Optogenetic Stimulation of Escape Behavior in Drosophila melanogaster
Summary
Genetically encoded optogenetic tools enable noninvasive manipulation of specific neurons in the Drosophila brain. Such tools can identify neurons whose activation is sufficient to elicit or suppress particular behaviors. Here we present a method for activating Channelrhodopsin2 that is expressed in targeted neurons in freely walking flies.
Abstract
A growing number of genetically encoded tools are becoming available that allow non-invasive manipulation of the neural activity of specific neurons in Drosophila melanogaster1. Chief among these are optogenetic tools, which enable the activation or silencing of specific neurons in the intact and freely moving animal using bright light. Channelrhodopsin (ChR2) is a light-activated cation channel that, when activated by blue light, causes depolarization of neurons that express it. ChR2 has been effective for identifying neurons critical for specific behaviors, such as CO2 avoidance, proboscis extension and giant-fiber mediated startle response2-4. However, as the intense light sources used to stimulate ChR2 also stimulate photoreceptors, these optogenetic techniques have not previously been used in the visual system. Here, we combine an optogenetic approach with a mutation that impairs phototransduction to demonstrate that activation of a cluster of loom-sensitive neurons in the fly’s optic lobe, Foma-1 neurons, can drive an escape behavior used to avoid collision. We used a null allele of a critical component of the phototransduction cascade, phospholipase C-β, encoded by the norpA gene, to render the flies blind and also use the Gal4-UAS transcriptional activator system to drive expression of ChR2 in the Foma-1 neurons. Individual flies are placed on a small platform surrounded by blue LEDs. When the LEDs are illuminated, the flies quickly take-off into flight, in a manner similar to visually driven loom-escape behavior. We believe that this technique can be easily adapted to examine other behaviors in freely moving flies.
Introduction
A growing arsenal of genetically encoded tools have been developed to manipulate neural activity in specific cells in Drosophila melanogaster1. These tools enable the noninvasive activation or silencing of specific neurons in the intact and freely moving animal. Among these, Channelrhodopsin2 (ChR2), a light-activated cation channel, offers key advantages, since it can be temporally controlled and quickly induced. When neurons that express ChR2 are exposed to bright blue (470 nm) light they rapidly depolarize and exhibit elevated firing rates3-5. Such targeted activation of specific neurons in freely moving animals has revealed the sufficiency of particular neurons for behaviors such as CO2 avoidance3, proboscis extension2,4, and giant-fiber mediated startle responses4. However, as the intense light sources necessary to stimulate ChR2 also stimulate photoreceptors, applying optogenetic techniques to the visual system has been limited. By combining an optogenetic approach with a mutation that impairs phototransduction, we have demonstrated that activation of a specific cluster of neurons in the fly’s optic lobe can drive the escape behavior used to avoid collision6.
Most, if not all, visual animals exhibit an escape behavior to avoid collisions with oncoming objects. Walking or stationary flies, when presented with a looming collision, take-off into flight, away from the oncoming collision7-9. These take-offs are characterized by raised wings prior to take-off and an unstable flight trajectory10,11. This response is distinct from the giant-fiber mediated startle response, jumps that are not preceded by raised wings, and usually result in a free-falling tumble4,9 . Having identified a specific cluster of loom sensitive neurons in the optic lobe, Foma-1 neurons, that are uniquely tuned to encode approaching objects, we sought to probe their involvement in the fly’s loom escape behavior. Here we demonstrate the use of optogenetics to selectively activate these neurons and elicit the fly’s escape behavior.
