Isolation of Low Endotoxin Content Extracellular Vesicles Derived from Cancer Cell Lines
Summary
The proposed protocol includes guidelines on how to avoid contamination with endotoxin during the isolation of extracellular vesicles from cell culture supernatants, and how to properly evaluate them.
Abstract
Extracellular vesicles (EVs) are a heterogeneous population of membrane vesicles released by cells in vitro and in vivo. Their omnipresence and significant role as carriers of biological information make them intriguing study objects, requiring reliable and repetitive protocols for their isolation. However, realizing their full potential is difficult as there are still many technical obstacles related to their research (like proper acquisition). This study presents a protocol for the isolation of small EVs (according to the MISEV 2018 nomenclature) from the culture supernatant of tumor cell lines based on differential centrifugation. The protocol includes guidelines on how to avoid contamination with endotoxins during the isolation of EVs and how to properly evaluate them. Endotoxin contamination of EVs can significantly hinder subsequent experiments or even mask their true biological effects. On the other hand, the overlooked presence of endotoxins may lead to incorrect conclusions. This is of particular importance when referring to cells of the immune system, including monocytes, because monocytes constitute a population that is especially sensitive to endotoxin residues. Therefore, it is highly recommended to screen EVs for endotoxin contamination, especially when working with endotoxin-sensitive cells such as monocytes, macrophages, myeloid-derived suppressor cells, or dendritic cells.
Introduction
Extracellular vesicles (EVs), according to the MISEV 2018 nomenclature, are a collective term describing various subtypes of cell-secreted membranous vesicles that play crucial roles in numerous physiological and pathological processes1,2. Moreover, EVs show promise as novel biomarkers for various diseases, as well as therapeutic agents and drug delivery vehicles. However, realizing their full potential is difficult as there are still many technical obstacles related to their acquisition3. One such challenge is the isolation of endotoxin-free EVs, which has been neglected in many cases. One of the most common endotoxins is lipopolysaccharide (LPS), which is a major component of gram-negative bacterial cell walls and can cause an acute inflammatory response, owing to the release of a large number of inflammatory cytokines by various cells4,5. LPS induces a response by binding to LPS binding protein, followed by interaction with the CD14/TLR4/MD2 complex on myeloid cells. This interaction leads to the activation of MyD88- and TRIF-dependent signaling pathways, which in turn triggers the nuclear factor kappa B (NFkB). Translocation of NFkB to the nucleus initiates the production of cytokines6. Monocytes and macrophages are highly sensitive to LPS, and their exposure to LPS results in a release of inflammatory cytokines and chemokines (e.g., IL-6, IL-12, CXCL8, and TNF-α)7,8. The CD14 structure enables the binding of different LPS species with similar affinity and serves as a co-receptor for other toll-like receptors (TLRs) (TLR1, 2, 3, 4, 6, 7, and 9)6. The number of studies being conducted on the effects of EVs on monocytes/macrophages is still increasing9,10,11. Especially from the perspective of studying the functions of monocytes, their subpopulations, and other immune cells, the presence of endotoxin and even their masked presence in EVs is of great importance12. The overlooked contamination of EVs with endotoxins may lead to misleading conclusions and hide their true biological activity. In other words, working with monocytic cells requires confidence in the absence of endotoxin contamination13. Potential sources of endotoxins can be water, commercially obtained media and sera, media components and additives, laboratory glassware, and plasticware5,14,15.
Therefore, this study aimed to develop a protocol for the isolation of low endotoxin-containing EVs. The protocol provides simple hints on how to avoid endotoxin contamination during EVs isolation instead of removing endotoxins from EVs. Previously, many protocols have been presented on how to remove endotoxins from, for example, engineered nanoparticles used in nanomedicine; however, none of them are useful for biological structures such as EVs. The effective depyrogenation of nanoparticles can be carried out by ethanol or acetic acid rinsing, heating at 175 °C for 3 h, γ irradiation, or triton X-100 treatment; however, these procedures lead to the destruction of EVs16,17.
The presented protocol is a pioneering study focused on avoiding endotoxin impurities in EVs, unlike previous studies on the effect of EVs on monocytes9. Applying proposed principles to laboratory practice may help to obtain reliable research results, which can be crucial when considering the potential use of EVs as therapeutic agents in the clinic12.
Protocol
1. Preparation of ultracentrifuge tubes
- Use sterile, single-use tubes. If this is not possible, reuse the ultracentrifuge tubes after washing them with a detergent using a sterile Pasteur pipette or other single-use applicators. Remember that ultracentrifuge tubes should be dedicated to one type of centrifuged material (cell culture supernatant/serum/plasma) and species (human/mouse/etc.).
- After a detergent wash, rinse the ultracentrifuge tubes with deionized, LPS-free water 3x.
