Purification of Active Photosystem I-Light Harvesting Complex I from Plant Tissues
Summary
This protocol describes the isolation of Photosystem I (PSI) – Light Harvesting Complex I (LHCI) from plant tissues. PSI along with PSII is responsible for the conversion of light to chemical energy in oxygenic photoautotrophs and has a quantum efficiency of ~1, making it a target for studying light-driven energy transfer.
Abstract
This method is used to isolate Photosystem I (PSI) together with the Light Harvesting Complex I (LHCI), its native antenna, from plants. PSI-LHCI is a large membrane protein complex coordinating hundreds of light harvesting and electron transport factors and is the most efficient light harvesting system found in nature. Photons absorbed by the four LHCA antenna proteins that make up LHCI are transferred through excitonic interaction to the PSI core reaction center and are used to facilitate light-driven charge separation across the thylakoid membrane, providing reducing power and energy for carbon fixation in photoautotrophic organisms. The high quantum efficiency of PSI makes this complex an excellent model to study light-driven energy transfer. In this protocol, plant tissue is mechanically homogenized, and the chloroplasts are separated from the bulk cellular debris by filtration and centrifugation. The isolated chloroplasts are then osmotically lysed, and the thylakoid membranes are recovered via centrifugation and solubilized using the detergent n-dodecyl-beta-maltoside. The solubilized material is loaded onto an anion exchange column to collect most of the chlorophyll-containing complexes. Larger complexes are precipitated from the solution, resuspended in a small volume, and loaded on sucrose gradients to separate the major chlorophyll-containing complexes. The resulting sucrose gradient fractions are characterized to identify the band of interest containing PSI-LHCI. This protocol is highly similar to the protocol used in the crystallization of plant PSI-LHCI with some simplifications and relies on methods developed over the years in the lab of Nathan Nelson.
Introduction
Oxygenic photosynthesis is one of the most important chemical reactions on our planet. The conversion of light to chemical energy occurs in the reaction centers of two photosystems, photosystem I (PSI) and photosystem II (PSII)1 (Figure 1A). PSI is a large, highly conserved multisubunit pigment-protein complex that evolved over 3.5 billion years ago2,3. This complex, which contains approximately 100 chlorophyll molecules and about 20 carotenoids, facilitates the transfer of electrons across the thylakoid membrane from plastocyanin to ferredoxin acting as the terminal electron acceptor of the photosynthetic electron transport chain1,4,5(Figure 1B, C). In plants, this light-driven charge separation is the result of light energy transferred from both PSI core antenna pigments and the peripheral antenna pigments of Light Harvesting complex I (LHCI) to the PSI reaction center (Figure 1D). LHCI is a PSI-specific antenna complex within the thylakoid membrane composed of four chlorophyll a/b binding LHCA antenna proteins6,7.
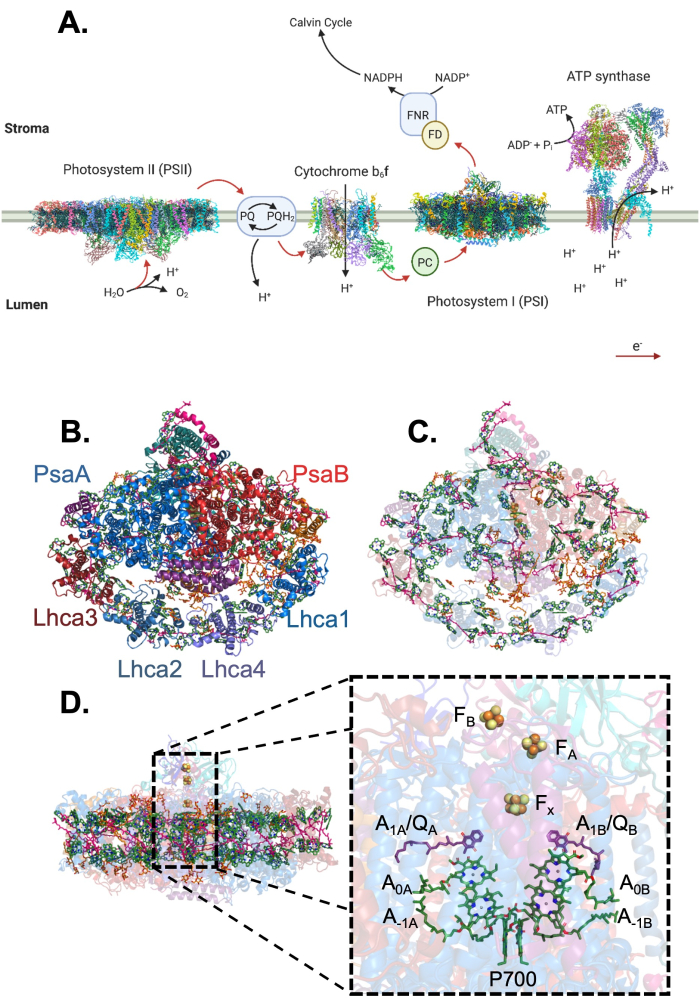
Figure 1: The photosynthetic electron transport chain and the overall structure of the PSI-LHCI complex. (A) The photosynthetic electron transport chain contains four main membrane-bound photosynthetic complexes and three soluble electron carriers. Electron flow (red arrows) through the transport chain and proton pumping (black arrows) into the lumen are used to create reducing power (NADPH) and produce ATP for carbon fixation37,38,39,40. Created with Biorender.com. (B) The structure of the plant PSI-LHCI from the lumenal side. PsaA and PsaB are the largest subunits of PSI and comprise the core of the complex. LHCI is the light-harvesting antenna complex associated with PSI and is composed of four antennae, LHCA1-4. (C) The PSI-LHCI complex coordinates over 150 ligands. Shown here are chlorophylls (green), carotenoids (pink), quinones (purple), lipids (orange), and the FeS clusters of the reaction center in yellow/orange. (D) The reaction center of PSI is split into two branches (A and B), starting from P700, the reaction center special chlorophyll pair, going into two accessory chlorophylls (A-1A/B) followed by another pair of chlorophylls (A0A/B). These chlorophylls are followed by a phylloquinone (A1A/B or QA/B in some publications) in each branch before joining together at the iron-sulfur cluster Fx followed by two more clusters, FA and FB, coordinated by the PsaC subunit. Please click here to view a larger version of this figure.
The first isolation of PSI from plants in 1966 shed light on the differences in light-harvesting pigment content between PSI and PSII, showing that PSI was highly enriched in β-carotene relative to PSII and that cytochromes f and b6 (part of the cytochrome b6f complexes) are not tightly bound to PSI but loosely associated within the thylakoid membrane8. Nine years later, with partial denaturation of isolated PSI via SDS treatment it was shown that dissociation of small PSI subunits quenched NADP+ photoreduction by PSI, while the P700 signal and most of the chlorophylls remained within the remaining large molecular weight PSI particle, identifying the necessity of some of PSI's small subunits for full biological function and the location of the PSI reaction center9. Research into the association between the PSI core and LHCI was first published in the early 1980s, when isolations of different-sized PSI species containing differing ratios of chlorophyll A to P700 were observed, suggesting the association of PSI with a chlorophyll-containing peripheral antenna system10,11,12,13. However, it wasn't until 2003 that the first crystal structure of the plant PSI was published14. The crystal structure of the plant PSI-LHCI highlighted the remarkable conservation between the PSI core of plants and cyanobacteria and provided the first picture of chlorophyll arrangement within the plant PSI core and LHCI antenna, furthering the understanding of the energy transfer pathways within the plant PSI-LHCI complex14. Over the past decade more plant PSI-LHCI structures were determined adding atomic levels details to the structural description of the super-complex15,16,17,18,19.
PSI not only has a quantum efficiency close to one, but boasts the most negative reduction potential in nature20,21. A complete understanding of PSI-LHCI and its properties is essential for understanding light driven energy transfer and applying bio-inspired solutions to future light harvesting technology. To further this understanding of how PSI-LHCI and its many subunits can achieve such efficient energy conversion, complexes isolated for study must be active and whole. This protocol allows for the gentle purification of the complex in this active state22,23.
In this method plant tissues are mechanically disrupted and chloroplasts containing the photosynthetic electron transport chain are isolated by centrifugation. The thylakoid membranes are separated after hypotonic chloroplast lysis and are then solubilized using the detergent n-dodecyl-beta-maltoside (β-DDM). The solubilized chlorophyll containing membrane complexes are separated using anion exchange chromatography and PSI-LHCI is further separated using sucrose gradient centrifugation. After removal from the gradient and after characterization by both spectroscopy and using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), the complex can be prepared for further experiments. This procedure is used to purify the PSI-LHCI complex from plants without the use of any affinity tags. With minor modifications it can be adapted for preparations of the complex from other organisms, stabilize alternative PSI complexes or other complexes of the photosynthetic electron transport chain. Similar protocols were used to obtain PSI complex suitable for high resolution structural analysis23,24,25,26,27,28,29,30.
