behavioral indices using the three behavioral tests. First, we explain the set-up and necessary apparatus. Next, we detail the step-by-step procedure for running the experiments, collecting data through video, and analyzing said data using freeware that we developed. Finally, we show representative results comparing the pool of selectively bred mice with a control group.
Protocol
All experiments were performed in accordance with the guidelines for animal experiments from the National Institute of Genetics (NIG), and all procedures were approved by the NIG Committee for Animal Care and Use (approval no. 26-9).
1. Animals
- Selective breeding
- Create wild-derived heterogeneous stock (WHS)6 mice by crossing 8 inbred strains (MSM, HMI, BLG2, PGN2, KJR, CHD, NJL, and BFM/2) while avoiding any inbreeding (mating between siblings or cousins)7. Conduct a selective breeding, starting in the third generation (G3), by selecting mice that display longer “contact time” (selected group, 16 families of mating pairs) according to the active tameness test (see step 3.1).
- Generate the non-selected control group (also 16 families of mating pairs) via random mating from G3 onward. For a representative experiment, use mice obtained from selected (S1) and non-selected (C1) groups of WHS mice to show the pattern of both active and passive tameness using the three tameness tests (see step 3.1 – 3.3). For the experiments, use 10 G15 mice per group (S1 and C1).
- Representative subject animals
- Wean all mice from their parents at 3 weeks of age and house them in same-sex groups with littermates, using standard-size plastic cages containing wood chips, until the test. Maintain all animals at an NIG animal facility under a 12/12 h light/dark cycle (lights on from 06:00 to 18:00) and a constant temperature (23 ± 2 °C).
- Ensure that food and water are available ad libitum. Test the mice at 6 weeks of age during the light period to analyze their interactions with humans during the part of the day when humans are typically active.
- Handling protocol
- Use large tweezers covered with silicon tubes to transfer the mice to new cages by picking them up gently by their tails. Use the tweezers to separate them from their parents at weaning and move them during the experiments. None of the mice should receive handling before the tameness tests.
- Transfer the mice to an experimental room just before the tameness tests.
- Keep the male mice separated from one another after the test, to avoid inter-male aggression in WHS mice.
2. Equipment
- Use an open-field apparatus (400 x 400 x 400 mm, grayish polyvinylchloride).
- Illuminate the open field using a clip light with a neutral white bulb (100 lux) fixed at a height of 450 mm above the open field (Figure 1).
- Record all behaviors using a commercially available compact digital camera fixed at 850 mm above the open field using a tripod (Figure 1).
- Wear cloth gloves under latex gloves to protect the hands from being bitten at during the passive tameness test (a rare reaction).
- Measure the test period using a stopwatch.
3. Tests
Note: The first two tests (active tameness and passive tameness) were conducted for 1 min each, and the stay-on-hand test was conducted for less than 1 min. All three tests were conducted in the open-field apparatus and recorded using a digital camera. Iodophore disinfection procedure should be performed on all apparats before any behavior studies.
- Procedures for active tameness test
- Turn on the digital camera and start recording.
- Pick up a mouse gently by the tail using a pair of large tweezers (see step 1.3.1) and transfer it from the home cage to the center of the open-field apparatus.
- Start the stopwatch.
- Place the left hand on the lower left corner of the apparatus.
- Keep the palm facing up and move the fingers slowly and steadily to ensure that the mouse connects the hand to a human. The average time to move all four fingers from the little finger to the index finger should be approximately 0.5 s, with a continuously repeated movement cycle.
- Move the hand toward the mouse slowly, at an average speed of about 13 cm/s, stopping at 10 cm from the side of the mouse’s body.
- Maintain the same distance if the mouse attempts to move away. If the mouse approaches, keep the hand in the same position and continue to move the fingers slightly. If the mouse attempts to climb up the arm, return it to the field using the free hand.
- Conclude the test after 1 min, withdraw the hand, and proceed to the passive tameness test.
- Procedures for passive tameness test
- Follow the same steps as for the active tameness test, but instead of stopping 10 cm away from the mouse’s body, slowly touch the mouse with the fingertips.
- Leave the fingers in the same position, contacting the mouse, until the mouse moves away. When the mouse attempts to break contact, follow it, moving at an average speed of about 23 cm/s, and reinitiate the touch.
- Conclude the test after 1 min. Restart the stopwatch immediately to begin the stay-on-hand test.