NOTE: Do not use low-quality water. - Dry the ultracentrifuge tubes, and then fill them up with 70% ethanol. Leave the tubes with 70% ethanol for overnight disinfection. Remove the ethanol and dry the tubes again.
- Place the tubes and caps in sterilization packaging and close them tightly. Use the plasma or gas (ethylene oxide) sterilization method18,19. Store the ultracentrifuge tubes enclosed in the sterilization packaging in a dry place, and use them before the expiration date.
NOTE: Perform monthly monitoring of the LPS level in phosphate buffered saline (PBS) and the water used for EVs isolation. Monitoring should include the tubes as well (e.g., by testing the water [wash control] which has been stored in the ultracentrifuge tubes overnight at room temperature [RT]).
2. Preparation of EV-depleted low-endotoxin fetal bovine serum (EE-FBS)
- Ensure to use ultra-low endotoxin FBS (commercially available; see Table of Materials; <0.1 EU/mL).
- Place a bottle of ultra-low endotoxin FBS in a water bath and incubate at 56 °C for 30 min. This step is required to inactivate the complement system.
- Pipette the inactivated FBS to ultracentrifuge tubes and centrifuge it at 100,000 x g for 4 h at 4 °C. Collect the supernatant into sterile 50 mL tubes, ensuring not to exceed 45 mL per tube to avoid cap contamination and wetting the ring of the tube.
- Store the EE-FBS serum prepared in this manner at -20 °C. Check the concentration of LPS in EE-FBS (step 6). The LPS concentration should be at the same level as before ultracentrifugation.
3. Cell culture
- For this study, culture SW480 and SW620 cell lines in Dulbecco's modified Eagle's medium (DMEM) with 4.5 g/L glucose, L- glutamine, sodium pyruvate, and gentamicin (50 µL/mL) supplemented with 10% EE-FBS accordingly in 75 cm2 culture flasks using aseptic technique. Seed nearly 4.5 x 106 cells and 6.5 x 106 cells per flask for SW480 and SW620, respectively.
- Collect the supernatants twice a week (~10 mL per flask) when the cells reach full confluency (~13.5 x 106 and 20 x 106 cells per flask for SW480 and SW620, respectively), and split. Ensure that the viability of cells is not less than 99% and that the cells are cultured at 37 °C in a 5% CO2 atmosphere.
- Collect supernatants from the cell culture into appropriately labeled 15 mL tubes. Centrifuge the collected supernatants at 500 x g for 5 min at RT to remove cell debris.
- Collect the supernatants carefully, avoid aspirating debris, place in labeled tubes, and centrifuge again at 3,200 x g for 12 min at 4 °C.
- Collect supernatants from the second centrifugation step into sterile, labeled 50 mL tubes. Avoid wetting the edges of tubes. Ensure that the volume of supernatant does not exceed 45 mL and that the tubes are kept vertically.
- Wrap the tube's cap with a sterile transparent film and store supernatants in the vertical position at -80 °C.
NOTE: Perform monthly control of Mycoplasma spp. and LPS contamination in fresh culture supernatants (step 6). If the level of endotoxin in the supernatants exceeds 0.05 EU/mL, verify at which stage contamination might have occurred and restart the process from the beginning. If contamination of the cell culture by Mycoplasma spp. is detected, isolation of the EVs from such supernatants should be discontinued.
4. Isolation of EVs from cell culture supernatants
- Prepare 0.22 µm syringe filters and syringes of proper volume. Prepare two tubes with culture supernatants (90 mL).
- Fill the syringe with the supernatant and attach the filter to the needle adapter. Place the filled syringe with the filter over a 50 mL tube. Push on the plunger flange and collect ~90 mL of filtrate.
- Pipette the filtrate (7 mL) to the ultracentrifuge tubes (ensuring that the same volume is pipetted to each tube for proper balance) and centrifuge at 100,000 x g for 2 h at 4 °C.
NOTE: The efficiency of EVs pelleting depends on many factors (e.g., rotor type, its k-factor, migration path length, the viscosity of the medium, etc.), which need to be optimized for better recovery of EVs20. - Discard the supernatant using a sterile Pasteur pipette. Collect the remaining pellets using long, filtered pipette tips and pool them into two ultracentrifuge tubes. Fill them up to 7 mL with filtered endotoxin-free PBS to rinse the EVs.
- Centrifuge the PBS-resuspended pellets at 100,000 x g for 2 h at 4 °C. Completely remove the supernatant using a sterile Pasteur pipette, and add 200 µL of filtered, endotoxin-free PBS to both tubes to resuspend the EVs. Gently pipette the EVs pellet to collect all of the EVs.
- Transfer the EVs suspension into a sterile 1.5 mL test tube (if possible, use a low protein binding tube). Retain 10 µL of the EV suspension for preparing a dilution for nanoparticle tracking analysis (NTA) and for other purposes (e.g., protein concentration measurement).
