从植物提取物和分数中识别新型抗菌和抗生物膜分子的系统方法,以防止牙科蛀牙
Summary
天然产品是开发新药和治疗剂的有希望的起点。然而,由于化学多样性高,从植物中寻找新的治疗化合物是一项具有挑战性和耗时的任务。我们描述了从植物提取物和分数中识别抗菌和抗生物膜分子的简化方法。
Abstract
天然产品提供结构不同的物质,具有无数的生物活动。然而,由于复杂的植物基质和耗时的隔离和鉴定程序,从植物中识别和分离活性化合物是具有挑战性的。因此,提出了一种从植物中筛选天然化合物的渐进方法,包括分离和识别潜在的活性分子。它包括植物材料的收集:原油提取物的准备和分馏;色谱和光谱学(UHPLC-DAD-HRMS和NMR)分析和化合物鉴定方法:生物分析(抗菌和抗生物膜活动;细菌”粘附力”到唾液颗粒和初始葡萄糖基质用选定的治疗治疗):和数据分析。该模型简单、可重复,可持续控制多种化合物的高通量筛选、浓度和处理步骤。获得的数据为今后的研究提供了基础,包括含有最活跃提取物和/或分数的配方、分子的分离、微生物细胞和生物膜中特定目标的分子建模。例如,控制致癌生物膜的一个目标是抑制链 球菌突变 体葡萄糖转移酶的活性,这种转移酶合成细胞外基质的葡萄糖。这些酶的抑制可以防止生物膜的积累,降低其毒性。
Introduction
社会中最早使用的药物模型以天然产品为基础。从那时起,人类一直在寻找新的化学物质在自然界中,可以转化为药物1。这一搜索使民族植物筛查的技术和方法不断改进。NPs 提供了结构多样化物质的丰富来源,具有广泛的生物活动,可用于开发替代疗法或辅助疗法。然而,固有的复杂植物矩阵使得活性化合物的分离和鉴定成为一项具有挑战性和耗时的任务。
基于NPs的药物或配方可用于预防和/或治疗影响口腔的几种疾病,包括牙科蛀牙4。牙科蛀牙是全球最普遍的慢性疾病之一,它源于牙齿表面形成的富含糖分的饮食和微生物生物膜(牙菌斑)的相互作用,导致微生物代谢产生的有机酸导致脱氧,如果不治疗,会导致牙齿脱落5、6。虽然其他微生物可能与7有关,但链球菌是一种关键的致癌细菌,因为它是酸性、酸性且细胞外基质构建者。该物种编码多个外酶(如糖素转移酶或Gtfs),使用蔗糖作为基材8,以建立富含外聚糖精的细胞外基质,这是一个毒性决定因素9。此外,真菌念珠菌可以推动细胞外矩阵7的生产。尽管氟化物以各种方式施用,仍然是预防蛀牙10的基础,但需要新的方法作为辅助剂,以提高其有效性。此外,现有的抗斑块模式是基于使用广谱微生物制剂(如氯河西丁)11。作为替代方案,NPs是控制生物膜和预防蛀牙12,13的潜在疗法。
从植物中发现新的生物活性化合物的进一步进展包括必要的步骤或方法,例如:(一) 使用可靠和可重复的协议进行采样,考虑到植物往往表现出特异性:(二) 编制小规模的综合提取物及其各自的分数:(三) 其化学特征的定性和/或复制认为获取多维数据,例如GC-MS、LC-DAD-MS或NMR:(四) 使用可行的高产模型来评估生物活性:(五) 根据多变量数据分析或其他统计工具选择潜在的新热门歌曲:(六) 对目标化合物或有前途的候选人进行隔离和净化:和(七)使用分离化合物2,14对应生物活动的验证。
复制是快速识别原油提取物中已知化合物的过程,允许将新化合物与已经研究过的化合物区分开来。此外,当某些化合物已经描述生物活性时,这个过程可以防止隔离,并且特别有助于检测”频繁的击球手”。它已用于不同的非目标工作流程,从主要化合物识别或活动引导分馏的加速到提取物集合的化学剖析。它可以与代谢学研究完全集成,用于CE的非目标化学分析或代谢物的有针对性识别。所有这些最终导致在隔离程序1,15,16,17之前优先考虑提取物。
因此,在本手稿中,我们描述了从植物提取物和分数中识别抗菌和抗生物膜分子的系统方法。它包括四个多学科步骤:(1) 收集植物材料:((二)制备粗制提取物和馏分,然后进行化学剖面分析:(3) 生物分析:(4)生物和化学数据分析(图1)。因此,我们提出了为分析 Cacasaria西尔维斯特里斯 提取物和针对 链球菌突变 体和 念珠菌13的分馏的抗菌和抗生物膜活动而制定的协议,以及植物化学特征和数据分析的程序。为了简单起眼,这里的重点是演示使用细菌筛选天然化合物的方法。
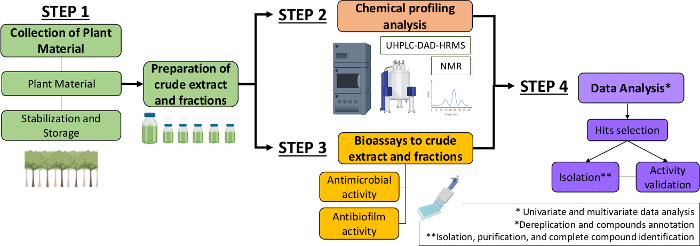
图1:系统方法的流程图,以识别植物提取物和分数中的活性分子。请点击这里查看此数字的较大版本。
Protocol
Representative Results
Discussion
与天然原油提取物工作相关的主要挑战包括其复杂的组成和经典生物制导隔离研究的不足。虽然这个过程是缓慢的,但它是有效的,并导致在NP研究的主要发现。为了合理化,需要优先级驱动的研究来合理化。因此,使用现代化学分析方法分析CE和分离前的复制是重要的特征研究材料,特别是有用的,以避免重新隔离已知化合物与已经描述的生物活性2,15。<…
