Modeling Neonatal Intraventricular Hemorrhage Through Intraventricular Injection of Hemoglobin
Summary
We present a model of neonatal intraventricular hemorrhage using rat pups that mimics the pathology seen in humans.
Abstract
Neonatal intraventricular hemorrhage (IVH) is a common consequence of premature birth and leads to brain injury, posthemorrhagic hydrocephalus (PHH), and lifelong neurological deficits. While PHH can be treated by temporary and permanent cerebrospinal fluid (CSF) diversion procedures (ventricular reservoir and ventriculoperitoneal shunt, respectively), there are no pharmacological strategies to prevent or treat IVH-induced brain injury and hydrocephalus. Animal models are needed to better understand the pathophysiology of IVH and test pharmacological treatments. While there are existing models of neonatal IVH, those that reliably result in hydrocephalus are often limited by the necessity for large-volume injections, which may complicate modeling of the pathology or introduce variability in the clinical phenotype observed.
Recent clinical studies have implicated hemoglobin and ferritin in causing ventricular enlargement after IVH. Here, we develop a straightforward animal model that mimics the clinical phenotype of PHH utilizing small-volume intraventricular injections of the blood breakdown product hemoglobin. In addition to reliably inducing ventricular enlargement and hydrocephalus, this model results in white matter injury, inflammation, and immune cell infiltration in periventricular and white matter regions. This paper describes this clinically relevant, simple method for modeling IVH-PHH in neonatal rats using intraventricular injection and presents methods for quantifying ventricle size post injection.
Introduction
Neonatal IVH originates from the germinal matrix, a site of rapid cell division that is adjacent to the lateral ventricles of the developing brain. This highly vascular structure is vulnerable to hemodynamic instability related to premature birth. Blood is released into the lateral ventricles in germinal matrix hemorrhage (GMH)-IVH when fragile blood vessels within the germinal matrix rupture. In the case of grade IV IVH, periventricular hemorrhagic infarction may also contribute to the release of blood products within the brain.1 The combination of GMH-IVH may cause PHH, particularly after high-grade hemorrhage (grades III and IV)1. PHH can be treated with the placement of a ventriculoperitoneal shunt, but shunt placement does not reverse the brain injury that may occur from IVH. Although modern neonatal intensive care has lowered rates of IVH2, 3, there are no specific treatments for the brain injury or hydrocephalus caused by IVH once it has occurred. A significant limitation in developing preventative treatments for IVH-induced brain injury and PHH is the incomplete understanding of IVH pathophysiology.
Recently, early CSF levels of key blood breakdown product hemoglobin have been shown to be associated with the later development of PHH in neonates with high-grade IVH4. Furthermore, CSF levels of iron-handling pathway proteins—hemoglobin, ferritin, and bilirubin—are associated with ventricle size in neonatal IVH. This was also shown in a multicenter cohort of infants with preterm PHH, where higher ventricular CSF levels of ferritin were associated with larger ventricle size5.
In this study, we developed a clinically relevant model of IVH-induced brain injury and hydrocephalus utilizing hemoglobin injection into the brain ventricles, which allows for quantification of brain injury and PHH and the testing of new therapeutic strategies (Figure 1)6, 7. This IVH model utilizes neonatal rat pups, which are placed under general anesthesia for the duration of the procedure. A midline incision is made on the scalp, and coordinates derived from skull landmarks—the bregma or lambda—are used to target the lateral ventricles for injection. Slow injection using an infusion pump delivers hemoglobin into the ventricle. This protocol is easy to use, versatile, and can model different components of IVH that result in PHH.
Protocol
NOTE: All animal protocols were approved by the institutions' Animal Care and Use Committee. See the Table of Materials for details about all materials, reagents, equipment, and software used in this protocol.
1. Preparation of hemoglobin and CSF solutions
- Prepare a sterile artificial CSF (aCSF) solution by adding 500 µL of the aCSF solution to a 1.5 mL microtube and store on ice.