- Dilute the EVs with filtered PBS (1:1,000) for measurements by NTA, according to the manufacturer's instructions. Secure the EV tubes by wrapping the cap with a sterile transparent film. Store the EVs at -80 °C.
5. Specific markers detection by western blotting
- Determine the protein level in the samples (e.g., by Bradford assay), and prepare the samples (20 µg) with loading buffer. Prepare the loading buffer by mixing 5 µL of sample buffer (4x) and 2 µL of sample reducing agent (10x) to a total volume of 20 µL. Incubate the samples at 70 °C for 10 min.
- Prepare polyacrylamide gels with SDS (10%-14%) and load the 20 µg of EVs to each well.
- Run the electrophoresis with running buffer (30 g of Tris, 144 g of glycine, 10% SDS per 1 L) for 45 min at 150 V.
- Perform semi-dry transfer of proteins from the gel onto the polyvinylidene difluoride (PVDF) membrane in a transfer machine with Towbin buffer (1.51 g of Tris, 7.2 g of glycine, 10% methanol per 0.5 L) at 25 V for 1 h.
- Place the membrane in TBST buffer (1 mL of Tween, 100 mL of Tris-buffered saline (TBS 10x) per 1 L) for blocking with 1% bovine serum albumin (BSA) in TBST buffer, and incubate for 1 h on the rocker at RT.
- Add diluted (1:1,000) antibodies, anti-CD91 (clone#D8O1A) or anti-Alix1 (clone#3A9), in 1% BSA and incubate with the membrane overnight at 4 °C on the rocker. Remove antibodies and wash membrane 3x with 10 mL of 1x TBST for 10 minutes.
- Add goat anti-rabbit or goat anti-mouse (dilution: 1:2,000) secondary antibody conjugated with horseradish peroxidase, depending on the primary antibody on the membrane, and incubate for 1 h on the rocker at RT. Remove antibodies and wash membrane 3x with 10 mL of 1x TBST for 10 minutes.
- Mix the substrate and luminol at a 1:1 ratio to obtain a 1 mL solution. Pour the solution on the membrane. Place the membrane immediately in the measuring chamber of the imaging system, choose the chemiluminescence module, and visualize protein bands on the screen.
6. Measurement of endotoxin level by Limulus Amebocyte Lysate test (LAL)
- Perform chromogenic LAL measurement of the endotoxin level, according to the manufacturer's recommendation. Briefly, use the method based on the interaction of the endotoxin with the limulus amebocyte lysate (LAL). The detection limit of this method is 0.005 EU/mL.
NOTE: LAL assay, despite its limitations, currently remains the standard method for detecting and quantifying endotoxin contamination in different kinds of solutions and other products used in science or medicine8. The limiting factor of this kit is the stability of the reconstituted amebocyte lysate solution, which is stable for only 1 week at -20 °C if frozen immediately after reconstitution. - Use a tenfold dilution of EVs and other samples and a 50-fold dilution of serum. For further experiments, use only low-endotoxin reagents such as EE-FBS, DMEM, PBS, RPMI (<0.005 EU/mL), and LPS-free culture supernatants.
7. Detection of prokaryotic 16S rRNA gene in EV samples
- Isolate DNA from the EV samples. Try to keep the reagents and the site of isolation aseptic. Measure the DNA concentration and quality (e.g., using a spectrophotometer).
- Perform polymerase chain reaction (PCR), as described previously21. Prepare 1.5% agarose gel with ethidium bromide or SYBR green to visualize the PCR products in ultraviolet light (UV).
- Run electrophoresis of the sample and weight marker with TRIS-acetate-EDTA buffer at 75 V for 45 min. Visualize DNA bands using the imaging system.
8. Determination of effective LPS concentration for stimulation in human monocyte model
- Prepare 2 x 106 monocytes/mL of monocyte suspension in RPMI 1640 supplemented with L-glutamine, glucose, and 2% EE-FBS. Add 50 µL of the suspension per well of a 96-well tissue culture plate22.
- Add a proper volume of LPS or culture medium to obtain the following final concentrations: 0 pg/mL (control), 10 pg/mL, 50 pg/mL, 100 pg/mL, 1 ng/mL, and 100 ng/mL, in 100 µL total volume of cell suspension. Make triplicates of each LPS concentration.
- Culture the cells for 18 h at 37 °C, 5% CO2. Collect the supernatant.
- Spin down the supernatant at 2,000 x g for 5 min. Transfer the supernatants into new tubes. Measure TNF8,23 and IL-1023 concentration in collected supernatants with the cytometric beads array (CBA) human cytokine kit, according to the manufacturer's procedure, with a flow cytometer.