- Procedures for stay-on-hand test
- Lift the mouse by the tail using a pair of large tweezers and place it gently on the left palm. Keep the hand close to the floor of the open-field apparatus.
- Stroke the mouse gently at an average speed of 0.5 s/stroke using the left thumb until it leaves the hand.
- Repeat steps 3.3.1 – 3.3.2 3x.
- After the third trial, stop the video recording; all tameness tests are now concluded.
- Post-experimental procedures
- Weigh the mouse and count the number of feces and urine left in the open field.
- Mark the mouse individually via an ear punch.
- Wipe the apparatus and experimenter gloves with a paper towel soaked in 0.2% Iodophor sanitizer.
- Clean the apparatus with a dry paper towel.
4. Behavioral analyses
Note: Video recordings were scored using tanaMove version 0.01 (Windows 7), event-recording software for behavioral analyses (Figure 2). The software is freely available (Supplemental Materials 1) and from our website (http://www.mgrl-lab.jp/MGRL_software.html). An individual who was blind to the test conditions scored all footage. The duration of all behavioral indices was measured in 0.1-s increments. During the scoring, the observer pressed the appropriate letter on the keyboard (see step 4.1) upon noting a given behavior.
- Assignment of keys for video analysis
- Assign keys for each behavior index. Using appropriate keys for each behavioral index is critical to summarize the data automatically in step 4.3.
Note: The assignments used in this study are shown in Figure 3.
- Assign keys for each behavior index. Using appropriate keys for each behavioral index is critical to summarize the data automatically in step 4.3.
- Video analysis
- Analyze each video recording 3x for different behavioral indices (Figure 3B). Self-grooming behavior was not scored. Make sure the built-in speaker of the computer is switched on in order to hear the timer sound in the video.
- Launch tanaMove.
- Drag and drop the video file to be analyzed into the window of tanaMove. Confirm that the file is loaded and that the path and name of the outputted CSV file in the Data File [.csv] box is correct.
- Click the Ready button. This activates a new window to enter the assigned keys.
- Click the Play button to start the video file. Click the Stop button to pause the video file.
- Press key A when the mouse is observed “heading” toward or approaching the hand, including standing to sniff. Press key R when the mouse is observed “locomoting” without heading toward the hand during the active tameness test.
- Press key W for at least 0.3 s (at least three intervals in the window) when the timer sound is heard at 60 s.
- Initiate the analysis of the passive tameness test after the left hand enters the video. Press key A when the mouse is observed heading toward or approaching the hand and key R when the mouse is observed traveling without heading toward the hand during the passive tameness test.
- Press key W for at least 0.3 s (at least three intervals in the window) when the timer sound is heard at 60 s.
- Press key T when the mouse is observed “staying” on the hand in the stay-on-hand test.
- After the stay-on-hand test, click the Stop button to stop the video observation.
- Click the Indietro button and type a new file name for the CSV data output.
- Click the Ready button to activate a new window to enter the assigned keys.
- Click the Play button to restart the video footage and initiate the observation of the active tameness test.
- Press key T when the mouse is observed “contacting” the human, which includes all voluntary contacting behavior but not accidental contacts, such as landing on a palm after jumping, during the active tameness test.
- Press key W for at least 0.3 s (at least three intervals in the window) when the timer sound is heard at 60 s.
- Press key T when the mouse allows the experimenter to touch its body during the passive tameness test.
- Press key W for at least 0.3 s (at least three intervals in the window) when the timer sound is heard at 60 s.
- Click the Stop button to stop the video.
- Click the Indietro button and type a new file name for the CSV data output.
- Click the Ready button to activate a new window to enter the assigned keys.
- Click the Play button to restart the video footage and initiate the observation of the active tameness test.
- Press key J when the mouse is observed “jumping” during the active tameness test.
- Press key W for at least 0.3 s (at least three intervals in the window) when the timer sound is heard at 60 s. Then start the observation of the passive tameness test.
- Press key J when the mouse is observed “jumping” during the passive tameness test.
- Click the Stop button to stop the video observation when the timer sound is heard at 60 s.
- Click the Indietro button and type a new file name for the CSV data output.
- Summarize the data
- Save all the raw data files (CSV) in the same folder with the Supplementary 2.xlsm file (Supplemental Materials 2).
- Open the Supplementary 2.xlsm file and click the Summarize button.
- Save the results as a separate file and use it for further analysis of the tameness behavior.