We use the Gal4-UAS transcriptional activator system to drive the expression of ChR2 in the Foma-1 neurons. ChR2 requires the cofactor all-trans-retinal and as this is found in low levels in the Drosophila central nervous system it must be supplemented in the flies’ diet.3,4 As bright light is used to activate ChR2 and flies exhibit strong phototactic behaviors12, we sought to eliminate the possibility of a visual response to the stimulus. To do this, we used animals that were homozygous mutant for a null allele of the norpA gene, which encodes a critical component of the phototransduction cascade, phospholipase C-β. Photoreceptors in such mutant flies are unable to respond to light13. To test the optogenetic stimulation of the escape response, we need to isolate a single fly and bathe it in bright blue light. To do this, we place individual flies in pipet tips. One pipet tip is placed in a custom holder, such that the fly will geotactically walk up the tip and out onto a rectangular platform. The fly is able to freely walk around on the top of this platform. The platform is surrounded by four blue LED arrays, each containing 3 LEDs, focused on the top of the platform. After the fly is on the platform, the LEDs are illuminated, and the fly’s response is recorded using a high-speed camera6.
Protocol
1. Generate Channelrhodopsin Flies
- Cross UAS-ChR2 flies with the Gal4 driver of your choosing, we use G105-Gal4, which is expressed in Foma-1 neurons in the optic lobe.
- To eliminate the possibility of a visual response to the blue light stimulation, both fly lines are in a w+norpA background.
- End result: w+norpA;G105-Gal4/UAS-ChR2 +
- After adult flies eclose, put selected females on fresh food, supplemented with 10 μM all-trans-retinal (a co-factor required for ChR2) and protected from light, for 3 days before performing the behavioral assay.
2. Make 10 μM All-trans-retinal Enhanced Food
- Dissolve 100 mg of all-trans-retinal in 17.6 ml of 95% ethanol to make 20 mM retinal. Keep all-trans-retinal protected from light at all times.
- Melt standard cornmeal fly food in microwave, and let cool until warm to touch.
- Mix 50 μl of 20 mM all-trans-retinal into vials of 10 ml of fly food.
- Let vials cool and keep protected from light.
3. Equipment
- Pipet tips: Standard 1,000 μl pipet tips are cut near the tip, creating a pore diameter of ~2.25 mm.
- Platform (see Figure 1).
- A Delrin base, 17 cm X 25 cm, was constructed with threaded holes at each corner to fit ¼” NPT coolant hose connectors.
- A vertical holder, made from Delrin, is attached to the center of the base. The overall dimensions are 25 mm X 40 mm X 65 mm (width X depth X height). A 10 mm wide groove runs the length of the holder, with a thumb screw at the bottom. A platform is attached to the top of the holder, 25 mm X 40 mm X 10 mm, with a 3.5 mm diameter hole aligned with the groove in the holder.
- LED arrays (see Figure 1).
- Four arms of coolant hose, ~18 cm long, are affixed to the platform base using the coolant hose connector. Coolant hose is used only as structural support and is not used for cooling purposes.
- Properly spaced grooves are cut into the final piece of coolant hosing of each arm to affix a heat sink to the end of each arm.
- A blue LED Rebel Tri-Stars is mounted to each heat sink using pre-cut thermal adhesive tape. A Carclo 18 ° Tri-lens is affixed to each Tri-Star.
- LED Tri-Stars are wired to the BuckPuck DC drivers and a power supply as specified. We have arranged our set-up with each BuckPuck powering two Tri-Stars in series.
- Illumination of all four LED Tri-Stars at 700 mA yielded an irradiance of 713 W/m2 on our platform.
- Camera: The camera is mounted on a small tripod and focused on the top of the platform.
4. Behavioral Assay
- Briefly anaesthetize flies on ice.
- Place individual flies in pipet tips, using tape to close both ends of the tip.
- After the flies have awoken and are actively exploring the pipet tip, remove the tape and place a pipet in the groove in vertical holder. The thumbscrew is used to secure the pipet tip in place and to close the bottom of the tip.
- As the fly explores the pipet tip (typically 30 – 60 sec), start the camera recording just before the fly emerges from the tip onto the platform.
- After the fly has emerged onto the platform, wait 1-2 seco, and turn on the blue LEDs. Use a timer to manually measure the time until the fly initiates flight.