Representative Results
A prerequisite or obligatory step for this protocol is the exclusion of possible endotoxin contamination from reagents. All the reagents being used, such as FBS, DMEM, RPMI, PBS, and even ultracentrifuge tubes, must be endotoxin-free (<0.005 EU/mL). Maintaining the regime of no endotoxin contamination is not easy as, for example, the regular/standard serum for cell culture can be its rich source (0.364 EU/mL; see Table 1).
Although this protocol was developed to isolate EVs with low endotoxin content, it is important to characterize the isolated EVs. In this study, the EVs were isolated from two colorectal cancer cell lines, SW480 and SW620. The expression of EVs markers, CD9 and Alix, was confirmed by western blot (Figure 1A). There was no difference in the mean size of EVs isolated from SW480 and SW620 cells (134 nm and 128.2 nm, respectively; Figure 1B). Additionally, there were no differences in the concentration of isolated EVs between these cell lines (Figure 1C). The exemplary distributions of EV size, as measured by NTA, are presented in Figure 1D. The time of ultracentrifugation was optimized according to the results of Cvjetkovic et al.20. Briefly, the mathematical formula for the conversion of centrifugal run parameters between two fixed angle rotors (70Ti and T-1270) was used. The effectiveness of small EVs depletion by spinning with a T-1270 rotor for 120 min at 100,000 x g was slightly less than that achieved by using a 70Ti rotor at 118,000 x g for 155 min; however, it was still in the range of effective RNA and protein pelleting. The calculation was confirmed by experimental data (Table S1), where the extended time of centrifugation (4 h vs. 2 h) had no meaningful impact on the number of EVs pelleted or still present in supernatants.
The level of LPS that caused a monocyte response was assessed. For this purpose, human monocytes were cultured with successive concentrations of LPS (0 pg/mL, 10 pg/mL, 50 pg/mL, 100 pg/mL, 1 ng/mL, and 100 ng/mL). After overnight culture, the level of IL-10 and TNF was determined in the culture supernatants. In this study, the lowest dose of LPS enabling the secretion of IL-10 and TNF in monocytes was 50 pg/mL, which corresponded to 0.5 EU/mL (Figure 2).
Finally, the last step was related to testing the level of LPS in the EVs isolated as above. The LPS contamination of the EV samples was around 0.5 EU/mL (50 pg/mL; Figure 3) for both cell lines (45.80 ± 20.39 pg/mL and 48.75 ± 7.412 pg/mL for SW480 and SW620, respectively). This means that 1 µL of EVs (around 109 EVs) contains less than 0.05 pg of endotoxin, and when added to 100 µL of monocyte suspension (ratio of 104 EVs per one monocyte) is diluted 100 times (the final concentration of LPS is around 0.5 pg/mL).
Additionally, the purity of EVs was tested by PCR for 16S rRNA gene21, which confirms the lack of bacterial contamination in EVs isolated from SW480 and SW620 cell lines (Supplementary Figure 1).
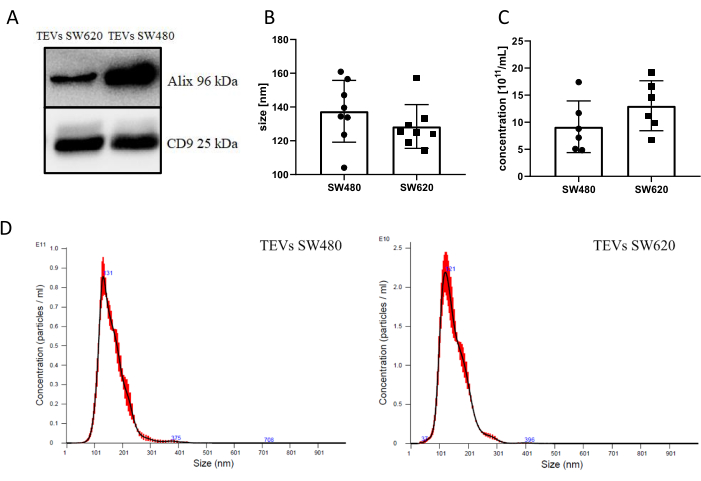
Figure 1: Characterization of isolated EVs. (A) Western blot analysis of the protein markers of EVs isolated from SW480 and SW620 cell lines. (B) NTA was used to measure the size of EVs isolated from SW480 and SW620 cell lines (nm). (C) NTA was used to measure the concentration of EVs isolated from SW480 and SW620 cell lines (EVs/mL). (D) Exemplary distributions of EVs size, as measured by NTA (blue numbers indicate particle size at a given point on the curve). Data in B and C are presented as mean ± SD (n = 10); t-test for statistical analysis was used. Please click here to view a larger version of this figure.