- Prepare a sterile 150 mg/mL hemoglobin solution by adding 75 mg of hemoglobin to 500 µL of aCSF in a 1.5 mL microtube and store on ice.
2. Preparation of the animal for injection
- Turn the heating pad to the medium setting to maintain the body temperature of the rat.
- Anesthetize postnatal day 4 (P4) rats in an induction chamber filled with 3% isoflurane.
NOTE: Confirm sufficient anesthesia using the toe/tail-pinch response every 15 min. Monitor anesthesia with visual observation of tissue color, body temperature, and respiratory rate. - Administer pain relief with a 5 mg/kg subcutaneous carprofen injection to the anesthetized rat.
- Place the anesthetized rat prone in the stereotactic apparatus with the nose positioned in the anesthesia adaptor with a constant flow of 1.5% isoflurane.
- Tighten non-rupture ear bars on the external auditory meatus to secure the head.
NOTE: Apply vet ointment to keep the eyes moisturized if the eyes are open at the age of injection. - Clean the head, alternating with sterile cotton-tipped applicators soaked in betadine and 70% ethanol.
- Touch the betadine-soaked applicator to the center of the scalp and spread the betadine in circles, moving outward.
- Repeat step 2.6.1.1 with the ethanol-soaked applicator.
- Repeat step 2.6.1.1 and step 2.6.1.2 3x.
- Apply a sterile surgical drape to protect the surgical field.
- Using a sterile scalpel, make a 0.3 cm incision vertically down the center of the head to expose the bregma of the skull.
NOTE: If injecting from the lambda, expose the lambda of the skull instead of the bregma. - Use a sterile cotton-tipped applicator to dry the area.
3. Setting up the stereotactic injector
- Draw the hemoglobin solution prepared in step 1.2 into a 0.3 mL sterile syringe with a 30G needle and place the syringe into the stereotactic injector system.
NOTE: If generating control conditions, draw aCSF solution prepared in step 1.1 into a 0.3 mL sterile syringe and proceed with the protocol. - Turn on the stereotactic injector interface and click on the Configuration button to input the injection volume and rate settings.
- Click on Volume and set the volume at 20,000 nL (20 µL).
- Click on Infusion rate and set the rate at 8,000 nL/min (8 µL/min).
- Exit Configuration by clicking on the Reset Pos button.
- Flush the needle tip by clicking on the Infuse button until a small bead of hemoglobin solution emerges at the needle tip.
- Gently wick the hemoglobin solution from the needle tip with a sterile cotton-tipped applicator.
4. Animal injection
- Set the bregma as zero on the stereotactic injector system by adjusting the mediolateral and anteroposterior positions of the syringe before lowering the tip of the flushed syringe needle to gently touch the skull at the bregma.
NOTE: If injecting from the lambda, set lambda as zero. - Identify the coordinates of choice.
- If injecting from the bregma, in P4 rats described here, use 1.5 mm lateral, 0.4 mm anterior, and 2.0 mm deep from bregma.
- If injecting from the lambda, use the following coordinates for P4 rats: 1.1 mm lateral, 4.6 mm anterior, and 3.3 mm deep from lambda.
- Raise the syringe needle 1 cm above the skull to clear the scalp. When the syringe is raised, proceed to set the mediolateral and anteroposterior coordinates.
- Lower the syringe needle to gently touch the skull. Check that the needle is touching the skull.
- Set the dorsoventral coordinate over a 30 s period.
NOTE: While setting the dorsoventral coordinate, the needle will puncture the skull. Care must be taken to assure that the syringe passes through the skull without deforming the skull. Skull deformation is avoided by slowly withdrawing the needle along the dorsoventral coordinate if deformation occurs, then placing the needle back along the same trajectory. This allows the needle to pass through the hole in the skull with less force and no deformation. - On the stereotactic injector interface, click on the Run button to begin injection.
- After the injection is finished, leave the syringe needle in place for 2 min to minimize backflow of the solution.
- Withdraw the syringe slowly along the dorsoventral coordinate over 2 min until the needle tip is 2 cm above the scalp.