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
我们感谢联合国ESP化学研究所(NuBBE)的比奥莱奥·德·比奥恩萨奥斯、比奥森特塞·埃·普罗杜托斯·纳图拉伊斯(NuBBE)为植物材料的编写提供实验室。我们还感谢牙科材料和普罗斯特霍顿学部、UNESP、阿拉夸拉/SP的应用微生物实验室。这项研究得到了圣保罗研究基金会(FAPESP #2013/07600–3 至 AJC)的研究资助和奖学金以及间接费用基金 (FAPESP #2017/07408-6 和 FAPESP #2019/23175-7 到 SMR) 的支持: #2011/21440–3 和#2012/21921–4 至 PCPB)。国家科学和技术发展委员会与FAPESP合作提供了额外支持(国家科学技术中心#465637/2014-0和FAPESP#2014/50926-0至AJC)。
Materials
| 96-well microplates | Kasvi | Flat bottom | |
| Activated carbon | LABSYNTH | Clean up and/or fractionation step | |
| Analytical mill | Ika LabortechniK | Model A11 Basic | |
| Blood agar plates | Laborclin | ||
| Chromatographic column C18 | Phenomenex Kinetex | 150 × 2.1 mm, 2.6 µm, 100Â | |
| Dimethyl sulfoxide | Sigma-Aldrich | Vehicle solution | |
| ELISA plate reader | Biochrom Ez | ||
| Ethanol | J. T. Baker | For extraction and fractionation steps, and mobile phase composition | |
| Ethanol | Sigma-Aldrich | Vehicle solution | |
| Ethyl acetate | J. T. Baker | Fractionation step | |
| GraphPad Software | La Jolla | GraphPad Prism7 | |
| Hexane | J. T. Baker | Fractionation step | |
| Incubator | Thermo Scientific | ||
| Isopropanol | J. T. Baker | For extraction step | |
| Lyophilizer (a freeze dryer) | Savant | Modulyo | |
| Nylon Millipore | LAC | 0.22 µm x 13 mm | |
| Orbital shaker | Quimis | Model G816 M20 | |
| Polyamide solid phase extraction cartridge | Macherey-Nagel | Clean up and/or fractionation step | |
| Silica gel | Merck | 40–63 μm, 60 Â | |
| Sodium Chloride (NaCl) | Synth | 0,89% in water | |
| Solid phase extraction cartridges (SPE) | Macherey-Nagel | Clean up and/or fractionation step | |
| Tryptone | Difco | ||
| UHPLC-DAD | Dionex | Ultimate 3000 RS | |
| Ultrasonic bath | UNIQUE | Model USC 2800 | |
| Yeast extract | Difco |
Riferimenti
- Newman, D. J., Cragg, G. M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. Journal of Natural Products. 83 (3), 770-803 (2020).
