创建维诺格拉茨基柱:在沉积物样本中丰富微生物物种的方法
English
Condividere
Panoramica
资料来源:伊丽莎白·苏特1,克里斯托弗·科尔博1,乔纳森·布莱泽1
1大学生物科学系,瓦格纳学院,1 校园路,纽约州斯塔顿岛,10301
维诺格拉茨基柱是一个微型的封闭生态系统,用于丰富沉积物微生物群落,特别是那些参与硫循环的微生物群落。该柱在19世纪80年代首次由谢尔盖·维诺格拉茨基使用,此后应用于生物地球化学中涉及的多种微生物的研究,如光合体、硫氧化剂、硫酸还原剂、甲烷原、铁氧化剂、氮循环器,以及更多 (1,2)。
地球上的大多数微生物被认为是不可培养的,这意味着它们不能被隔离在试管或培养皿(3)。这是由于许多因素,包括微生物依赖于其他代谢产物。维诺格拉茨基柱中的条件与微生物的自然栖息地(包括它们与其他生物的相互作用)紧密地模仿,并允许它们在实验室中生长。因此,这项技术允许科学家研究这些生物体,并了解它们对地球生物地球化学循环的重要性,而不必孤立地生长。
地球的环境充满了微生物,它们生长在所有类型的栖息地,如土壤、海水、云层和深海沉积物。在所有栖息地,微生物相互依赖。随着微生物的生长,它消耗特定的基质,包括富含碳的燃料,如糖以及营养物质、维生素和氧气等呼吸气体。当这些重要的资源耗尽时,具有不同代谢需求的不同微生物就会开花并茁壮成长。例如,在维诺格拉茨基柱中,微生物首先消耗添加的有机物质,同时消耗柱底层中的氧气。一旦氧气被消耗,厌氧生物就可以接管并消耗不同的有机物质。随着时间推移,不同微生物群落的连续发展称为继承(4)。微生物继承在维诺格拉茨基柱中很重要,微生物活动会改变沉积物的化学成分,进而影响其他微生物的活性等等。土壤和沉积物中的许多微生物也沿着梯度生活,梯度是两种不同类型的生境之间的过渡区,基于基质的浓度(5)。在梯度的正确点上,微生物可以接收不同基质的最佳量。随着维诺格拉茨基柱的发展,它开始模仿这些自然梯度,特别是在氧气和硫化物中(图1)。
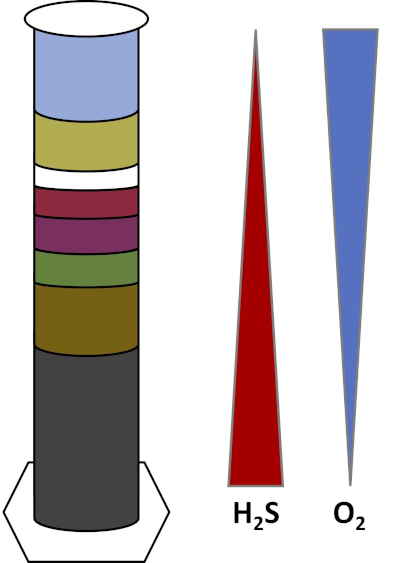
图 1:在维诺格拉茨基柱中发育的氧(O2)和硫化物(H2S)梯度的表示。
在维诺格拉茨基柱中,池塘或湿地的泥浆和水混合在透明的柱子中,允许孵育,通常是在光线下。在柱中加入额外的基质,为社区提供碳源,通常以纤维素和硫的形式存在。光合器通常开始在沉积物的顶层生长。这些光合微生物主要由蓝藻组成,蓝藻产生氧气,呈绿色或红褐色层(图2,表1)。光合作用产生氧气,而氧气对水的可溶性并不高,而且低于此层(图1)。这将产生氧气梯度,从顶层的高浓度氧气到底层的零氧。含氧层称为有氧层,无氧层称为厌氧层。
在厌氧层中,许多不同的微生物群落可以增殖,这取决于可用的基质的类型和数量、初始微生物的来源和沉积物的孔隙度。在柱的底部,厌氧分解有机物的生物体可以茁壮成长。微生物发酵产生有机酸从纤维素的分解。然后,硫酸盐还原剂可以使用这些有机酸,利用硫酸盐氧化这些有机物,并生产硫化物作为副产品。如果沉积物变黑,硫酸盐还原剂的活性表示,因为铁和硫化物会反应形成黑色硫化铁矿物(图2,表1)。硫化物也会向上扩散,从而产生另一个梯度,其中硫化物浓度高在柱的底部,低在柱的顶部(图1)。
在柱的中间附近,硫氧化剂利用从上面供应的氧气和下面的硫化物。光合硫氧化剂在适当的光量下可以开发在这些层中。这些生物体被称为绿色和紫色硫细菌,通常显示为绿色、紫色或紫红色细丝和斑点(图2,表1)。绿色硫磺细菌对硫化物的耐受性较高,通常发展在紫硫细菌正下方的层中。紫硫细菌以上,紫无硫菌也可能发展。这些有机体使用有机酸作为电子捐赠者而不是硫化物进行光合,通常表现为红色、紫色、橙色或棕色层。非光合硫氧化剂可以发展在紫色非硫细菌之上,这些通常表现为白色细丝(图2,表1)。此外,在维诺格拉茨基列中也可能形成气泡。有氧层中的气泡表示蓝藻产生的氧气。厌氧层中的气泡很可能是由于甲烷原的活性,这种生物体有氧分解有机物,形成甲烷作为副产品。
| 列中的位置 | 功能组 | 有机体示例 | 视觉指示器 |
| 返回页首 | 光合成器 | 蓝藻 | 绿色或红褐色图层。有时是氧气的气泡。 |
| 非光合硫氧化剂 | 比吉亚托亚, 蒂奥巴奇鲁斯 | 白色图层。 | |
| 紫色无硫细菌 | 罗多微比姆, 罗多斯皮里卢姆, 罗多普苏德蒙纳斯 | 红色、紫色、橙色或棕色图层。 | |
| 紫色硫磺细菌 | 铬 | 紫色或紫色-红色图层。 | |
| 绿色硫磺细菌 | 氯比 | 绿色图层。 | |
| 硫酸盐还原细菌 | 脱苏尔福维布里奥、脱硫素、脱硫杆菌、脱硫菌 | 黑色层。 | |
| 底部 | 梅萨诺根 | 美他球菌,美他拉诺沙西纳 | 有时是甲烷的气泡。 |
表 1:可能出现在经典维诺格拉茨基柱中,从上到下的主要细菌群。给出了每组生物体的例子,并列出了每一层生物体的可视指标。根据佩里等人(2002年)和罗根等人(2005年)。
Procedura
Risultati
In this experiment, water and sediment were collected from a freshwater habitat. Two Winogradsky columns were constructed and allowed to develop: a classical Winogradsky column incubated in the light at room temperature (Fig. 2A) and a Winogradsky column incubated in the dark at room temperature (Fig. 2B).