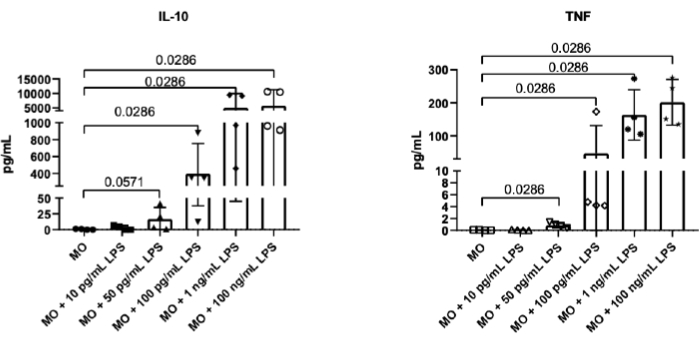
Figure 2: IL-10 and TNF secretion by monocytes stimulated with different doses of LPS. Secretion of cytokines by monocytes was determined after stimulation with LPS doses as indicated: 10 pg/mL, 50 pg/mL, 100 pg/mL, 1 ng/mL, and 100 ng/mL, in comparison to control monocytes (MO). Data are presented as mean ± SD (n = 4); Mann-Whitney U test was used. TNF and IL-10 concentrations were measured in culture supernatants by the cytometric beads array (CBA) method. Please click here to view a larger version of this figure.
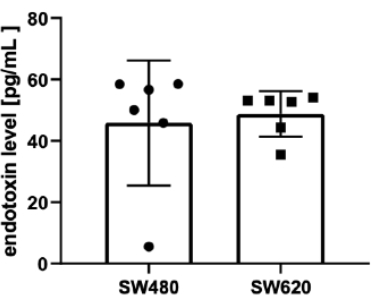
Figure 3: Measurement of LPS level in isolated EV samples by chromogenic LAL test. LPS content in EV samples obtained from SW480 and SW620 cell lines (pg/mL). Data are presented as mean ± SD (n = 6). Please click here to view a larger version of this figure.
| S.No. | sample | endotoxin level [EU/mL] | endotoxin level [pg/mL] | ||
| 1 | DMEM | <0.005 | <0.5 | ||
| 2 | RPMI1640 | <0.005 | <0.5 | ||
| 3 | FBS ultra low endotoxin | <0.005 | <0.5 | ||
| 4 | FBS ultra low endotoxin after 4 h centrifugation (EE-FBS) | <0.005 | <0.5 | ||
| 5 | regular FBS | 0.368 | 36.8 | ||
| 6 | filtered PBS | <0.005 | <0.5 | ||
| 7 | culture supernatant form SW480 cells after 2 h centrifigation | <0.005 | <0.5 | ||
| 8 | culture supernatant from SW620 cells after 2 h centrifugation | <0.005 | <0.5 | ||
| 9 | wash control (water stored in ultracentrifuge tubes) | <0.005 | <0.5 | ||
Table 1: LPS level in various reagents and samples determined by chromogenic LAL test. Measurements are presented as EU/mL and pg/mL. Samples included DMEM, RPMI, EE-FBS, FBS, filtered PBS, and wash control (water from ultracentrifugation tubes).
Supplementary Table 1: Representative examples of EVs concentration (EVs/mL) and size (nm) measurements in pellets and supernatants after 2 h or 4 h centrifugation of culture supernatants from SW480 and SW620 cell lines and PBS. Measurements were performed by NTA. Please click here to download this File.
Supplementary Figure 1: PCR results of pan-prokaryote 16S rRNA gene amplification in the following samples, from the left: molecular weight ladder (MW, 100-1000 bp), NC-negative control, EVs derived from SW480 and SW620, and PC (positive control-bacterial DNA) Please click here to download this File.
Discussion
In the last few years, methods for proper EVs isolation have become increasingly important, enabling their further reliable analyses, for example, in the context of obtaining reliable omics and functional data24. Based on previous research experience, it seems that not only the type of isolation method, but also other conditions during this procedure may be important. The use of EV-depleted FBS is widely recognized as a necessity25,26; however, the monitoring of endotoxin contamination in EVs is often neglected.
Nevertheless, all methods used for the isolation of EVs should have developed standards for rigorous aseptic handling and quality control at every stage of the procedure. This is particularly significant due to EVs' further downstream application. The proposed protocol is the result of previous experience with endotoxin-sensitive cells, such as monocytes and macrophages, which are endotoxin sensitive. CD14, a marker of monocytes, is capable of binding LPS at picomolar concentrations. Obtained results indicate that monocytes, after stimulation with 50 pg/mL (0.5 EU/mL) of LPS secrete TNF and IL-10. Yang et al., however, reported that even 0.1 EU/mL (10 pg/mL) of endotoxin might induce a potent reaction (upregulation of the inflammatory IL1B gene)27. Moreover, Chaiwut et al. demonstrated that the intracellular production of TNF and IL-6 in monocytes might be induced by even lower concentrations of LPS, 2.5 pg/mL or 5 pg/mL, respectively23. Alvarez et al. confirmed that the monocyte activation rate (calculated value) rises after a small concentration of LPS, such as 5 pg/mL28. Therefore, limiting endotoxin contamination in EVs at every possible stage of the isolation protocol is crucial for further experiments conducted with LPS-sensitive cells. Currently, ultracentrifugation is the most widely used method for EV isolation. However, to the best of our knowledge, there are no studies on the LPS contamination of EVs isolated by this method. Hence, the above protocol focuses on obtaining low-endotoxin EVs without external EVs contamination from the culture supernatants.