- Wolfender, J. L., Litaudon, M., Touboul, D., Queiroz, E. F. Innovative omics-based approaches for prioritisation and targeted isolation of natural products – new strategies for drug discovery. Natural Product Report. 36 (6), 855-868 (2019).
- Michel, T., Halabalaki, M., Skaltsounis, A. New Concepts, Experimental Approaches, and Dereplication Strategies for the Discovery of Novel Phytoestrogens from Natural Sources. Planta Medica. 79 (7), 514-532 (2013).
- Jeon, J. G., Rosalen, P. L., Falsetta, M. L., Koo, H. Natural products in caries research: current (limited) knowledge, challenges and future perspective. Caries Research. 45 (3), 243-263 (2011).
- Tonetti, M. S., Jepsen, S., Jin, L., Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. Journal of Clinical Periodontology. 44 (5), 456-462 (2017).
- Peres, M. A., et al. Oral diseases: a global public health challenge. Lancet. 394 (10194), 249-260 (2019).
- Bowen, W. H., Burne, R. A., Wu, H., Koo, H. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiology. 26 (3), 229-242 (2018).
- Paes Leme, A. F., Koo, H., Bellato, C. M., Bedi, G., Cury, J. A. The role of sucrose in cariogenic dental biofilm formation–new insight. Journal of Dental Research. 85 (10), 878-887 (2006).
- Koo, H., Falsetta, M. L., Klein, M. I. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. Journal of Dental Research. 92 (12), 1065-1073 (2013).
- Cury, J. A., de Oliveira, B. H., dos Santos, A. P., Tenuta, L. M. Are dental fluoride releasing materials clinically effective on caries control. Dental Materials. 32 (3), 323-333 (2016).
- Mattos-Graner, R. O., Klein, M. I., Smith, D. J. Lessons Learned from Clinical Studies: Roles of Mutans Streptococci in the Pathogenesis of Dental Caries. Current Oral Health Reports. 1, 70-78 (2014).
- Rocha, G. R., Florez Salamanca, E. J., de Barros, A. L., Lobo, C. I. V., Klein, M. I. Effect of tt-farnesol and myricetin on in vitro biofilm formed by Streptococcus mutans and Candida albicans. BMC Complementary and Alternative Medicine. 18 (1), 61 (2018).
- Ribeiro, S. M., et al. Antimicrobial and antibiofilm activities of Casearia sylvestris extracts from distinct Brazilian Biomes against Streptococcus mutans and Candida albicans. BMC Complementary and Alternative Medicine. 19 (1), 308 (2019).
- Pilon, A. C., et al. Metabolômica de plantas: métodos e desafios. Quimica Nova. 43 (3), 329-354 (2020).
- Wolfender, J. L., Nuzillard, J. M., Hooft, J. J. J., Renault, J. H., Bertrand, S. Accelerating Metabolite Identification in Natural Product Research: Toward an Ideal Combination of Liquid Chromatography-High-Resolution Tandem Mass Spectrometry and NMR Profiling, in Silico Databases, and Chemometrics. Analytical Chemistry. 91 (1), 704-742 (2019).
- Allard, P. M., et al. Pharmacognosy in the digital era: shifting to contextualized metabolomics. Current opinion in biotechnology. 54, 57-64 (2018).
- Hubert, J., Nuzillard, J., Renault, J. Dereplication strategies in natural product research: How many tools and methodologies behind the same concept. Phytochemistry Reviews. 16, 55-95 (2017).
- Bueno, P. C. P., Pereira, F. M. V., Torres, R. B., Cavalheiro, A. J. Development of a comprehensive method for analysing clerodane-type diterpenes and phenolic compounds from Casearia sylvestris Swartz (Salicaceae) based on ultra-high performance liquid chromatography combined with chemometric tools. Journal of separation science. 38 (10), 1649-1656 (2015).
- Bueno, P. C. P., Lopes, N. P. Metabolomics to Characterize Adaptive and Signaling Responses in Legume Crops under Abiotic Stresses. American Chemical Society omega. 5 (4), 1752-1763 (2020).