Figure 2B: A photo of classical Winogradsky column (left), incubated at room temperature in light for 68 days and a Winogradsky column incubated at room temperature in the dark for 68 days (right).
After allowing the columns to develop for 7-9 weeks, the layers in the classical column can be compared to the column incubated in the dark (Fig. 2B). In the classical Winogradsky column, a green cyanobacterial layer can be observed near the top of the tube. Near the center of the tube, a red-purple layer can be observed, indicative of purple nonsulfur bacteria. Under this layer, a purple-red layer is observed, indicative of purple sulfur bacteria. Directly under this layer, black sediment can be observed in the anaerobic region of the column, indicative of sulfate reducing bacteria.
The column grown in the dark (Fig. 2B) developed differently than the classical Winogradsky column. Like the classical column, the dark column yielded black sediment near the bottom of the column, indicative of sulfate reducing bacteria. The dark column did not yield the green cyanobacterial layer, nor the red, purple, or green layers indicative of purple nonsulfur, purple sulfur, and green sulfur bacteria, respectively. These groups are dependent on light for growth, and therefore unable to grow in the dark.
The precise results of each Winogradsky column will vary widely with their incubation conditions and their source habitats.
Microbial communities originating from freshwater habitats will not be accustomed to high salt concentrations and the addition of salt may slow down or inhibit growth. Conversely, there may be sufficient halophilic bacteria in brackish and saltwater habitats so that the addition of salts makes no difference or even enhances the growth of particular layers when compared to a column without added salts.
Sandy sediments are more porous than muddy sediments. If enough sulfide is produced in such porous sediments, sulfides can diffuse all the way to the top of the column and inhibit growth of aerobic organisms. In this case, the column may only contain layers indicative of anaerobes and may not contain any aerobes, such as the cyanobacteria.