- Rotate the stereotactic injector arm away from the operative field.
5. Postoperative care
- Close the scalp with a 6-0 monofilament suture. Make one simple interrupted suture at the center of the 0.3 cm incision.
- Remove the pup from anesthesia and place it onto a secure area on the heating pad.
- Return the rodent to the home cage to recover from the anesthesia under the care of its dam.
NOTE: Timely return to the care of the dam reduces early postoperative mortality. - Monitor the animals for anesthesia by loss of the righting reflex hourly post surgery for 3 h.
- Monitor the animals daily for 7 days for normal activity, food intake, and weight gain. Monitor the incision site for wound healing, closure, and reappearance of fur at the site of surgery.
NOTE: In the rare case that neurological changes such as seizures, central depression, or decreased appetite are observed during monitoring, euthanize the animal using intravascular perfusion or cervical dislocation under anesthesia. - To prevent infection once the suture is closed and the wound is healing, apply topical triple-antibiotic at the incision site.
6. MRI acquisition and quantification
- Perform MRI on a 4.7T or 9.4T small-animal scanner.
- Turn the heating pad to the medium setting to maintain the body temperature of the rat.
- Induce anesthesia in a chamber using 3% isoflurane.
NOTE: Confirm sufficient anesthesia using the toe/tail-pinch response every 15 min. Monitor anesthesia with visual observation of tissue color, body temperature, and respiratory rate. - Place the anesthetized rat prone in the MRI with the nose positioned in the anesthesia adaptor with a constant flow of 1.5% isoflurane.
- Perform T2-weighted imaging by selecting a T2-weighted fast spin echo sequence.
- If using a 4.7T MRI scanner, enter the following parameters into the MRI software: repetition time = 3,000 ms, echo time = 27.50 ms, number of averages = 3, field of view = 18.0 mm x 18.0 mm, matrix = 128 x 128, number of axial slices = 24, thickness = 0.50 mm.
- If using a 9.4T MRI scanner, enter the following parameters into the MRI software: repetition time = 5,000 ms, echo time = 66.00 ms, echo spacing = 16.50 ms, number of averages = 2, repetitions = 1, rare factor = 8, field of view = 16.0 mm x 16.0 mm, matrix = 256 x 256, number of axial slices = 32, thickness = 0.50 mm.
- Click on the Continua button to start the sequence.
7. Image processing and analysis
- Use native T2-weighted data to analyze brain volume. Use segmentation software to manually delineate the lateral ventricles6. Click on Paintbrush Mode and select square brush style. Adjust the brush size to 1. Click Layout inspector and select axial view. Click zoom to fit. Place the cursor on the image; trace and fill the lateral ventricle space.
- Click on Segmentation in the toolbar | Volume and Statistics to view the segmented volumes.
Representative Results
The success of injection was confirmed by radiologic and immunohistochemical means. Animals that underwent hemoglobin injection developed moderate acute ventriculomegaly when assessed via MRI (Figure 2A), with significantly larger lateral ventricles at 24 h and 72 h post hemoglobin injection compared to aCSF-injected animals (Figure 2B,C). While there was no significant difference in lateral ventricle volume between hemoglobin-injected and aCSF-injected animals 38 days post injection (Figure 2D), it is important to note that 44% (4/9) of the animals in the hemoglobin-injected group that were followed to 38 days post injection displayed unresolved ventriculomegaly at this timepoint (Figure 2D). This wide distribution in ventricle sizes is a pattern that is consistent with the clinical course of IVH-PHH. In addition, white matter volume was quantified at 38 days post injection (Figure 3) and was significantly decreased in the hemoglobin-injected group compared to the aCSF-injected group (Figure 3B).
