- 00:00Panoramica
- 01:06Principles of Gas Chromatography
- 03:54Instrument Initialization
- 05:37Running the GC
- 06:32Representative Results: Quantification of Caffeine and Palmitic Acid in Coffee
- 07:28Applications
- 08:59Summary
炎イオン化検出ガスクロマトグラフィー (GC)
English
Condividere
Panoramica
ソース: 研究所博士 b. ジル Venton – ヴァージニアの大学の
ガス ・ クロマトグラフィー (GC) が分離し、ガス相で分子量が小さい化合物を検出する使用されます。サンプルは、ガスまたは注入口で気化した液体です。通常、分析化合物は、大きな化合物を気化しにくい未満 1,000 Da です。それは非常に信頼性が高く、ほぼ継続的に実行することができますので、GC は環境モニタリングおよび産業用アプリケーションで人気です。小さく、揮発性の分子が検出されたアプリケーションと非水溶液、GC は、通常使用されます。液体クロマトグラフィーは、水溶液サンプルでの測定より人気があり、分子が蒸発させる必要がないのでより大きい分子を研究に使用できます。LC は極 analytes の分離のより一般的な非極性分子の GC は支持されます。
ガス ・ クロマトグラフィーの移動相はキャリアガス、通常ヘリウムの低分子量、化学的に不活性のためです。圧力が適用され、モバイルのフェーズで列を検体に移動します。分離は、液相をコーティングしたカラムを使用して行われます。中空毛細管カラムは最も人気のある列で、毛細血管の壁にコーティングした固定相を持っています。固定相はポリジメチルシロキサンの誘導体の分離をチューニングする機能グループの 5-10% と多い。典型的な官能基、フェニル、シアノプロピル, または trifluoropropyl のグループです。キャピラリーカラムは、通常 5-50 メートルです。狭い列のより高い解像度がより高い圧力を必要とします。詰められたコラムは、ビーズを充填したカラムの固定相のコーティングでも使用できます。詰められたコラムが短く、1-5 メートル、高効率、高速解析を許可する高い容量を持つ開いている尿細管毛細血管が一般に好ましい。
炎イオン化検出 (FID) は、サンプルの炭素の量を検出する GC における有機化合物の良い一般的な器です。列の後のサンプルはホット、水素-空気火炎で焼かれて。炭素イオンは、燃焼によって生成されます。プロセスの全体的な効率が低い (1 の 105炭素イオンを炎のイオン生成) のみイオンの総量は、サンプル中の炭素の量に直接比例。電極は、イオンからの電流を測定するために使用されます。FID は、サンプル全体を熱分解した破壊的な検出器です。FID は不燃ガスと水によって影響を受けるです。
Principi
Procedura
Applications and Summary
GC is used for a variety of industrial applications. For example, it is used to test the purity of a synthesized chemical product. GC is also popular in environmental applications. GC is used to detect pesticides, polyaromatic hydrocarbons, and phthalates. Most air quality applications use GC-FID to monitor environmental pollutants. GC is also used for headspace analysis, where the volatiles that are evaporated from a liquid are collected and measured. This is useful for the cosmetic and food and beverage industries. GC is used for forensic applications as well, such as detecting drugs of abuse or explosives. In addition, GC is useful in the petroleum industry for measuring hydrocarbons. The extensive applications makes GC a billion dollar per year worldwide market.
Figure 3 shows an example of how GC could be used in the food industry. Figure 3 shows a chromatograph of artificial vanilla (black) and real vanilla (red). GC can be used to identify the real sample, which contains a large peak for vanillin but does not contain a second peak for ethylvanillin.
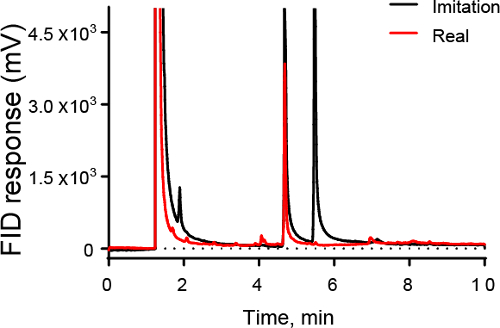
Figure 3. GC-FID chromatogram of vanilla samples. Both imitation and real vanilla show large peaks at 4.7 min due to vanillin, the principle component of vanilla. However, imitation vanilla also has a large peak at 5.3 min, which is due to ethylvanillin, a compound not present in large quantities in real vanilla.
Trascrizione
Gas Chromatography, or GC, is a technique that is used to separate, detect, and quantify small volatile compounds in the gas phase.
In GC, liquid samples are vaporized, then carried by an inert gas through a long, thin column. Analytes are separated based on their chemical affinity with a coating on the inside of the column.
Because GC requires that analytes are vaporized to the gas phase, the instrument is ideally suited for volatile, nonpolar chemicals less than 1,000 daltons in mass. For larger, aqueous, or polar molecules that are difficult to vaporize, liquid chromatography is a useful alternative. This video will introduce the basics of gas chromatography, and illustrate the steps required to analyze the chemical species in a non-aqueous mixture sample using a gas chromatograph.