Freshwater generally contains less sulfate than saltwater. Sulfate is important for the growth of sulfate-reducing bacteria. Sulfate reducers create sulfide as a byproduct and are indicated by the development of a black layer in the bottom of the column. If sulfate is not supplemented to freshwater communities, sulfate reducers may not produce enough sulfide. The creation of the sulfide byproduct is important for the growth of green and purple sulfur bacteria and the nonphotosynthetic sulfur oxidizers. In these cases, sulfur oxidizers can still grow using the egg yolk as a source of sulfur, even if the sulfate reducers (black layer) never develop.
Different wavelengths of light should select for organisms with different absorption pigments. A column kept in the dark will only allow for nonphotosynthetic organisms to grow, including sulfate reducers, iron oxidizers, and methanogens. Photosynthesizers have pigments that absorb light at different wavelengths within the visible range (~400-700nm). By covering a column with, for example, blue cellophane, blue light (~450-490nm) is blocked from entering the column. All of the photosynthesizers in the column have pigments which require the blue wavelengths (6) and their growth should be inhibited. On the other hand, red cellophane will block light of ~635-700nm. These wavelengths are important for the pigments used by cyanobacteria (6), while purple sulfur, green sulfur, and purple nonsulfur bacteria may still be able to grow.
Different microbial communities may have vastly different adaptive abilities to cope with changes in temperatures. High temperatures can enhance rates of microbial activity when sufficient thermophiles are present. On the other hand, in the absence of thermophiles, high temperatures may decrease overall microbial activity. Similarly, low temperatures may decrease overall microbial activity unless the microbial community contains sufficient psychrophiles.
Applications and Summary
The Winogradsky column is an example of an interdependent microbial ecosystem. After mixing mud, water, and additional carbon and sulfur substrates in a vertical column, the stratified ecosystem should stabilize into separate, stable zones over several weeks. These zones are occupied by different microorganisms which flourish at a particular spot along the gradient between the sulfide-rich sediment in the bottom and the oxygen-rich sediment at the top. By manipulating the conditions and substrates within the Winogradsky column, the presence and activity of different microorganisms such as halophiles, thermophiles, psychrophiles, sulfur oxidizers, sulfur reducers, iron oxidizers, and photosynthesizers can be observed.
Riferimenti
- Zavarzin G. (2006). Winogradsky and modern microbiology. Microbiology 75(6): 501-511. doi: 10.1134/s0026261706050018
- Esteban DJ, Hysa B, and Bartow-McKenney C (2015). Temporal and Spatial Distribution of the Microbial Community of Winogradsky Columns. PLoS ONE 10(8): e0134588. doi:10.1371/journal.pone.0134588
- Lloyd KG, Steen AD, Ladau J, Yin J, and Crosby L. (2018). Phylogenetically novel uncultured microbial cells dominate Earth microbiomes. mSystems 3(5): e00055-18. doi:10.1128/mSystems.00055-18
- Anderson DC, and Hairston RV (1999). The Winogradsky Column & Biofilms: Models for Teaching Nutrient Cycling & Succession in an Ecosystem. The American Biology Teacher, 61(6): 453-459. doi: 10.2307/4450728
- Dang H, Klotz MG, Lovell CR and Sievert SM (2019) Editorial: The Responses of Marine Microorganisms, Communities and Ecofunctions to Environmental Gradients. Frontiers in Microbiology 10(115). doi: 10.3389/fmicb.2019.00115
- Stomp M, Huisman J, Stal LJ, and Matthijs HCP. (2007) Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. ISME Journal. 1(4): 271-282. doi:10.1038/ismej.2007.59
- Perry JJ, Staley JT, and Lory S. (2002) Microbial Life, First Edition, published by Sinauer Associates
- Rogan B, Lemke M, Levandowsky M, and Gorrel T. (2005) Exploring the Sulfur Nutrient Cycle Using the Winogradsky Column. The American Biology Teacher, 67(6): 348-356. doi: 10.2307/4451860
Trascrizione
Most of the Earth’s microorganisms cannot be cultured in a lab, often because they rely on other microbes within their native communities. A Winogradsky column, named for its inventor Sergei Winogradsky, is a miniature, enclosed ecosystem which enriches the microbial communities within a sediment sample, enabling scientists to study many of the microbes that play a vital role in Earth’s biogeochemical processes, without needing to isolate and culture them individually.
Typically, mud and water from an ecosystem, such as a pond or a marsh, are mixed. As an optional experiment, salt can be added to this mixture to enrich various halophile species. Next, a small portion of the mixture is supplemented with carbon, usually in the form of cellulose from newspaper, and sulfur, usually from an egg yolk. For another optional experiment, a nail can be added to this mixture to enrich certain Gallionella species. This new mixture is then added to a transparent column, so that the column is one quarter full. Finally, the rest of the mud mixture and more water is added to the column until it is most of the way full.