Representative Results
Blind flies expressing either ChR2 or the G105 driver alone show a low rate of take-off following their illumination with bright blue light. Blind flies exhibited the same rate of take-off regardless of illumination (Figure 2), suggesting that these take-offs were spontaneous rather than due to the illumination with blue light. When the ChR2 is expressed in the Foma1 neurons, however, illumination with blue light elicits the escape response. Over 50% of the flies tested took off within 1 sec of illumination, and 75% within 5 sec (Figure 2). In contrast, only 10% of control flies took off within 1 sec, and 20% within 5 sec. As the G105 driver is expressed both in Foma-1 neurons and in the γ-lobe of the mushroom body, we used a driver specifically expressed in this part of the mushroom body (201Y-Gal4) as an additional control. These flies exhibited a rate of take-off similar to the other controls performed (Figure 2), indicating specifically that the optogenetic activation of Foma-1 neurons elicited the escape response.
High speed imaging taken at 200 frames per second of the optogenetically induced responses revealed that these responses were similar to the loom-evoked escape behavior (Figure 3). Namely, 90% of the flies filmed raised their wings prior to take-off (n = 30)6. Further, the ensuing flight trajectory was less stable than voluntary take-offs10, with the fly’s body being in a somewhat vertical orientation (Figure 3), but more stable than giant fiber mediated responses9.
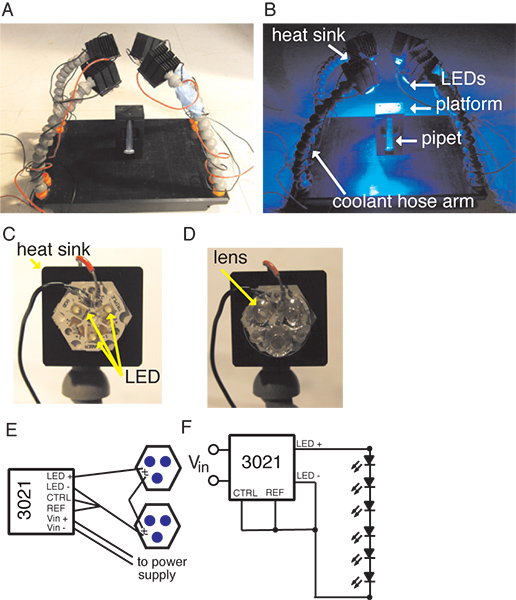
Figure 1. Experimental set-up showing the platform with the vertical holder and the four coolant hosing arms holding heat sinks with LED arrays affixed to them. A, the set-up under ambient illumination. B, the set-up when the LEDs are illuminated. C, a close up view of a Tri-Star LED on the heat sink. D, a close up of the Tri-Star with the tri-lens attached. E, a schematic of the Buckpuck Driver and LED circuit. F, circuit diagram for the BuckPuck and LED circuit.
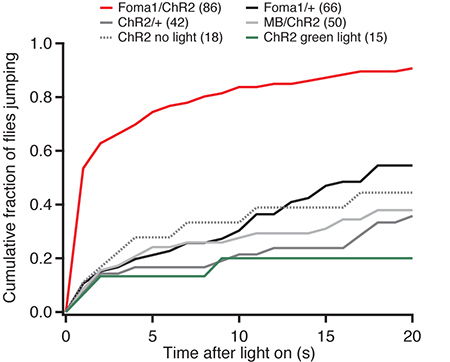
Figure 2. Cumulative histogram of time of escape jump for experimental and control fly lines. All flies express w+norpA to render them visually blind. “Foma-1” flies have the G105-Gal4 driver; “MB” flies have the 201Y-Gal4 driver that drives expression in the mushroom body.