We previously published a study detailing the acute inflammatory reaction that occurs after hemoglobin injection9. In this present study, proinflammatory cytokines were evaluated for tumor necrosis factor-alpha (TNFα) production in vivo (Figure 4), and immune cell infiltration into the periventricular areas and white matter were evaluated using glial fibrillary acidic protein (GFAP) immunofluorescence (Figure 5). Injection of 15 µL of hemoglobin, whole blood, or saline into the lateral ventricle of postnatal day 5 rats resulted in higher levels of proinflammatory cytokine TNFα 3 h after hemoglobin injection compared to whole blood and saline (Figure 4). There were significantly more reactive astrocytes in the corpus callosum of hemoglobin-injected animals compared to aCSF-injected animals (Figure 5). Finally, other blood breakdown products have been utilized in this fashion including iron and ferritin to reliably result in ventriculomegaly and hydrocephalus6,7.
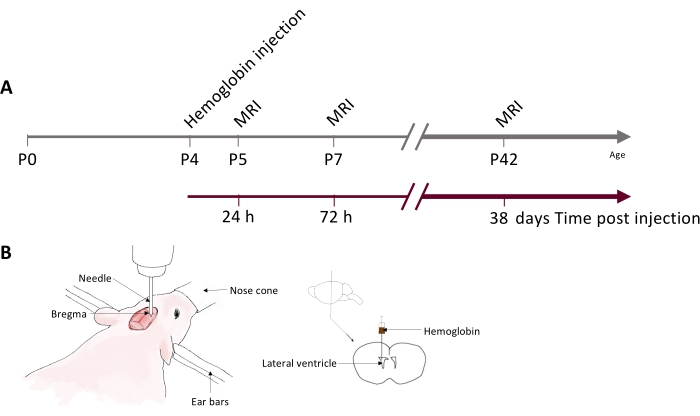
Figure 1: Experimental timeline and schematic of the neonatal rat IVH model. (A) Schematic showing hemoglobin injection and MRI timeline used for data generated in this study. (B) Schematic of stereotactic setup for injection (left) and hemoglobin injection location into the right lateral ventricle (right). Abbreviations: IVH = intraventricular hemorrhage; MRI = magnetic resonance imaging; PN = postnatal day N. Please click here to view a larger version of this figure.
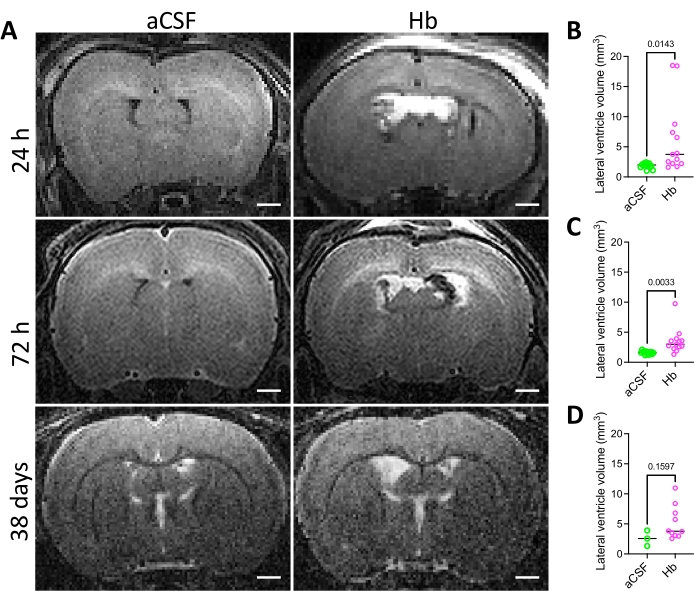
Figure 2: Lateral ventricle volumes in the intraventricular hemorrhage rat model. (A) Representative in vivo T2 coronal MRI images of rat brains 24 h, 72 h, and 38 days after intraventricular injection of aCSF (left) or 150 mg/mL Hb (right) into the right lateral ventricle at postnatal day 4. Scale bars = 1 mm. (B–D) Quantification of lateral ventricle volumes (B) 24 h, (C) 72 h, and (D) 38 days following aCSF or Hb injection. Hb-injected animals had significantly larger ventricles at 24 h and 72 h. Data in B and C are mean ± s.e.m., n = 13 per group, unpaired two-tailed t-test. Data in D are mean ± SEM, n = 3 in aCSF group and n = 9 in Hb group, unpaired two-tailed t-test. Abbreviations: MRI = magnetic resonance imaging; PN = postnatal day N; aCSF = artificial cerebrospinal fluid; Hb = hemoglobin; SEM = standard error of the mean. Please click here to view a larger version of this figure.