Representative Results
We previously conducted selective breeding for active tameness in miceandsuccessfully produced mice that showed a high level of active tameness6. The representative results of the tameness tests for the selected and non-selected groups of mice at the 15th generation (G15) are shown in Figure 4. Mice from the selected group scored higher on the active tameness test, exhibiting a significantly longer duration of contact with a human hand than the control mice did. This contact duration was an index used for selective breeding. The two groups did not differ significantly in heading, locomotion, or jumping duration (Figure 4A, Table 1), but the selected mice scored higher on the passive tameness test, tolerating human contact for significantly longer periods of time than the control mice did. In addition, the selected mice exhibited a significantly shorter locomotion and jumping duration (Figure 4B, Table 1). Finally, the selected mice remained on a human hand significantly longer than the control mice did in the stay-on-hand-test (Figure 4C, Table 1). Together, these results demonstrated that the selectively bred mice were tamer than the control mice.
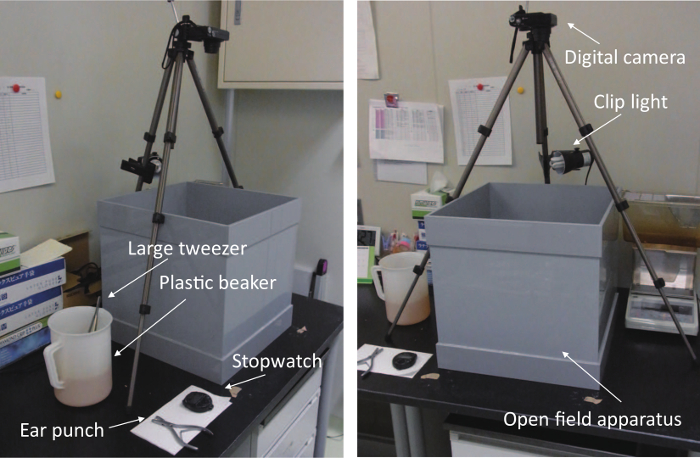
Figure 1: Tameness test apparatus. These panels show the apparatus from different angles (left and right). The set-up consisted of an open field, a tripod, a clip light, a digital camera, a stopwatch, an ear punch, large tweezers, and a plastic beaker containing 0.2% Iodophor sanitizer. The clip light was turned on during the test. The tripod height was adjusted so that the digital camera could capture the entire space within the apparatus. Ear punching and body weight measurements were conducted immediately after the test. Please click here to view a larger version of this figure.
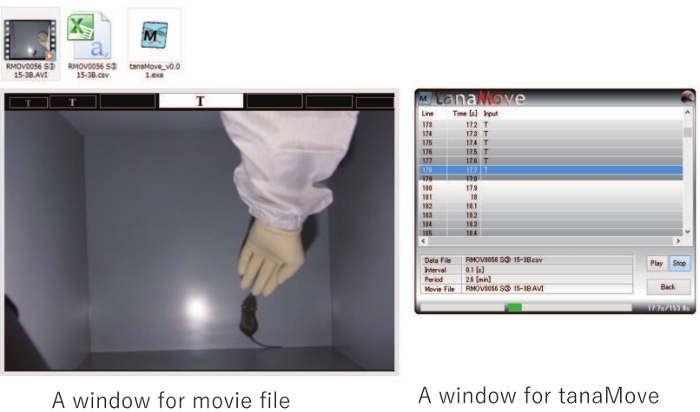
Figure 2: Measurement of behavioral indices through video scoring. Behavioral indices in the tameness tests were scored using the software tanaMove. This figure shows a still shot from a video recording, 17.7 s after the start of an active tameness test. The mouse is in contact with the experimenter, so the observer recorded the key T, as shown in the editing window of tanaMove. Please click here to view a larger version of this figure.
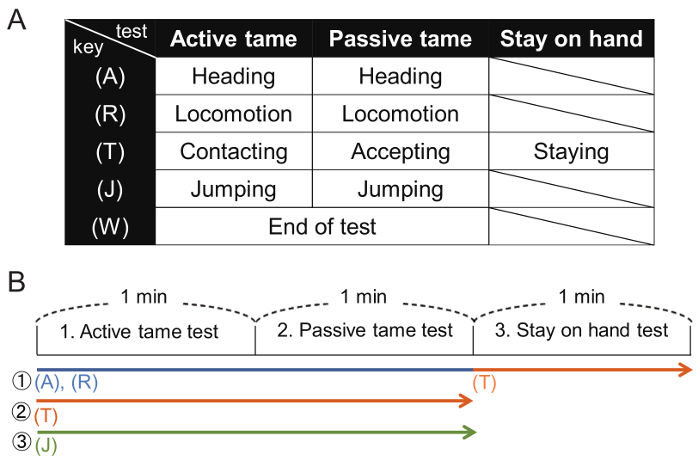
Figure 3: Analysis of the video data using tanaMove. (A) Each behavioral index was assigned to a single key on the keyboard. (B) The active and passive tameness tests were conducted in succession for 1 min each and followed by the stay-on-hand test. The observation indices were measured in chronological order, 1 to 3. Please click here to view a larger version of this figure.
