Summary
This manuscript describes a method to quantify tumor cell accumulation in the lungs in an animal model of tumor metastasis.
Abstract
To investigate the molecular mechanisms governing tumor metastasis, various assays using the mouse as a model animal have been proposed. Here, we demonstrate a simple assay to evaluate tumor cell extravasation or micrometastasis. In this assay, tumor cells were injected through the tail vein, and after a short period, the lungs were dissected and digested to count the accumulated labeled tumor cells. This assay skips the initial step of primary tumor invasion into the blood vessel and facilitates the study of events in the distant organ where tumor metastasis occurs. The number of cells injected into the blood vessel can be optimized to observe a limited number of metastases. It has been reported that stromal cells in the distant organ contribute to metastasis. Thus, this assay could be a useful tool to explore potential therapeutic drugs or devices for prevention of tumor metastasis.
Introduction
Tumor metastasis accounts for high mortality in cancer-related diseases. From the viewpoint of investigations at the molecular level, metastasis can be divided into multiple steps: tumor initiation at the primary tumor site, primary tumor growth and invasion into surrounding tissues, intravasation, circulation through blood vessels, extravasation at distant organs, and tumor re-growth. Each step involves different sets of molecules/signals1.
Studies to date have been concerned with events occurring in distant organs before the tumor cells start circulating in the blood, which is also known as the pre-metastatic phase2,3,4,5,6. Our mouse model studies revealed that the pre-metastatic phase facilitates lung metastasis by creating long-lasting and low-level inflammatory responses in the lungs. The pre-metastatic phase is characterized by hyperpermeability of themicrovasculature, recruitment of bone marrow derived cells (BMDC), and upregulation of cytokine/chemokine-like molecules including S100A8 and SAA33,4. It has been reported that lysyl oxidase modifies the extracellular matrix in the lungs to recruit BMDCs7. Another report has revealed the importance of Angpt2, MMP3, and MMP10 in the pre-metastatic phase8. In other words, the intricate interactions between primary tumors, bone marrow, and remote organs need to be understood and properly modulated (for instance, by using drugs that target proteins involved in these interactions) to prevent future metastasis9. To regulate the pre-metastatic phase, it should first be visualized and evaluated for diagnostic or therapeutic interventions. Here, we report a lung tumor cell recruitment assay that enables the study of the pre-metastatic phase in the lungs.
Tumor cells directly injected into the blood vessel of the mouse are similar to circulating tumor cells in cancer patients, although there may be differences between cultured cells and circulating tumor cells. Circulating tumor cells themselves undergo some characteristic changes when they enter circulation from the primary tumor. Nevertheless, cultured cells can form tumors in immunodeficient mice when they are injected in the tail vein. Additionally, in the mouse model system, fluorescence labeled tumor cells can be used that allow tracking of these cells in the body. The behavior of circulating tumor cells is critical as they determine the ultimate consequences in cancer patients10. Blood is not a good place for survival of tumor cells2. They need to escape from attack by the host immune system and need anti-apoptotic signals to prevent apoptosis due to a lack of physical support. Tumor cells with higher abilities of tumor initiation at metastatic sites generate more tumor nodules. If the tumor cells show specific organ tropism, metastasis should be observed in those particular organs. In this assay, the initial steps of the metastatic cascade (primary tumor growth and invasion) are not included, and so the focus is on the interaction between circulating tumor cells and the stromal cells (endothelial cells, epithelial cells, and blood cells). In conventional tumor cell injection assays, researchers have to wait until tumor nodules grow to a tangible size to detect the metastasis. In this case, the exact number of tumor cells in the blood vessel cannot be quantified. Additionally, this process includes a tumor regrowth phase, indicating that other factors in the tumor cells may affect the outcome.
Counting labeled tumor cells under a fluorescence stereomicroscope is a simple process. The stereomicroscope provides a wide field of view so that the whole lung can be scanned. Flow cytometry is also a useful tool that instantly gives an accurate number of tumor cells in the tissue. Counting fluorescent cells in the tissue under a confocal microscope is also possible and displays the most accurate pictures of metastatic tumor cells, but it is time-consuming and may give biased results if only a selected area is counted. The ultimate goal of this assay is to observe how easily tumor cells accumulate in the lungs. As reported previously3,4, if the lungs are in pre-metastatic phase due to inflammatory signaling from the primary tumor, the injected tumor cells are prone to accumulate in the lungs, thus implying higher chances of lung metastasis. Other conditions (rheumatoid arthritis, asthma, etc.), and their treatment with anti-inflammatory agents (such as anti-TNFα) may attenuate lung metastasis. Thus, we conclude that this assay is a powerful tool to assess the possibility of lung metastasis.