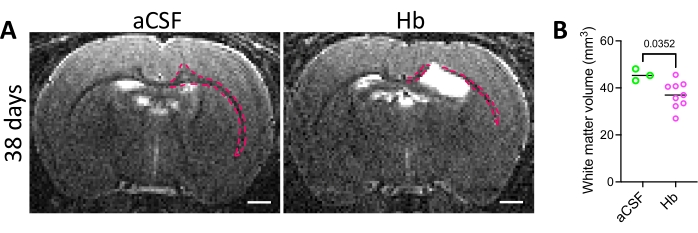
Figure 3: White matter injury in the intraventricular hemorrhage rat model. (A) Representative in vivo T2 coronal MRI images of rat brains 38 days after intraventricular injection of aCSF (left) or 150 mg/mL Hb (right) into the right lateral ventricle at postnatal day 4. White matter is outlined in red. Scale bars = 1 mm. (B) Quantification of white matter volumes 38 days following aCSF or Hb injection. Hb-injected animals had decreased white matter volumes. Data in B are mean ± SEM, n = 3 in aCSF group, n = 9 in Hb group. Unpaired two-tailed t-test. Abbreviations: MRI = magnetic resonance imaging; PN = postnatal day N; aCSF = artificial cerebrospinal fluid; Hb = hemoglobin; SEM = standard error of the mean. Please click here to view a larger version of this figure.
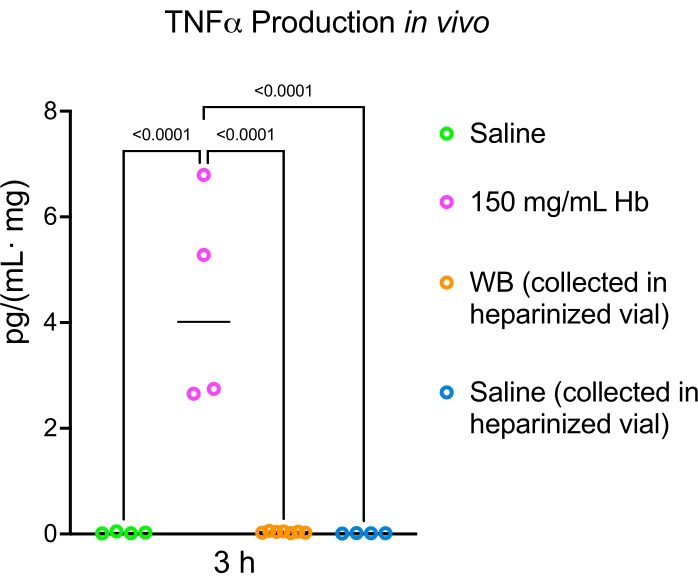
Figure 4: Hemoglobin induces more TNFα production than whole blood in vivo. Administration of 15 µL of hemoglobin, whole blood, or saline into the lateral ventricle of postnatal day 5 rats resulted in higher levels of the proinflammatory cytokine TNFα 3 h after hemoglobin injection compared to whole blood and saline. Data are mean ± SEM, n = 4 in all groups, one-way ANOVA with post-hoc Tukey's test. Abbreviations: Hb = hemoglobin; TNFα = tumor necrosis factor-alpha; WB = whole blood; ANOVA = analysis of variance. Please click here to view a larger version of this figure.