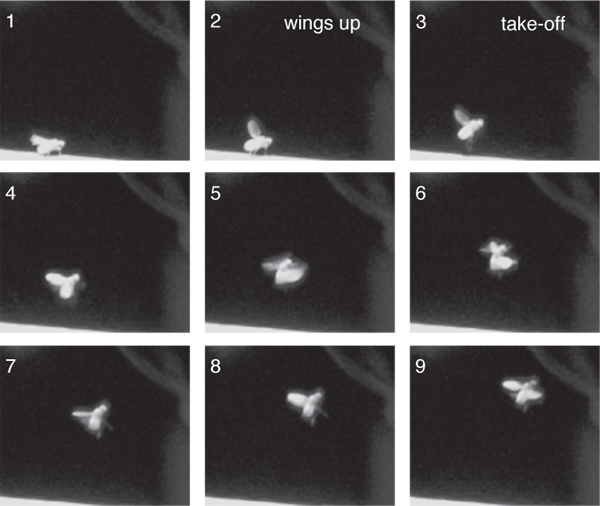
Figure 3. High speed video frames of ChR2 induced escape responses. Frames are numbered sequentially with 5 msec between frames.
Discussion
We have demonstrated optogenetic stimulation of escape behaviors by bathing freely walking flies in bright blue light. This approach can be easily adapted to examine other behaviors in freely walking flies, and can be scaled to larger platforms by simply tiling the LED arrays we used over a larger area. Using either the inexpensive camera we describe, or other available camera systems, the user can tailor the frame rate and spatial resolution of the images acquired to suit the behavior of interest. Additionally, our imaging is limited to the time after the LEDs are illuminated, as the blue LEDs provide the illumination for the camera as well as the activation of ChR2. This is sufficient for our behavior, but if the position or movement of the fly prior to LED illumination needs to be recorded, additional light sources providing signals in the infrared range, combined with appropriate filtering of the camera, could be incorporated.
Optogenetics has been widely hailed as a non-invasive way to manipulate neural activity. Yet, this technique has been largely unavailable to the visual system as the light used to activate ChR2 will also activate visual pathways. Additionally, the use of optogenetics to probe non-visual behaviors could also be hampered by the flies’ phototactic responses to bright lights. The use of norpA flies that are visually blind enables us to use optogenetic tools in the visual system, and prevents phototaxis from obscuring other behaviors.
This protocol requires that ChR2 be expressed in the neurons of choice, that the flies be fed all-trans-Retinal, and that the flies be bathed in bright blue light. The protocol we developed has met these requirements, yet has not been fully optimized. For instance, we believe the amount of light we use might be brighter than necessary, and that a higher concentration of all-trans-Retinal, or longer feeding time, could result in a higher rate of take-off. We have not systematically explored these parameters.
The Foma-1 neurons we activate are found in the lobula complex of the fly, and are thus situated close to the back surface of the brain. It is possible that the success of this experiment relies on the neurons being close to the surface, as the light must penetrate through the fly’s cuticle to activate ChR2. Thus, cells situated deeper in the brain may not be successfully activated using this method.
The G105 driver is expressed in a cluster of 5 neurons on each side of the optic lobe, the Foma-1 neurons, as well as in neurons in the γ-lobe of the mushroom body. Fortunately, we had a driver to do control experiments to eliminate a role of the mushroom body neurons in the escape behavior. However, whether the activation of all 10 Foma-1 neurons are required for this behavior, or whether there are only one or two specific cells are sufficient, cannot be determined at this time. As more specific drivers are developed, and intersectional strategies for limiting expression are refined1,14, we expect to be able to target neurons with greater specificity.
Cryptochromes are photoreceptors involved in circadian rhythms and behaviors that are sensitive to blue light. In this protocol, cryptochromes are likely activated by the blue light used to stimulate ChR2. This does not appear to influence the take-off behavior observed here, as the low rate of take-off observed in control flies (for which the blue light activates the cryptochromes but not ChR2 in Foma-1 neurons) closely matches the rate of take-off observed in control flies without illumination or when illuminated with green light (which does not activate cryptochromes). However, for other behaviors that are more directly influenced by cryptochromes, this might prove problematic. One potential improvement to avoid this could be the use of a red-shifted channelrhodopsin that is activated by yellow, 589 nm, light15.