Protocol
Representative Results
Discussion
Using this protocol, the PSI-LHCI complex from plant tissues can be purified in its active state. Spinach leaves were used here, but these methods can be applied to preparations from various plants23,40. In all cases, care must be taken while performing this protocol to protect the complex from damage. This preparation should be done in the dark or under a green light, on ice with pre-chilled buffers, and all resuspension steps should be performed gently.
<p …Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Y.M. acknowledges the support by the National Science Foundation under Award No. 2034021 and the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences under Award No. DE-SC0022956. C.G. is supported by the National Science Foundation under Award No. 00036806.
Materials
| 15 mL Falcon tube | VWR | 62406-200 | Used for storing thylakoids |
| Bio rad Econo-Column 1.5 X 30 cm | biorad | 7374153 | |
| Cheesecloth grade 50, 100% cotton | Arkwright LLC | B07D1FZZMB | From Amazon |
| Glass rods | Millipore Sigma | BR135825 | Any similar rod will suffice |
| Low profile 64 oz vitamix blender | Vitamix | ||
| NaCl | Sigma-Aldrich | S7653 | |
| Open top polyallomer centrifugation tubes | Seton Scientific | 5030 | |
| Optima XE Ultracentrifuge | beckman coulter | A94471 | |
| Polyethylene glycol 6,000 | Hampton Research | HR2-533 | |
| Potter-Elvehjen Tissue Grinder, 30 ml. | WHEATON | 358049 | |
| Sucrose | Sigma-Aldrich | S7903 | |
| SW 40 Ti | beckman coulter | 331301 | |
| TOYOPEARL DEAE-650C | Tosoh Bioscience | 7988 | |
| Tricine | Sigma-Aldrich | T0377 | |
| β-DDM | Glycon – Biochemicals GmbH | D97002 | Stored as 10% stocks at -20 °C |
Riferimenti
- Nelson, N., Junge, W. Structure and energy transfer in photosystems of oxygenic photosynthesis. Annual Review of Biochemistry. 84, 659-683 (2015).
- Fischer, W. W., Hemp, J., Johnson, J. E. Evolution of oxygenic photosynthesis. Annual Review of Earth and Planetary Sciences. 44 (1), 647-683 (2016).
- Yoon, H. S., Hackett, J. D., Ciniglia, C., Pinto, G., Bhattacharya, D. A molecular timeline for the origin of photosynthetic eukaryotes. Molecular Biology and Evolution. 21 (5), 809-818 (2004).
- Busch, A., Hippler, M. The structure and function of eukaryotic photosystem i. Biochimica et Biophysica Acta – Bioenergetics. 1807 (8), 864-877 (2011).
- Rochaix, J. D. Regulation of photosynthetic electron transport. Biochimica et Biophysica Acta – Bioenergetics. 1807 (3), 375-383 (2011).
- Croce, R., Morosinotto, T., Castelletti, S., Breton, J., Bassi, R. The Lhca antenna complexes of higher plants photosystem I. Biochimica et Biophysica Acta-Bioenergetics. 1556 (1), 29-40 (2002).
- Croce, R., Van Amerongen, H. Light-harvesting in photosystem I. Photosynthesis Research. 116 (2-3), 153-166 (2013).
- Anderson, J. M., Boardman, N. K. Fractionation of the photochemical systems of photosynthesis I. Chlorophyll contents and photochemical activities of particles isolated from spinach chloroplasts. Bibliotek for Laeger. 112 (3), 403-421 (1966).
- Bengis, C., Nelson, N. Purification and properties of the photosystem I reaction center from chloroplasts. Journal of Biological Chemistry. 250 (8), 2783 (1975).
- Lam, E., Ortiz, W., Malkin, R. Chlorophyll a/b proteins of Photosystem I. FEBS Letters. 168 (1), 10-14 (1984).
- Kuang, T. Y., Argyroudi-Akoyunoglou, J. H., Nakatani, H. Y., Watson, J., Arntzen, C. J. The origin of the long-wavelength fluorescence emission band (77°K) from photosystem I. Archives of Biochemistry and Biophysics. 235 (2), 618-627 (1984).
- Mullet, J. E., Burke, J. J., Arntzen, C. J. A developmental study of Photosystem I peripheral chlorophyll proteins. Plant Physiology. 65 (5), 823-827 (1980).
- Mullet, J. E., Burke, J. J., Arntzen, C. J. Chlorophyll a/b proteins of Photosystem I. Plant Physiology. 65 (5), 814-822 (1980).
- Ben-Shem, A., Frolow, F., Nelson, N. Crystal structure of plant photosystem I. Nature. 426 (6967), 630-635 (2003).
- Mazor, Y., Borovikova, A., Nelson, N. The structure of plant photosystem I super-complex at 2.8 A resolution. eLife. 4, 07433 (2015).