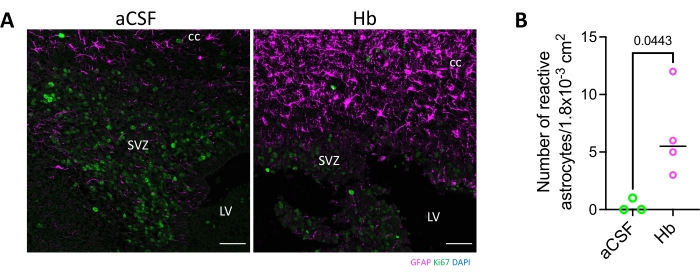
Figure 5: Astrocyte activation in the corpus callosum following hemoglobin injection into the lateral ventricle. (A) GFAP immunostaining shows hemoglobin injection into the lateral ventricle of postnatal day 4 rodents resulted in astrocyte activation in the corpus callosum and subventricular zone 72 h following injection. Scale bars = 50 µm. (B) The number of reactive astrocytes was significantly increased in hemoglobin-injected animals compared to aCSF-injected animals. Data are mean ± SEM, n = 3 in aCSF group, n = 4 in Hb group, unpaired two-tailed t-test. Abbreviations: GFAP = glial fibrillary acidic protein; DAPI = 4',6-diamidino-2-phenylindole; LV = lateral ventricle; SVZ = subventricular zone; cc = corpus callosum; aCSF = artificial cerebrospinal fluid; Hb = hemoglobin; SEM = standard error of the mean. Please click here to view a larger version of this figure.
Figure 6: Comparison of 4.7T and 9.4T MRI image quality. (A) 4.7T and 9.4T T2-weighted MRI taken 72 h following hemoglobin injection into the right lateral ventricle of postnatal day 4 rats. Scale bars = 1 mm. Abbreviation: MRI = magnetic resonance imaging. Please click here to view a larger version of this figure.
Discussion
This IVH model utilizing hemoglobin injection allows for the study of the pathology of IVH specifically mediated by hemoglobin. For complementary studies, hemoglobin can also be easily delivered in vitro and does not confound biochemical assays for proteins made by microglia/macrophages that are present in whole blood.
The leading theories of IVH-PHH include the mechanical obstruction of CSF circulation, the disruption of cilia lining the ependymal walls, inflammation, fibrosis, and iron toxicity10. Existing animal models for IVH such as the collagenase-induced rat pup model induce IVH through direct injury and disruption of the extracellular matrix11, while others such as the glycerol-induced rabbit pup model induce IVH as an effect of intracranial hypotension12. Additional models use autologous and donor rat blood injection into the lateral ventricles13, 14. While these and other existing models present important features for the study of IVH-PHH, they focus on the impact of blood within the ventricle without considering the role of specific components of the blood released during hemorrhage on the development of neurological sequelae following IVH.
Early CSF levels of hemoglobin following IVH have been shown to be associated with PHH, and CSF levels of iron metabolism pathway proteins—hemoglobin, ferritin, and bilirubin—are associated with ventricle size following IVH4. This suggests that the pathogenesis of PHH may be associated specifically with the hemoglobin and iron components of the blood released into the ventricles during IVH. Thus, this model presents an important avenue for targeted investigation into the role of hemoglobin on PHH development and allows for further studies into therapeutics targeting hemoglobin and iron metabolism pathways following IVH.
Ventricular enlargement after IVH in humans can be due to brain parenchymal loss (sometimes referred to as hydrocephalus ex vacuo) or hydrocephalus, which indicates increased CSF pressure. These processes can occur together15 and it can be difficult to determine to what extent ventricular changes are due to increased CSF pressure versus volume loss without invasive procedures. This model, which allows for both the assessment of ventricular size radiologically in living animals and the assessment of tissue injury via histology, can help researchers understand the relationship between the two and, more importantly, assess to what extent potential therapies reverse brain injury.