In our experiments, we observed a low level of spontaneous take-offs such that ~10% of control flies took off within 1 sec of LED illumination, and 13-28% within 5 sec. As we observed this same rate of take-off when the flies were illuminated with green light that did not effectively activate ChR2, as well as flies without any illumination, we believe that these were spontaneous flights rather than light induced take-offs. The effect of ChR2 activation of Foma-1 neurons on escape behavior must therefore be measured on top of this spontaneous activity. For other behaviors that do not involve take-off, however, this may result in terminated trials if flies escape from the platform prior to execution of the tested behavior. The extent to which these spontaneous escapes represent an experimental inconvenience is obviously dependent on timescale of the behavior of interest.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by a Stanford Dean’s Fellowship (SEJdV), a National Institutes of Health Director’s Pioneer Award (TRC DP0035350), a McKnight Foundation Scholar’s Award (TRC) and R01 EY022638 (TRC).
Materials
| Reagent | |||
| All-trans Retinal | Advance Scientific & Chemical Inc | R3041 | |
| Equipment | |||
| Heat Sink 9.2 C/W | Luxeonstar | LPD30-30B | 30 mm square X 30 mm high |
| Carclo 18 ° Tri-Lens | Luxeonstar | 10507 | |
| Blue Rebel LED on Tri-Star Base | Luxeonstar | MR-B0030-20T | 470 nm, 174 lm @ 700 mA. |
| 700 mA BuckPuck DC Driver | Luxeonstar | 3021-D-E-700 | |
| Wiring Harness for BuckPuck Driver | Luxeonstar | 3021-HE | |
| Pre-cut thermal adhesive tape | Luxeonstar | LXT-S-12 | 20 mm Hex Base |
| Snap-Loc Coolant Hose, ¼” ID | McMaster-Carr | 5307K49 | |
| Snap-Loc Coolant Hose Connector | McMaster-Carr | 5307K39 | ¼” NPT Male |
| Laboratory Grade Switching Mode Programmable DC Power Supply | BK Precision | 1698 | |
| Exilim camera | Casio | EX-FH20 |
References
- Venken, K., Simpson, J., Bellen, H. Genetic manipulations of genes and cells in the nervous system of the fruit fly. Neuron. 72, 202-230 (2011).
- Gordon, M., Scott, K. Motor control in a Drosophila taste circuit. Neuron. 61, 373-384 (2009).
- Suh, G. S. B., et al. Light activation of an innate olfactory avoidance response in Drosophila. Current Biology. 17, 905-908 (2007).
- Zhang, W., Ge, W., Wang, Z. A toolbox for light control of Drosophila behaviors through Channelrhodopsin 2-mediated photoactivation of targeted neurons. European Journal of Neuroscience. 26, 2405-2416 (2007).
- Nagel, G., et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proceedings of the National Academy of Science. 100, 13940-13945 (2003).
- de Vries, S., Clandinin, T. Loom-sensitive neurons link computation to action in the Drosophila visual system. Current Biology. 22, 353-362 (2012).
- Card, G. Escape behaviors in insects. Current Opinion in Neurobiology. 22, 1-7 (2012).
- Card, G., Dickinson, M. H. Visually mediated motor planning in the escape response of Drosophila. Current Biology. 18, 1300-1307 (2008).
- Fotowat, H., Fayyazuddin, A., Bellen, H. J., Gabbiani, F. A novel neuronal pathway for visually guided escape in Drosophila melanogaster. Journal of Neurophysiology. 102, 875-885 (2009).
- Card, G., Dickinson, M. H. Performance trade-offs in the flight initiation of Drosophila melanogaster. The Journal of Experimental Biology. 211, 341-353 (2008).
- Hammond, S., O’Shea, M. Escape flight initiation in the fly. Journal of Comparative Phsyiology A. 193, 471-476 (2007).
- Benzer, S. Behavioral mutants of Drosophila isolated by countercurrent distribution. PNAS. 58, 1112-1119 (1967).
- Bloomquist, B., et al. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 54, 723-733 (1988).
- Gohl, D., et al. A versatile in vivo system for directed dissection of gene expression patterns. Nature Methods. 8, 231-237 (2011).
- Zhang, F., et al. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox cateri. Nature Neuroscience. 11, 631-633 (2008).