Protocol
All procedures performed with mice were approved by the Animal Research Committee of Tokyo Women's Medical University.
1.Tail Vein Injection of Lewis Lung Carcinoma (LLC) Cells
- Maintain LLC cells in DMEM supplemented with 10% FCS, in a humidified 5% CO2 incubator at 37 °C. Cells should contain a fluorescent protein expression system (e.g., GFP).
- Remove cells from culture dish by using a non-enzymatic cell dissociation reagent. Follow manufacturer's protocol.
- Collect cells in a 15 mL tube, and centrifuge at 400 x g for 3 min. Resuspend the cells in 5 mL of PBS by pipetting, and centrifuge the tube (400 x g, 3 min). Then, remove the supernatant.
- Repeat this washing step one more time.
- After the second wash, pass the cells through a cell strainer (40 µm pore size), and resuspend in PBS to give a final cell density of 1 x 106 cells/mL.
- Inject 0.1 mL of cell suspension (1 x 105 cells, 29G needle) into each C57BL/6 mouse (8 – 10 week old males) via the tail vein.
2. Isolation of the Lungs and Counting the Cells Under a Fluorescence Stereomicroscope
- Euthanize the mice by CO2 inhalation.
- Remove the skin on the ventral surface of the chest with scissors. Then, cut the ribs and the diaphragm to expose the thoracic cavity. Flush PBS (100 cmH2O, total 15 mL) through the right ventricle with a winged infusion 25G needle and tubing.
- Cut out the heart and the thymus. Grip the trachea with forceps, and pull up, dissecting the connective tissues around trachea with scissors. Dissect out the lungs and wash them in PBS.
- Detach each lobe and observe it under a fluorescence stereomicroscope.
3. Lung Digestion
- Dissolve 10 mg of collagenase, 10 mg of dispase, and 10 µg of DNase in 10 mL of serum-free DMEM. Filter through a 0.22 µm syringe filter to prepare the enzymatic digestion solution.
- Dice the isolated caudal lobe with scissors. Place the lung pieces in a 10 mL syringe, followed by the plunger.
- Aspirate the digestion solution (5 mL) into the syringe by pulling back on the plunger. Operate the plunger up and down until the lung sinks into the solution. Then, place the lung and digestion buffer in a 50 mL tube.
- Shake the tube for 15 min at 37 °C on a reciprocal shaker (150 rpm). Lungs may become tattered during this incubation.
- Pipette up and down until lung cells are completely dispersed. Shake the tube for additional 30 min at 37 °C on a reciprocal shaker (150 rpm).
- Pass the lung cells through a 40 µm cell strainer. Spin down the cells at 400 x g for 3 min.
4. Flow Cytometry of Lung Cells
- Dissolve 100 mg of BSA in 10 mL of PBS and filter through a 0.22 µm syringe filter to prepare PBS-1% BSA.
- Remove supernatant of the sample obtained at step 3.6 using a pipette and add 2 mL of red blood cell lysis solution. Then, spin down the cells at 400 x g for 3 min. Discard the supernatant.
- Resuspend the cells in 5 mL of PBS-1% BSA by pipetting, and centrifuge the tube (400 x g, 3 min). Discard the supernatant.
- Repeat this washing step one more time.
- Suspend cells in 1 mL of PBS-1% BSA and pass through a new 40 µm cell strainer.
- Analyze the cells with a flow cytometer to count tumor cells (using filter setup FL1 for GFP-LLC).
Representative Results
The lungs present many GFP-LLC cells 2 h after injection (Figure 1A). It should be noted that the fluorescent spots also detected in the red filter should be excluded from the cell number count. The vast majority of LLC cells disappear from the lungs 24 h after injection (Figure 1C).
To confirm the number of the GFP-LLC in the lungs, one of the lobes can be used for flow cytometric analysis (Figure 2).