Traditionally, interspecies comparisons of brain development have relied primarily on postmortem brain mass. A seminal study by Dobbing and Sands conducted with this method estimated P7 to be a major period of brain growth in rodents, comparable to the changes observed in human neonates born at term16. More recently, studies comparing developmental milestones such as oligodendrocyte maturation and establishment of the blood-brain barrier have defined P1–P3 in rodents to be analogous to 23–32 weeks gestation in human neonates17,18,19,20,21. In addition, the germinal matrix does not involute until P7 in rats22. Therefore, the P4 rats used in this model correspond to a period of brain maturation in which the germinal matrix is present, with characteristics that we believe are representative of the human population at risk for IVH-PHH. Furthermore, the rate of unresolved ventriculomegaly at 38 days after hemoglobin injection in this study (44%) is comparable with clinical rates of PHH after IVH (30%)23.
Limitations of our postnatal rat IVH model include the use of a lissencephalic animal and the lack of direct injury to the germinal matrix and/or periventricular parenchyma. However, this model has several benefits including good reproducibility, low cost, and versatility that allows for the use of different aged animals and radiologic (Figure 6), biochemical, and histological analyses. Future laboratory work on the pathophysiology of IVH may lead to better treatments for this condition.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
JMS received funding from NIH/NINDS R01 NS110793 and K12 (Neurosurgeon Research Career Development Program). BAM received funding from NIH/NINDS K08 NS112580-01A1, University of Kentucky Neuroscience Research Priority Area Award, and a Hydrocephalus Association Innovator Award.
Materials
| 0.3 mL insulin syringe | BD Microfine + Insulin Syringe | 230-4533 | 0.3-0.5 mL synringes will work |
| 1.5 mL microtube | USA Scientific | 1615-5500 | Lot No. K194642H -3 511 |
| 4.7T MRI | Agilent/Varian | 4.7T/33 cm | Agilent/Varian DirectDrive 4.7-T (200-MHz) MRI system |
| 6-0 monofilament suture | ETHICON | 667G | |
| 9.4T MRI | Bruker | BioSpec 94/20 | Used in this protocol without the cryoprobe |
| Analytical balance | CCURIS Instruments | W3200-320 | |
| Artificial CSF (aCSF) | Tocris Bioscience | 3525 | Batch No: 72A |
| Betadine | Purdue Products L.P. | 301005-00 | NDC 67618-150-09 |
| Carprofen (injectable) | Zoetis Inc. | PI 4019448 | Rimadyl |
| Ethanol | Decon Laboratories | 2701 | |
| Heating pad | Sunbeam | E12107-819 | UL 612A, Z-1228-001 |
| Hemoglobin | MP Biomedicals | 100714 | LOT NO. SR02321 |
| Isoflurane | Piramal Critical Care | NDC 66794-017-25 | |
| Isoflurane vaporizer | VETEQUIP | 911103 | |
| Light for stereotactic insturment | Dolan-Jenner industries | Fiber-Lite MI-150 | |
| Microinjection syringe pump | World Precision Instruments | MICRO21 | Serial 184034 T08K |
| MRI software | Bruker BioSpin | Paravision 360 3.2 | |
| Oxygen | Airgas Healthcare | UN1072 | LOT NUMBER S1432080XA02 |
| Sprague Dawley rats | Charles River Laboratories | Strain code: 001 | |
| Stereotactic instrument | KOPF Instuments | Model 900LS Lazy Susan | |
| Sterile cotton tipped applicator | Fischerbrand | 23-400-118 | |
| Surgical blade | covetrus | #10 | |
| Topical triple antibiotic | Triple Antibiotic Ointment | NDC 51672-2120-1 | |
| Ventricle volume quantification software | ITK-SNAP | ITK-SNAP 4.0.0 beta |
Riferimenti
- Robinson, S. Neonatal posthemorrhagic hydrocephalus from prematurity: Pathophysiology and current treatment concepts: A review. Journal of Neurosurgery: Pediatrics. 9 (3), (2012).
- Hasselager, A. B., Børch, K., Pryds, O. A. Improvement in perinatal care for extremely premature infants in Denmark from. Danish Medical Journal. 63 (1), (1994).
- Johnston, P. G., Gillam-Krakauer, M., Fuller, M. P., Reese, J. Evidence-Based Use of Indomethacin and Ibuprofen in the Neonatal Intensive Care Unit. Clinics in Perinatology. 39 (1), (2012).