As mentioned above, endotoxin contamination may have various sources. First, it should be stressed that endotoxin is a tough oppponent, and not all methods of sterilization of glass or plasticware are effective in its removal or degradation. For example, the standard autoclaving procedure cannot eliminate LPS due to endotoxin's high heat stability27. Also, washing, even extensively, cannot remove endotoxin completely. By applying more depyrogenic methods to laboratory practice, such as plasma or ETO (ethylene oxide) sterilization18,19, endotoxin contamination can be limited. Next, permanent monitoring and prevention of possible contamination are crucial. The presented results indicate the importance of using ultra-low endotoxin reagents, such as serum, for culture supplementation. In addition, routine control of endotoxin contamination of all reagents (such as PBS, DMEM, and RPMI) and even plasticware (e.g., tubes) is highly recommended. It is also necessary to pre-check the culture supernatants before EVs isolation to prevent endotoxin accumulation. As presented above, an interesting alternative to the standard measurement of endotoxin levels by LAL assay is PCR amplification of the bacterial 16s rRNA gene. Determination of permissive (which does not induce monocyte stimulation) endotoxin levels of LPS in EVs is crucial. Considering culture conditions (concentration of monocytes and contamination with LPS of the applied EVs, the concentration of EVs; Figure 3), the final concentration of endotoxin that affects monocytes stimulated with EVs is not higher than 0.5 pg/mL (EVs are usually diluted 100-1,000 times). This dose is five times lower than that postulated by Chaiwut et al. as the least effective dose (2.5 pg/mL) able to induce the production of TNF by monocytes23. In the Chaiwut et al. study, cytokine production was determined using flow cytometry and presented as an MFI (mean fluorescent intensity) shift without quantification. Furthermore, monocyte stimulation with very low doses of LPS was performed in medium supplemented with 10% FBS, which may be an additional source of LPS23. This may suggest that the effective dose of LPS used for monocyte stimulation was higher. Thus, considering the presented results and literature data, it seems that the concentration of endotoxin up to 50 pg/mL (0.5 EU/mL) in EVs is permissive, and is not the cause of monocytes' activation. The next step in optimizing this protocol is further reduction of endotoxin contamination, even to the level undetected by LAL or cell-based assays. Recently, the importance of using biological models to detect masked endotoxin contamination, called low endotoxin recovery (LER), is strongly recommended8.
Thus, this protocol is innovative in this respect because it collects all aspects necessary for the isolation of EVs with the lowest possible endotoxin content, which has not yet been addressed in other studies.
As with each method, this protocol has some limitations. First, the proposed protocol reduces endotoxin contamination but does not eliminate it. Second, it is unknown whether the purity achieved is sufficient for other immune cells. Therefore, each protocol should be adapted for further application. Establishing such a protocol will allow obtaining results that will correspond more to the biologically active components of EVs than to their accidental contamination. Finally, the procedure is more expensive than usual because of the need to purchase several endotoxin testing kits and low endotoxin serum. As a promising alternative, Bussolati presented a new, sophisticated method for EVs isolation by tangential flow filtration. This method allows the acquisition of EVs with low endotoxicity (0.1-0.7 EU/mL; 10-70 pg/mL)29. Until now, this novel method has not been commonly used for EVs isolation, and requires specialized equipment in the form of a tangential flow filtration (TFF) system. Recently, Gałuszka et al. proposed a different way to eliminate endotoxin from air pollutants (particulate matter with the size of EVs but without membrane structure) by using polymyxin B30. However, the usefulness of this protocol for cell-derived vesicles needs to be verified through biological models, especially when thinking about in vivo experiments. Polymixin B and sodium deoxycholate (also applicable to endotoxin removal) have been previously described as neuro- and nephrotoxic31. Other possibilities are affinity columns with poly(ε-lysine), which selectively bind endotoxins. This method is quick and efficient, however, dedicated to protein samples/solutions; therefore, applying it to such complicated structures as EVs needs validation32. Moreover, these columns are intended for samples with high initial endotoxin levels, and their effectiveness in removing relatively small amounts of LPS is unknown and requires verification. The final anticipated concentration of LPS after purification with LPS-depleting columns may still be too high for immune cell tests (for example, <5 EU/mL).