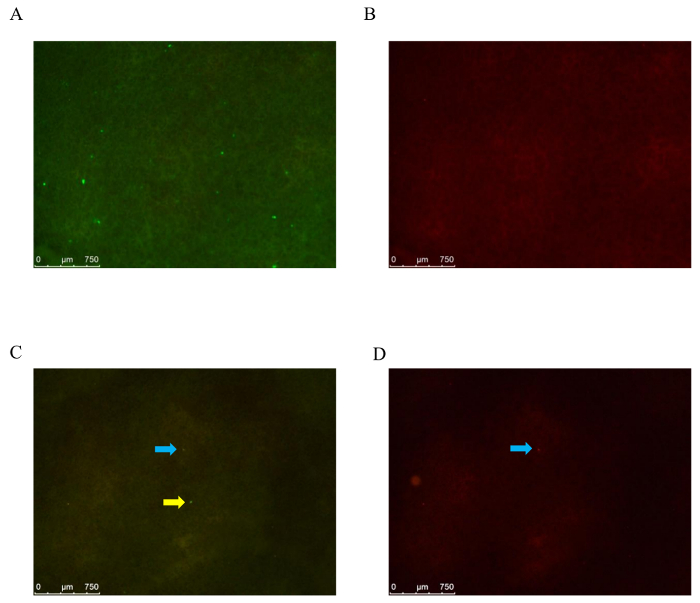
Figure 1: Detection of GFP-LLC cells under a fluorescence stereo microscope. (A) Lungs are isolated 2 h (A: filter set GFPLP, B: filter set ET/CY3) or 24 h (C: filter set GFPLP, D: filter set ET/CY3) after injection of 3 x 104 GFP-LLC cells. In (C), a GFP-LLC cell is marked by a yellow arrow. The spots marked by blue arrows are not GFP-LLC cells. Scale bar indicates 750 µm. Please click here to view a larger version of this figure.
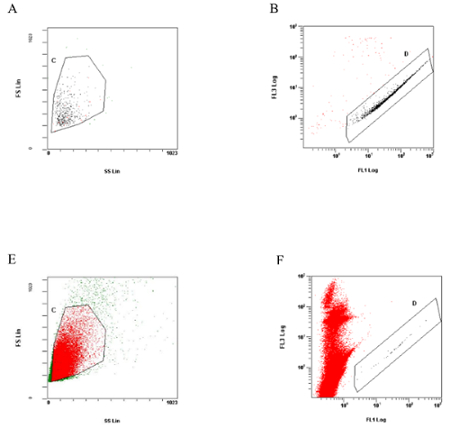
Figure 2: Flow cytometry analysis of lung cells. (A) and (B); Flow cytometric analysis of GFP-LLC cells used for tail vein injection. To exclude dead cells the cell suspension buffer contained propidium iodide (1 µg/mL). (A) shows FSC vs. SSC plot, and cells in the region of (C) are developed in (B) to show FL1 vs. FL3 plot. Cells in the region (D) are considered to be GFP-LLC cells. (E) and (F); Dot plots of caudal lobe cells isolated 24 h after the tail vein injection. The GFP-LLC cells are observed in region (D). Please click here to view a larger version of this figure.
Discussion
Pre-treatment of mice with antibodies, drugs, a high-fat diet or tumor-conditioned medium prior to tumor cell injection is possible. The most difficult step in this assay is the tail vein injection. Incomplete injection results in inconclusive data. The tail vein in C57BL/6 is particularly difficult to identify, resulting in failed injections. Placing the mice on a heating pad (37 °C) helps dilate the tail vein so that injection becomes easier.
This assay uses a large number of (1 x 104 – 1 x 105) tumor cells, which is much larger than that observed in patient blood. A one-time injection of such a large number of the cells may bring about unwanted responses from the host animals. In the fluorescence stereo microscopy counting, tumor cells deep inside the tissue are hard to detect. In flow cytometry analysis, lungs are completely digested to count the tumor cells in the lungs. This process causes loss of valuable information about tumor cell location (inside or outside of the blood vessel). On the contrary, detection of tumor cells under a confocal fluorescence microscope allows the distinguishing tumor of cells in the blood vessel from the others10.