Succession, which refers to the consecutive development of different microbial communities over time, can be observed in real time with a Winogradsky column. As microbes grow within the column, they consume specific substrates and change the chemistry of their environment. When their substrates are depleted, the original microbes die off and microbes with different metabolic needs can flourish in the altered environment. Over time, visibly distinct layers begin to form, each containing parts of a bacterial community with different microenvironmental needs.
For example, photosynthetic microbes, largely composed of cyanobacteria, form green or red-brown layers near the top of the column. Since photosynthesis produces oxygen, often seen as bubbles in the top portion of the column, a gradient is formed with the highest oxygen concentrations near the top, and the lowest towards the bottom. Depending upon the available substrates, different microbial communities can grow in the anaerobic bottom layer. Bubbles in this layer can indicate the presence of methanogens, which create methane gas via fermentation. Here, the microbial fermentation of cellulose results in organic acids. Sulfate reducers oxidize those acids to produce sulfide, and their activity is indicated by black sediment. Sulfide diffuses upward in the column, creating yet another gradient where sulfide concentrations are highest towards the bottom of the column, and lowest near the top. Towards the middle of the column, sulfur oxidizers utilize the oxygen from above and sulfide from below. With adequate light, photosynthetic sulfur oxidizers, such as green and purple sulfur bacteria, develop. Green sulfur bacteria tolerate higher sulfide concentrations. Thus, they grow directly below the purple sulfur bacteria. Directly above this layer, purple non-sulfur bacteria form a red-orange layer. Nonphotosynthetic sulfur oxidizers are indicated by the presence of white filaments.
Conditions such as light and temperature can also be varied to enrich other communities. In this video, you will learn how to construct a Winogradsky column, and vary the growing conditions and substrates to enrich specific microbial communities.
First, locate an appropriate aquatic ecosystem, such as a pond or marsh. The sediment samples should come from the area near the water’s edge, and be completely saturated with water. Then, use a shovel and a bucket to collect one to two liters of the saturated mud. Next, obtain approximately three liters of fresh water from the same source and return to the lab with the field samples.
In the lab, put on the appropriate personal protective equipment, including a lab coat and gloves. Now, transfer approximately 750 milliliters of mud to a mixing bowl. Then, sift through the mud to remove large rocks, twigs, or leaves and use a spoon to break apart any clumps. Next, add some of the fresh water to the mixing bowl, and stir with a large spoon. Add water until the consistency of the water-mud mixture is similar to a milkshake. Continue to make sure there are no clumps.
As an optional experiment, select for halophilic bacteria by adding 25 to 50 milligrams of salt to the mud mixture.
Then, transfer approximately 1/3 of the water-mud mixture to a second mixing bowl. Add one egg yolk and a handful of shredded newspaper to the bowl. Next, add this mixture to the column, until it is about 1/4 full. Next, add the water-mud mixture without the egg and newspaper to the column, until it is approximately 3/4 full. Then, add more water to the column, leaving a 1/2 inch space on top. Cover the column with plastic wrap and secure it with a rubber band.
Incubate the column in the light near a window at room temperature for the next four to eight weeks. Throughout the incubation period, monitor changes in the Winogradsky column at least once a week for the development of different colored layers and the formation of bubbles. Additionally, record the time it takes for different layers to develop.
Another modification that can be done is incubating the column near a radiator to select for thermophilic bacteria, or in a refrigerator to select for psychrophilic bacteria. Vary the light conditions by placing different columns in high light, low light, or darkness to incubate. Alternatively, limit the wavelength of incoming light by covering the column with different shades of cellophane to determine which colors select for different bacterial groups. For another optional experiment, to enrich iron-oxidizing bacteria, add a nail to the mud-water mixture prior to the addition of newspaper and an egg yolk.
After one to two weeks, growth of the cyanobacterial layer is indicated by a green or red-brown film on top of the mud layer of the classical Winogradsky column. Over time, the appearance and evolution of the different layers is monitored, each indicative of the different types of bacteria present. When comparing a column grown in the dark to a traditional Winogradsky column, we see the dark treatment yields the black layer at the bottom of the column, indicative of sulfate-reducing bacteria.
The dark column may also yield other layers, depending on other incubation conditions. Additionally, the dark column doesn’t yield the green cyanobacterial layer, nor the red, purple, or green layers indicative of purple non-sulfur, purple sulfur, and green sulfur bacteria respectively. These groups are dependent on light for growth.