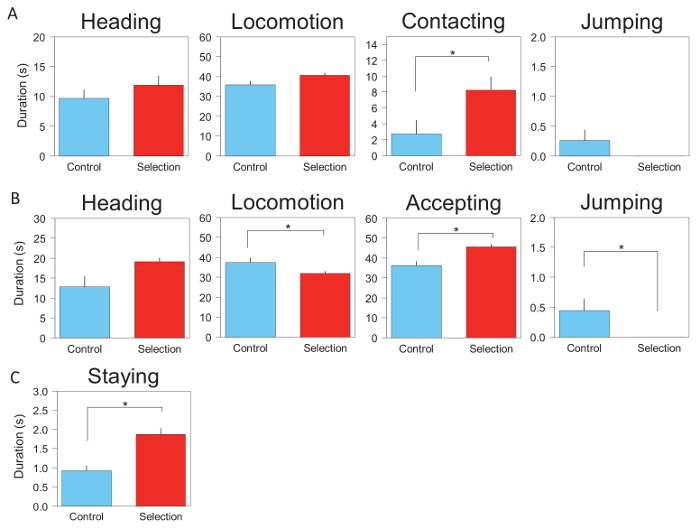
Figure 4: Representative results of the tameness tests. For the representative experiment, we used mice obtained from selected (S1) and non-selected (C1) groups of WHS, to show the pattern of both active and passive tameness using the three tameness tests. For the experiments, 10 G15 mice per group (S1 and C1) were used. (A) These panels show the results of the active tameness test. Four behavioral indices were compared between the selected and control groups: heading, locomotion, contact, and jumping. (B) These panels show the results of the passive tameness test. Four behavioral indices were compared between the selected and control groups: heading, locomotion, accepting, and jumping. (C) This panel shows the result of the stay-on-hand test. A single index was compared between the selection and control groups: staying on the hand. All values are averages of three trials. Error bars indicate SEM. Please click here to view a larger version of this figure.
| Tame test | Behavior index | Group | Shapiro-Wilk test | Mann-Whitney U test |
| Active tame test | Heading | Control | 0.3991 | 0.5922 |
| Selected | 0.4907 | |||
| Locomotion | Control | 0.3832 | 0.0853 | |
| Selected | 0.3599 | |||
| Contacting | Control | < 0.0001 | 0.0049 | |
| Selected | 0.1336 | |||
| Jumping | Control | < 0.0001 | 0.2105 | |
| Selected | ― | |||
| Passive tame test | Heading | Control | 0.0408 | 0.0600 |
| Selected | 0.7725 | |||
| Locomotion | Control | 0.1470 | 0.0373 | |
| Selected | 0.4356 | |||
| Accepting | Control | 0.5834 | 0.0023 | |
| Selected | 0.7742 | |||
| Jumping | Control | 0.0048 | 0.0108 | |
| Selected | ― | |||
| Stay on hand test | Staying | Control | 0.6683 | 0.0003 |
| Selected | 0.1897 |
Table 1: Results of a statistical analysis comparing behavioral indices between the selected and control groups. The p-values from Shapiro-Wilk and Mann-Whitney U-tests are shown. Given that some behavioral indices had non-normal distributions, we used the Mann-Whitney U-test to examine the differences between the selected and control groups. A dash (-) means that the statistics could not be calculated because all individuals exhibited zero scores.
Discussion
The representative results cover nine behavioral indices from the active tameness, passive tameness, and stay-on-hand tests. A comparison of the scores between the selected and control groups revealed clear differences in tameness. These results show that the three tests are valid for measuring tameness in mice.