Fluorescence stereo microscopy is a very simple method. Using flow cytometry to count the tumor cells has no bias compared with microscope scanning. It is possible to scan entire lung sections under a confocal fluorescence microscope; however, the assay will then be laborious. Other types of tumor cells or stromal cells may be mixed with the injected tumor cells. Co-injection of cancer-associated cells enhances the possibility of metastasis. The tumor metastasis rate heavily depends on the type of the tumor cell and the animal used.
As mentioned above, the most critical step in this protocol is the tail vein injection. An accurate number of cells needs to be injected with no leakage, but is technically difficult. Carefully observing the handling by skilled researchers may be of great help.
A series of mouse model studies have reported that 20% of the injected tumor cells underwent extravasation, 3% of them formed micrometastases, and only 0.02% developed into tangible metastatic nodules2. In case of GFP-LLC cell injection (3 x 104 cells), 150-300 GFP-LLC cells were detected by flow cytometry analysis in the caudal lobe isolated 2 h after injection. The total number of GFP-LLC in the lungs is assumed to be 600-1,200, indicating that 2-4% of the injected cells remained alive. This ratio decreases in a time-dependent manner3,11. The other cells are assumed to undergo apoptosis due to lack of physical support or nutrition, or due to exclusion by the immune system. In our recent data, 24 h after the injection of GFP-LLC (6 x 104 cells), the number of GFP-LLC in the lungs was found to be about 20. These results may be affected by the mouse strain, tumor cell, and other factors. For instance, it has been reported that the number of the tumor cells in lungs was elevated in the pre-metastatic phase12, or in gene modified mice13,14.
Disclosures
The authors have nothing to disclose.
Acknowledgements
None
Materials
| Collagenase | Sigma | C6885 | |
| Dispase | Gibco | 17105-041 | |
| Bovine serum albumin | Sigma | A7030 | |
| DPBS | Gibco | 14190-144 | no calcium, no magnesium |
| Deoxyribonuclease I | Sigma | DN-25 | trace amount |
| RBC lysis buffer | Sigma | R7757 | |
| Cell strainer | BD | 352340 | 40 micrometer mesh |
| Fluorescence labeling kit | Sigma | MIN26-KIT | |
| DMEM | Gibco | 11965-092 | |
| 0.22 μm syringe filter | sartorius | 17597K | |
| O.C.T. compound | Sakura Finetek | 4583 | |
| 4% Paraformaldehyde Phosphate Buffer Solution | Wako | 163-20145 |
References
- Valastyan, S., Weinberg, R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell. 147 (2), 275-292 (2011).
- Kaplan, R. N., et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 438 (7069), 820-827 (2005).
- Hiratsuka, S., Watanabe, A., Aburatani, H., Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature Cell Biology. 8 (12), 1369-1375 (2006).
- Hiratsuka, S., et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nature Cell Biology. 10 (11), 1349-1355 (2008).
- Tomita, T., Sakurai, Y., Ishibashi, S., Maru, Y. Imbalance of Clara cell-mediated homeostatic inflammation is involved in lung metastasis. Oncogene. 30 (31), 3429-3439 (2011).
- Deguchi, A., et al. Serum amyloid A3 binds MD-2 to activate p38 and NF-κB pathways in a MyD88-dependent manner. Journal of Immunology. 191 (4), 1856-1864 (2013).
- Erler, J. T., et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 15, 35-44 (2009).
- Huang, Y., et al. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Research. 69 (19), 7292-7237 (2009).
- Sceneay, J., Smyth, M. J., Möller, A. The pre-metastatic niche: finding common ground. Cancer Metastasis Reviews. 32 (3-4), 449-464 (2013).
- Castle, J., Shaker, H., Morris, K., Tugwood, J. D., Kirwan, C. C. The significance of circulating tumor cells in breast cancer: A review. The Breast. 23, 552-560 (2014).
- Wolf, M. J., et al. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell. 22 (1), 91-105 (2012).
- Hiratsuka, S., et al. Primary tumours modulate innate immune signalling to create pre-metastatic vascular hyperpermeability foci. Nature Communications. 4, 1853 (2013).
- Deguchi, A., et al. Eritoran inhibits S100A8-mediated TLR4/MD-2 activation and tumor growth by changing the immune microenvironment. Oncogene. 35 (19), 1445-1456 (2016).
- Ieguchi, K., et al. ADAM12-cleaved ephrin-A1 contributes to lung metastasis. Oncogene. 33 (17), 2179-2190 (2014).