In summary, this protocol proposed how to avoid endotoxin contamination by applying several principles to laboratory practice, such as rigorous aseptic technique during all steps of EVs isolation, using ultra-low endotoxin reagents, monitoring endotoxin impurities at all procedure steps, and changing the sterilization method. Moreover, the presented protocol provides hints on how to control endotoxin levels at every step of the isolation procedure. LPS present in EVs affects the function of immune cells, and therefore may cause false results in assays conducted with these cells.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Science Centre, Poland, grant number 2019/33/B/NZ5/00647. We would like to thank Professor Tomasz Gosiewski and Agnieszka Krawczyk from the Department of Molecular Medical Microbiology, Jagiellonian University Medical College for their invaluable help in the detection of bacterial DNA in EVs.
Materials
| Alix (3A9) Mouse mAb | Cell Signaling Technology | 2171 | |
| 1250ul Filter Universal Pipette Tips, Clear, Polypropylene, Non-Pyrogenic | GoogLab Scientific | GBFT1250-R-NS | |
| BD FACSCanto II Flow Cytometr | BD Biosciences | ||
| CBA Human Th1/Th2 Cytokine Kit II | BD Biosciences | 551809 | |
| CD9 (D8O1A) Rabbit mAb | Cell Signaling Technology | 13174 | |
| ChemiDoc Imaging System | Bio-Rad Laboratories, Inc. | 17001401 | |
| DMEM (Dulbecco’s Modified Eagle’s Medium) | Corning | 10-013-CV | |
| ELX800NB, Universal Microplate Reader | BIO-TEK INSTRUMENTS, INC | ||
| Fetal Bovine Serum | Gibco | 16000044 | |
| Fetal Bovine Serum South America Ultra Low Endotoxin | Biowest | S1860-500 | |
| Gentamicin, 50 mg/mL | PAN – Biotech | P06-13100 | |
| Goat anti-Mouse IgG- HRP | Santa Cruz Biotechnology | sc-2004 | |
| Goat anti-Rabbit IgG- HRP | Santa Cruz Biotechnology | sc-2005 | |
| Immun-Blot PVDF Membrane | Bio-Rad Laboratories, Inc. | 1620177 | |
| LPS from Salmonella abortus equi S-form (TLRGRADE) | Enzo Life Sciences, Inc. | ALX-581-009-L002 | |
| Mini Trans-Blot Electrophoretic Transfer Cell | Bio-Rad Laboratories, Inc. | 1703930 | |
| Nanoparticle Tracking Analysis | Malvern Instruments Ltd | ||
| NuPAGE LDS Sample Buffer (4X) | Invitrogen | NP0007 | |
| NuPAGE Sample Reducing Agent (10x) | Invitrogen | NP0004 | |
| Parafilm | Sigma Aldrich | P7793 | transparent film |
| Perfect 100-1000 bp DNA Ladder | EURx | E3141-01 | |
| PierceTM Chromogenic Endotoxin Quant Kit | Thermo Scientific | A39552 | |
| PP Oak Ridge Tube with sealing caps | Thermo Scientific | 3929, 03613 | |
| RPMI 1640 | RPMI-1640 (Gibco) | 11875093 | |
| SimpliAmp Thermal Cycler | Applied Biosystem | A24811 | |
| Sorvall wX+ ULTRA SERIES Centrifuge with T-1270 rotor | Thermo Scientific | 75000100 | |
| Sub-Cell GT Horizontal Electrophoresis System | Bio-Rad Laboratories, Inc. | 1704401 | |
| SuperSignal West Pico PLUS Chemiluminescent Substrate | Thermo Scientific | 34577 | |
| SW480 cell line | American Type Culture Collection(ATCC) | ||
| SW480 cell line | American Type Culture Collection (ATCC) | ||
| Syringe filter 0.22 um | TPP | 99722 | |
| Trans-Blot SD Semi-Dry Transfer Cell | Bio-Rad Laboratories, Inc. | 1703940 | Transfer machine |
| Transfer pipette, 3.5 mL | SARSTEDT | 86.1171.001 |
Riferimenti
- Théry, C., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles. 7 (1), 1535750 (2018).
- Yáñez-Mó, M., et al. Biological properties of extracellular vesicles and their physiological functions. Journal of Extracellular Vesicles. 4, 27066 (2015).
- van Niel, G., et al. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nature Reviews Molecular Cell Biology. 23 (5), 369-382 (2022).
- Ngkelo, A., Meja, K., Yeadon, M., Adcock, I., Kirkham, P. A. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Giα dependent PI-3 kinase signalling. Journal of Inflammation. 9 (1), (2012).
- Schwarz, H., Schmittner, M., Duschl, A., Horejs-Hoeck, J. Residual endotoxin contaminations in recombinant proteins are sufficient to activate human CD1c+ dendritic cells. PLOS ONE. 9 (12), 113840 (2014).