Tameness levels in animals can be difficult to quantify empirically. Several methods have been developed for evaluating tameness in deer, mice, and rats8,9,10. However, these methods did not quantify active and passive tameness separately. In a previous study, we developed three tameness tests to quantify tameness in mice5. Using scores from the active tameness test, we conducted selective breeding experiments and successfully bred mice that were actively tame6. In addition to being more willing to approach and touch humans during the active tameness test, selectively bred mice also tolerated human contact longer during the passive tameness test, even though results from that test were not used as the basis of selection. These results suggested that the two tests are not entirely independent and share some overlap.
In order to accurately measure the innate tameness of mice to humans, it is critical to avoid handling the mice until the tameness tests. To avoid direct contact between humans and mice, we used large tweezers covered with silicon tubes to pick up the mice by their tails and transfer them to new cages5. The silicon tubing cover makes the transfer less painful for the mice.
We did not conduct tameness tests during the dark phase of each 24-h period. However, it might be interesting to test whether the light and dark phases affect tameness. As the open-field apparatus is known to induce fear and stress responses in mice, and for this reason is frequently used to measure emotionality, some degree of tameness may have been suppressed during the test by the fearful/stress responses of the mice. Although we were able to establish groups of mice that exhibited a high level of active tameness to humans under the given test conditions, future studies should seek to reduce the fear and stress that mice feel during the test, for instance by conducting the tests during the dark phase or by habituating the mice to the apparatus before the tests.
In addition, conducting the tameness test with an experimenter who is blind to the status of the test animals (selected or non-selected) could make the test more reliable8.
Notably, the three tests may be applied to both laboratory and wild mouse strains5. Because the tests could be applied to wild strains, we were able to establish a mouse population exhibiting high active tameness using a genetically heterogeneous stock descended from eight wild mouse strains6.
Tameness test results were quantified through video scoring. To ensure consistency, a single person analyzed all video data for the selective breeding experiments in the present study. However, this method requires the observer to be an expert who can produce reliable and reproducible results, so care should be taken to properly train video scorers. Future work on tameness in mice could potentially take advantage of automatic analysis software that replaces human observers, an approach that was used in a previous study of social behavior11.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI) as follows: 15H05724, 16H01491, and 15H04289.
Materials
| open-field apparatus | O’Hara & Co. Ltd. | OF-3102 | 400×400×400 mm, grayish polyvinylchloride |
| compact digital camera | Ricoh Company, Ltd. | CX-5 | production in the company is over |
| latex pure gloves | AS ONE | BioLab, IKG-8002 | |
| stopwatch | SEIKO | SSBJ018 | |
| Iodophor sanitizer | Ecolab G.K. (CLEA Japan) | CL-4123 | Mikro Klene |
Riferimenti
- Albert, F. W., et al. Phenotypic differences in behavior, physiology and neurochemistry between rats selected for tameness and for defensive aggression towards humans. Hormones and Behavior. 53 (3), 413-421 (2008).
- Belyaev, D. K. Destabilizing selection as a factor in domestication. Journal of Heredity. 70 (5), 301-308 (1978).
- Trut, L. Early canid domestication: the farm-fox experiment. American Scientist. 87 (2), 160-169 (1999).
- Price, E. O. . Animal Domestication and Behavior. , (2002).
- Goto, T., Tanave, A., Moriwaki, K., Shiroishi, T., Koide, T. Selection for reluctance to avoid humans during the domestication of mice. Genes, Brain and Behavior. 12 (8), 760-770 (2013).
- Matsumoto, Y., et al. Selective breeding and selection mapping using a novel wild-derived heterogeneous stock of mice revealed two closely linked loci for tameness. Scientific Reports. 7, 4607 (2017).
- Koide, T., Goto, T., Takano-Shimizu, T. Genomic mixing to elucidate the genetic system of complex traits. Experimental Animals. 61 (5), 503-509 (2012).
- Albert, F. W., et al. Genetic architecture of tameness in a rat model of animal domestication. Genetica. 182 (2), 541-554 (2009).
- Cottle, C. A., Price, E. O. Effects of the nonagouti pelage-color allele on the behavior of captive wild Norway rats (Rattus norvegicus). Journal of Comparative Psychology. 101 (4), 390-394 (1987).
- Hayssen, V. Effects of the nonagouti coat-color allele on behavior of deer mice (Peromyscus maniculatus): a comparison with Norway rats (Rattus norvegicus). Journal of Comparative Psychology. 111 (4), 419-423 (1997).
- Arakawa, T., et al. A male-specific QTL for social interaction behavior in mice mapped with automated pattern detection by a hidden Markov model incorporated into newly developed freeware. Journal of Neuroscience Methods. 234, 127-134 (2014).