- Blaženović, I., Kind, T., Ji, J., Fiehn, O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites. 8 (2), 31 (2018).
- Eloff, J. N. Quantifying the bioactivity of plant extracts during screening and bioassay-guided fractionation. Phytomedicine: International Journal Of Phytotherapy And Phytopharmacology. 11 (4), 370-371 (2004).
- Rios, J. L., Recio, M. C. Medicinal plants and antimicrobial activity. Journal of Ethnopharmacology. 100 (1-2), 80-84 (2005).
- Eloff, J. N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica. 64, 711-714 (1998).
- Eloff, J. N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complementary and Alternative Medicine. 19 (1), 106 (2019).
- Klein, M. I., Xiao, J., Heydorn, A., Koo, H. An analytical tool-box for comprehensive biochemical, structural and transcriptome evaluation of oral biofilms mediated by mutans streptococci. Journal of Visualized Experiments. (47), e2512 (2011).
- Lemos, J. A., Abranches, J., Koo, H., Marquis, R. E., Burne, R. A. Protocols to study the physiology of oral biofilms. Methods in molecular biology. 666, 87-102 (2010).
- Venkitaraman, A. R., Vacca-Smith, A. M., Kopec, L. K., Bowen, W. H. Characterization of glucosyltransferase B, GtfC, and GtfD in solution and on the surface of hydroxyapatite. Journal of Dental Research. 74, 1695-1701 (1995).
- Vacca-Smith, A. M., Venkitaraman, A. R., Quivey, R. G., Bowen, W. H. Interactions of streptococcal glucosyltransferases with alpha-amylase and starch on the surface of saliva-coated hydroxyapatite. Archives of Oral Biology. 41, 291-298 (1996).
- Van Dijck, P., et al. Methodologies for in vitro and in vivo evaluation of efficacy of antifungal and antibiofilm agents and surface coatings against fungal biofilms. Microbial Cell. 5 (7), 300-326 (2018).
- Marsh, P. D. Are dental diseases examples of ecological catastrophes. Microbiology. 149 (2), 279-294 (2003).
- Bowen, W. H., Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Research. 45 (1), 69-86 (2011).
- Lobo, C. I. V., et al. Dual-species biofilms of Streptococcus mutans and Candida albicans exhibit more biomass and are mutually beneficial compared with single-species biofilms. Journal of Oral Microbioly. 11 (1), 1581520 (2019).
- Kim, D., et al. Candida albicans stimulates Streptococcus mutans microcolony development via crosskingdom biofilm-derived metabolites. Scientific reports. 7, 41332 (2017).
- Ferreira, P. M. Folk uses and pharmacological properties of Casearia sylvestris: a medicinal review. Anais da Academia Brasileira de Ciencias. 83 (4), 1373-1384 (2011).
- Xia, L., Guo, Q., Tu, P., Chai, X. The genus Casearia: a phytochemical and pharmacological overview. Phytochemistry Reviews. 14, 99-135 (2015).
- Ferreira, P. M. P., et al. Toxicological findings about an anticancer fraction with casearins described by traditional and alternative techniques as support to the Brazilian Unified Health System (SUS). Journal of Ethnopharmacol. 15, 241 (2019).
- Koo, H., Xiao, J., Klein, M. I., Jeon, J. G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. Journal of Bacteriology. 192 (12), 3024-3032 (2010).
- Maske, T. T., van de Sande, F. H., Arthur, R. A., Huysmans, M. -. C. D. N. J. M., Cenci, M. S. In vitro biofilm models to study dental caries: a systematic review. Biofouling. 33 (8), 661-675 (2017).
- Fu, Y., Luo, J., Qin, J., Yang, M. Screening techniques for the identification of bioactive compounds in natural products. Journal of Pharmaceutical and Biomedical Analysis. 168, 189-200 (2019).
- Sarker, S. D., Nahar, L. An introduction to natural products isolation. Methods in molecular biology. 864, 1-25 (2012).
- Clinical Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. Clinical Laboratory Standards Institute (CLSI). , (2015).
- Saputo, S., Faustoferri, R. C., Quivey, R. G. A drug repositioning approach reveals that Streptococcus mutans is susceptible to a diverse range of established antimicrobials and nonantibiotics. Antimicrobial Agents and Chemotherapy. 62 (1), 01674 (2018).