- Qin, X., Suga, M., Kuang, T., Shen, J. R. Photosynthesis. Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science. 348 (6238), 989-995 (2015).
- Mazor, Y., Borovikova, A., Caspy, I., Nelson, N. Structure of the plant photosystem I supercomplex at 2.6 resolution. Nature Plants. 3, 17014 (2017).
- Pan, X., et al. Structure of the maize photosystem I supercomplex with light-harvesting complexes I and II. Science. 360 (6393), 1109-1113 (2018).
- Wang, J., et al. Structure of plant photosystem I−light harvesting complex I supercomplex at 2.4 Å resolution. Journal of Integrative Plant Biology. 63 (7), 1367-1381 (2021).
- Jensen, P. E., et al. function, and regulation of plant photosystem I. Biochimica et Biophysica Acta – Bioenergetics. 1767 (5), 335-352 (2007).
- Nelson, N. Plant photosystem I – The most efficient nano-photochemical machine. Journal of Nanoscience and Nanotechnology. 9 (3), 1709-1713 (2009).
- Amunts, A., Toporik, H., Borovikova, A., Nelson, N. Structure determination and improved model of plant photosystem I. Journal of Biological Chemistry. 285 (5), 3478-3486 (2010).
- Gorski, C., et al. The structure of the Physcomitrium patens photosystem I reveals a unique Lhca2 paralogue replacing Lhca4. Nature Plants. 8 (3), 307-316 (2022).
- Perez-Boerema, A., et al. Structure of a minimal photosystem I from the green alga Dunaliella salina. Nature Plants. 6 (3), 321-327 (2020).
- Toporik, H., et al. The structure of a red-shifted photosystem I reveals a red site in the core antenna. Nature Communications. 11 (1), 5279 (2020).
- Toporik, H., Li, J., Williams, D., Chiu, P. L., Mazor, Y. The structure of the stress-induced photosystem I-IsiA antenna supercomplex. Nature Structural and Molecular Biology. 26 (6), 443-449 (2019).
- Caspy, I., et al. Structure and energy transfer pathways of the Dunaliella Salina photosystem I supercomplex. Biochimica et Biophysica Acta – Bioenergetics. 1861 (10), 148253 (2020).
- Naschberger, A., et al. Algal photosystem I dimer and high resolution model of PSI:plastocyanin complex. Nature Plants. 8 (10), 1191-1201 (2022).
- Furukawa, R., et al. Formation of a PSI-PSII megacomplex containing LHCSR and PsbS in the moss Physcomitrella patens. Journal of Plant Research. 132 (6), 867-880 (2019).
- Gisriel, C. J., et al. High-resolution cryo-electron microscopy structure of photosystem II from the mesophilic cyanobacterium, Synechocystis sp. PCC 6803. Proceedings of the National Academy of Sciences. 119 (1), 2116765118 (2021).
- Porra, R. J., Thompson, W. A., Kriedemann, P. E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochemica et Biophysica Acta. 12 (2), 103-107 (2004).
- Bassi, R., Simpson, D. Chlorophyll-protein complexes of barley photosystem I. European Journal of Biochemistry. 163 (2), 221-230 (1987).
- Hiyama, T., Ke, B. Difference spectra and excitation coefficients of P700. Biochimica et Biophysica Acta. 267 (459), 160-171 (1972).
- Anderson, J. M. P-700 content and polypeptide profile of chlorophyll-protein complexes of spinach and barley thylakoids. Biochimica et Biophysica Acta. 591 (1), 113-126 (1980).
- Caspy, I., et al. Dimeric and high-resolution structures of Chlamydomonas Photosystem I from a temperature-sensitive Photosystem II mutant. Communications Biology. 4 (1), 1-10 (2021).
- Caffarri, S., Kouřil, R., Kereïche, S., Boekema, E. J., Croce, R. Functional architecture of higher plant photosystem II supercomplexes. EMBO Journal. 28 (19), 3052-3063 (2009).
- Su, X., et al. Structure and assembly mechanism of plant C2S2M2-type PSII-LHCII supercomplex. Science. 357 (6353), 815-820 (2017).
- Malone, L. A., et al. Cryo-EM structure of the spinach cytochrome b 6 f at 3.6 Å resolution. Nature. 575 (7783), 535-539 (2019).
- Guo, H., Rubinstein, J. L. Structure of ATP synthase under strain during catalysis. Nature Communications. 13 (1), 2-10 (2022).
- Mazor, Y., Borovikova, A., Caspy, I., Nelson, N. Structure of the plant photosystem I supercomplex at 2.6 Å resolution. Nature Plants. 3, 17014 (2017).

.