- Zanoni, I., Granucci, F. Role of CD14 in host protection against infections and in metabolism regulation. Frontiers in Cellular and Infection Microbiology. 3, 32 (2013).
- Takashiba, S., et al. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor kB. Infection and Immunity. 67 (11), 5573-5578 (1999).
- Schwarz, H., et al. Biological activity of masked endotoxin. Scientific Reports. 7, 44750 (2017).
- Danesh, A., et al. Granulocyte-derived extracellular vesicles activate monocytes and are associated with mortality in intensive care unit patients. Frontiers in Immunology. 9, 956 (2018).
- Barry, O. P., Pratico, D., Savani, R. C., FitzGerald, G. A. Modulation of monocyte-endothelial cell interactions by platelet microparticles. The Journal of Clinical Investigation. 102 (1), 136-144 (1998).
- Sadallah, S., Eken, C., Martin, P. J., Schifferli, J. A. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. Journal of Immunology. 186 (11), 6543-6552 (2011).
- Soekmadji, C., et al. The future of Extracellular Vesicles as Theranostics – an ISEV meeting report. Journal of Extracellular Vesicles. 9 (1), 1809766 (2020).
- Gioannini, T. L., et al. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proceedings of the National Academy of Sciences. 101 (12), 4186-4191 (2004).
- Roslansky, P. F., Dawson, M. E., Novitsky, T. J. Plastics, endotoxins, and the Limulus amebocyte lysate test. Journal of Parenteral Science and Technology. 45 (2), 83-87 (1991).
- Fishel, S., Jackson, P., Webster, J., Faratian, B. Endotoxins in culture medium for human in vitro fertilization. Fertility and Sterility. 49 (1), 108-111 (1988).
- Schulz, E., Karagianni, A., Koch, M., Fuhrmann, G. Hot EVs – How temperature affects extracellular vesicles. European Journal of Pharmaceutics and Biopharmaceutics. 5 (146), 55-63 (2020).
- Osteikoetxea, X., et al. Differential detergent sensitivity of extracellular vesicle subpopulations. Organic & Biomolecular Chemistry. 13 (38), 9775-9782 (2015).
- Sakudo, A., Yagyu, Y., Onodera, T. Disinfection and sterilization using plasma technology: fundamentals and future perspectives for biological applications. International Journal of Molecular Sciences. 20 (20), 5216 (2019).
- Shintani, H. Ethylene oxide gas sterilization of medical devices. Biocontrol Science. 22 (1), 1-16 (2017).
- Cvjetkovic, A., Lötvall, J., Lässer, C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. Journal of Extracellular Vesicles. 3, (2014).
- Salamon, D., et al. Comparison of iSeq and MiSeq as the two platforms for 16S rRNA sequencing in the study of the gut of rat microbiome. Applied Microbiology and Biotechnology. 106 (22), 7671-7681 (2022).
- Baj-Krzyworzeka, M., Szatanek, R., Weglarczyk, K., Baran, J., Zembala, M. Tumour-derived microvesicles modulate biological activity of human monocytes. Immunology Letters. 113 (2), 76-82 (2007).
- Chaiwut, R., Kasinrerk, W. Very low concentration of lipopolysaccharide can induce the production of various cytokines and chemokines in human primary monocytes. BMC Research Notes. 15 (1), 42 (2022).
- Van Deun, J., et al. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. Journal of Extracellular Vesicles. 3, 24858 (2014).
- Lehrich, B. M., Liang, Y., Fiandaca, M. S. Foetal bovine serum influence on in vitro extracellular vesicle analyses. Journal of Extracellular Vesicles. 10 (3), 12061 (2021).
- Pham, C. V., et al. Bovine extracellular vesicles contaminate human extracellular vesicles produced in cell culture conditioned medium when ‘exosome-depleted serum’ is utilised. Archives of Biochemistry and Biophysics. 708, 108963 (2021).
- Li, Y., Boraschi, D. Endotoxin contamination: a key element in the interpretation of nanosafety studies. Nanomedicine. 11 (3), 269-287 (2016).
- Álvarez, E., et al. A system dynamics model to predict the human monocyte response to endotoxins. Frontiers in Immunology. 8, 915 (2017).
- Busatto, S., et al. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells. 7 (12), 273 (2018).
- Gałuszka, A., et al. Transition metal containing particulate matter promotes Th1 and Th17 inflammatory response by monocyte activation in organic and inorganic compounds dependent manner. International Journal of Environmental Research and Public Health. 17 (4), 1227 (2020).
- Falagas, M. E., Kasiakou, S. K. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Critical Care. 10 (1), 27 (2006).
- Petsch, D., Anspach, F. B. Endotoxin removal from protein solutions. Journal of Biotechnology. 76 (2-3), 97-119 (2000).