The GC instrument has five essential components. First, an injection port is used to introduce the sample into the instrument. Next, a heating chamber vaporizes the sample and mixes it with an inert gas. The inert gas, such as helium or nitrogen, carries the vaporized sample through the system. Combined, the carrier gas and sample make up the mobile phase. Next, the mobile phase enters the heated column, separating the analytes as they flow through. Lastly, a detector records the gases as they exit the column, or elute, and sends data to a computer for analysis. The most critical component of the instrument is the column. The column is a capillary with a stationary phase matrix coating the inner walls. Alternatively, columns can be packed with matrix-coated beads. The stationary phase is usually modified polydimethylsiloxane, which is ideal for resolving nonpolar molecules. Its separation properties are refined by adding 5–10% phenyl, cyanopropyl, or trifluoropropyl groups.
Analytes with low chemical affinity for the stationary phase move quickly through the column, while molecules with high affinity are slowed as they adsorb to the column walls.The length of time a compound spends inside the column is called its retention time, or Rt, and allows compounds to be identified. The detector sits at the end of the column and records gases as they elute. Flame-ionization detection, or FID, is widely used because it senses carbon ions, allowing it to detect virtually any organic compound. In FID, analytes combust in a hydrogen-air flame as they exit the column, producing carbon ions that induce a current in nearby electrodes. The current is directly proportional to the carbon mass, thus, the concentration of the compound can be determined. The final result is a chromatogram, which is a plot of FID signal vs time, showing each eluted component as they exit the column. Ideally, each peak will have a symmetrical, Gaussian shape. Asymmetrical features, such as peak tailing and peak fronting, can be due to overloading, injection problems, or the presence of functional groups that stick to the column, such as carboxylic acids.
Now that the principles of gas chromatography have been discussed, let’s take a look at how to carry out and analyze a gas chromatography analysis in the laboratory.
Before running an experiment, turn on the helium gas tank. Open the software on the computer, then bake out the column to remove any potential contaminants. Set the oven to a high temperature, typically 250 °C or above, and bake the column for at least 30 min.
Next, adjust the autosampler settings. Set the number of pre- and post-run rinses to clean the column between samples.
Use a sample volume of 1 μL and set the split ratio setting to program the instrument to accept only a fraction of the input. Adjust the flow rate of the carrier gas, and use established settings or trial and error to find the ideal pressure.
Now enter the temperature settings for the experiment. For an isothermal run, enter the temperature and the time for the separation. Alternatively, for a temperature gradient, enter the starting temperature and hold time, the ending temperature and hold time, and the ramp speed in °C per min.
Set the time for the column to cool between runs for either a gradient or isothermal run.
Finally, set the sampling rate and the detector temperature. The detector must always be hotter than the column to prevent condensation. After all the settings are programmed, save the methods file.
Activate the detector by opening the hydrogen tank valve and ignite the flame of the FID. The instrument is now ready for sample analysis.
To run the sample on the GC, first fill a vial with a wash solvent, such as acetonitrile or methanol. Prepare the sample, being certain to use glass syringes and glass vials as plastic residues can contaminate the GC.
Now add the prepared sample to a vial with a pipette. Fill at least half way, so that the autosampler syringe will be fully submerged. Then, load the wash and sample vials into the autosampler rack. Before running the sample, zero the baseline of the chromatogram on the computer software. Data can be collected either as a single run or using a batch table for multiple runs. Press “start” to run the sample.
In this example, caffeine and palmitic acid levels in coffee were analyzed using GC with FID. Caffeine is smaller and less polar, so it is less attracted to the column, and elutes first. Palmitic acid, which has a long alkane chain tail, elutes later due to a higher affinity with the stationary phase.
Because peak dimensions are proportional to carbon mass, the concentration of each component can be determined from its respective peak area on the chromatograph and compared to standards of known concentration.
The effect of column temperature was also explored. At 200 °C, samples moved through the column twice as fast as the sample run at 180 °C. Note that while peak heights change, the area under the curve remains constant.
GC is an important technique for chemical analysis, and is widely used in scientific, commercial, and industrial applications.
Due to the simplicity of GC, chemists routinely use it to monitor chemical reactions and product purity. Reactions can be sampled over time to show product formation and reactant depletion. The chromatograph reveals product concentrations and also the presence of unintended or side products.
GC is commonly used in tandem with mass spectrometry, called GS-MS, to unambiguously identify chemicals in samples or air. Mass spectrometry, or MS, separates molecules based on their mass to charge ratio, and enables the determination of compound identities. GC-MS is a powerful tool, as GC first separates complex mixtures into individual components, and MS gives precise mass information and chemical identity.
GC is routinely used in air monitoring to detect volatile organic compounds, or VOCs, which may arise from environmental pollution, pesticides and explosives. GC can be used to track and identify VOCs both indoors for headspace analysis and outdoors, for health, safety, and security.
You’ve just watched JoVE’s introduction to gas chromatography with FID. You should now understand the basic principles of gas chromatography and FID detection.
Thanks for watching!