- Mahaney, K. B., Buddhala, C., Paturu, M., Morales, D., Limbrick, D. D., Strahle, J. M. Intraventricular Hemorrhage Clearance in Human Neonatal Cerebrospinal Fluid: Associations with Hydrocephalus. Stroke. , (2020).
- Strahle, J. M., et al. Longitudinal CSF Iron Pathway Proteins in Posthemorrhagic Hydrocephalus: Associations with Ventricle Size and Neurodevelopmental Outcomes. Annals of Neurology. 90 (2), (2021).
- Strahle, J. M., et al. Role of Hemoglobin and Iron in hydrocephalus after neonatal intraventricular hemorrhage. Neurosurgery. 75 (6), (2014).
- Garton, T. P., He, Y., Garton, H. J. L., Keep, R. F., Xi, G., Strahle, J. M. Hemoglobin-induced neuronal degeneration in the hippocampus after neonatal intraventricular hemorrhage. Brain Research. 1635, (2016).
- Goulding, D. S., Caleb Vogel, ., Gensel, R., Morganti, J. C., Stromberg, J. M., Miller, A. J., A, B. Acute brain inflammation, white matter oxidative stress, and myelin deficiency in a model of neonatal intraventricular hemorrhage. Journal of Neurosurgery: Pediatrics. 26 (6), (2020).
- Strahle, J., Garton, H. J. L., Maher, C. O., Muraszko, K. M., Keep, R. F., Xi, G. Mechanisms of Hydrocephalus After Neonatal and Adult Intraventricular Hemorrhage. Translational Stroke Research. 3, (2012).
- Jinnai, M., et al. A Model of Germinal Matrix Hemorrhage in Preterm Rat Pups. Frontiers in Cellular Neuroscience. 14, (2020).
- Georgiadis, P., et al. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke. 39 (12), (2008).
- Cherian, S. S., Love, S., Silver, I. A., Porter, H. J., Whitelaw, A. G. L., Thoresen, M. Posthemorrhagic ventricular dilation in the neonate: Development and characterization of a rat model. Journal of Neuropathology and Experimental Neurology. 62 (3), (2003).
- Balasubramaniam, J., Xue, M., Buist, R. J., Ivanco, T. L., Natuik, S., del Bigio, ., R, M. Persistent motor deficit following infusion of autologous blood into the periventricular region of neonatal rats. Experimental Neurology. (1), (2006).
- Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. The Lancet Neurology. 8 (1), (2009).
- Dobbing, J., Sands, J. Comparative aspects of the brain growth spurt. Early Human Development. 3 (1), (1979).
- Craig, A., et al. Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Experimental Neurology. 181 (2), (2003).
- Lodygensky, G. A., Vasung, L., Sv Sizonenko, ., Hüppi, P. S. Neuroimaging of cortical development and brain connectivity in human newborns and animal models. Journal of Anatomy. 217 (4), (2010).
- Dean, J. M., et al. Strain-specific differences in perinatal rodent oligodendrocyte lineage progression and its correlation with human. Developmental Neuroscience. 33 (34), (2011).
- Engelhardt, B. Development of the blood-brain barrier. Cell and Tissue Research. 314 (1), (2003).
- Daneman, R., Zhou, L., Kebede, A. A., Barres, B. A. Pericytes are required for bloodĝ€"brain barrier integrity during embryogenesis. Nature. 468 (7323), (2010).
- Alles, Y. C. J., Greggio, S., Alles, R. M., Azevedo, P. N., Xavier, L. L., DaCosta, J. C. A novel preclinical rodent model of collagenase-induced germinal matrix/intraventricular hemorrhage. Brain Research. 1356, (2010).
- Christian, E. A., et al. Trends in hospitalization of preterm infants with intraventricular hemorrhage and hydrocephalus in the United States. Journal of Neurosurgery: Pediatrics. 17 (3), 2000-2010 (2016).

