An Experimental Model to Study Tuberculosis-Malaria Coinfection upon Natural Transmission of Mycobacterium tuberculosis and Plasmodium berghei
Summary
Tuberculosis and malaria are two of the most prevalent infections in humans and major causes of morbidity and mortality in impoverished populations in the tropics. We established an experimental model system to study outcome of malaria-tuberculosis coinfection in mice after challenge with both pathogens via their natural route of infection.
Abstract
Coinfections naturally occur due to the geographic overlap of distinct types of pathogenic organisms. Concurrent infections most likely modulate the respective immune response to each single pathogen and may thereby affect pathogenesis and disease outcome. Coinfected patients may also respond differentially to anti-infective interventions. Coinfection between tuberculosis as caused by mycobacteria and the malaria parasite Plasmodium, both of which are coendemic in many parts of sub-Saharan Africa, has not been studied in detail. In order to approach the challenging but scientifically and clinically highly relevant question how malaria-tuberculosis coinfection modulate host immunity and the course of each disease, we established an experimental mouse model that allows us to dissect the elicited immune responses to both pathogens in the coinfected host. Of note, in order to most precisely mimic naturally acquired human infections, we perform experimental infections of mice with both pathogens by their natural routes of infection, i.e. aerosol and mosquito bite, respectively.
Introduction
Human populations are rarely exposed to one pathogen only. Particularly in regions with high incidence of infections such as sub-Saharan Africa, coinfections represent a prime but highly underappreciated public health problem. Tuberculosis and malaria are the most prevalent bacterial and parasitic infections in humans, respectively and continue to be major causes of morbidity and mortality in impoverished populations in the tropics. Despite the wide geographic overlap between tuberculosis and malaria and the large number of individuals at risk of coinfection, very little is known about the interactions between the various and often counteracting immune regulators and effectors simultaneously elicited against malaria parasites and tubercle bacilli in coinfected individuals.
Rodent models of mixed infections allow characterizing differential immune reactions against distinct pathogens in one host, thus shedding light on the mutual influences modulating pathology and clinical outcome of the individual disease, which will also help to identify new recommendations for therapy and prevention. We have established an experimental mouse model which enables us to investigate the ramifications caused by concurrent infection with Mycobacterium tuberculosis (Mtb) and rodent Plasmodium species within the same host1. Importantly, to emulate naturally acquired human infections as closely as possible, our model implements the natural routes of infection used by both pathogens. Tuberculosis is an airborne infection, which therefore primarily manifests in the lungs. The causative agent, Mtb is transmitted by people with active disease via the aerosol route when they expel infectious aerosol droplets during coughing or sneezing. Thus, aerosol infection is the method of choice to mimic natural infection. Generation of airborne particles that contain Mtb can be achieved by the use of an inhalation exposure system. This whole body exposure chamber allows exposing experimental animals to Mtb-containing infectious aerosol droplets (Figure 1) which are inhaled into the alveoli of the lungs where infection is initiated2.
Malaria on the other hand is a vector-borne disease caused by the protozoan, apicomplexan parasite Plasmodium that is naturally transmitted by the bite of a female Anopheline mosquito. During the blood meal, infectious sporozoites are deposited under the host skin and subsequently reach the liver via the blood stream. Within hepatocytes they develop and rapidly multiply into liver-stage schizonts. Eventually, mature schizonts rupture and release thousands of pathogenic first-generation merozoites into the blood stream where they initiate a progressive cycle of red cell invasion, replication, red cell rupture, and reinvasion3. The Plasmodium life cycle continues as some merozoites develop into the sexual parasite stages, the male and female gametocytes, which can be taken up by mosquitoes during blood meals. In the mosquito, Plasmodium sporozoites develop within oocysts residing in the mosquito midgut and eventually migrate to the salivary glands for onward transmission into another host3,4.
While in experimental animal models the delivery of Mtb via the aerosol and thus most relevant route is very common, many experimental rodent Plasmodium infection studies including studies on malaria-tuberculosis coinfection5-7 have been carried out by infecting mice with parasitized erythrocytes, giving rise to blood-stage malaria infection while excluding the clinically-silent liver-stage phase. However, the liver stage is an obligatory step during infection and relevant for anti-plasmodial immunity8-11. We consider it therefore important to include the hepatic phase of malaria transmission and maintenance in studies of both malaria and malaria-tuberculosis coinfection, respectively. In addition, it has been shown that naturally transmitted sporozoites are more infectious than those transmitted via needle infections12, which prompted us to establish the malaria infection by mosquito bite instead of injecting isolated salivary gland derived sporozoites. The only way to obtain infectious mosquitoes for natural transmission of malaria parasites is to maintain the whole life cycle of the parasite in both the vertebrate host (here mouse) and the mosquito vector. Thus, access to an insectary for parasite maintenance is inevitable in order to perform natural transmission by bite.
Our protocol described herein was developed to investigate how coinfection with Plasmodium sporozoites impacts on chronic tuberculosis1. To do so, mice are aerosol infected with M. tuberculosis and 40 days later, when M. tuberculosis infection has reached the chronic phase, mice are exposed to malaria-infectious mosquitoes. The outcome of both malaria and tuberculosis in the coinfected animals can be followed by monitoring blood parasitemia and bacterial load in tissue, respectively. In our protocol, we will describe in detail how to infect mice with both pathogens via their natural infection route and how to confirm successful pathogen transmission. In our hands, the commonly achieved infection rate for both experimental infections is 100%. These protocols can be applied to study both infections separately or, as we do, to model and study coinfection between two of the most challenging human infectious diseases. This model is applicable to other Mycobacterium and rodent Plasmodium species beyond the ones described in this protocol.
Protocol
Safety Measures and Ethics Statement
The studies presented herein involve work with the human pathogen Mtb and the rodent malaria parasite Plasmodium berghei (P. berghei). Experiments with Mtb (strain H37Rv) and P. berghei must be carried out under the appropriate biosafety conditions. Biosafety level (BSL) 2 laboratories are required for rodent malaria parasites such as P. berghei and BSL 3 for Mtb and hence, for all coinfection studies described herein. NOTE: Mice infected with Mtb do not spread the infection to other mice within immediate vicinity (our own observation). Spread to the experimenter can therefore be excluded; however appropriate protective clothing has to be worn inside the BSL 3 laboratory at all times. According to our rules, experimenters wear surgical scrubs, hair nets, disposable respirators, and two pairs of gloves, of which the outer one is discarded anytime arms are withdrawn from the cabinet. New ones are put on before entering the cabinet again. Two containers, one with 2% Buraton (NOTE: other disinfectants approved to inactivate Mtb can be used at the approved concentration) for liquid and infectious waste material and one for surface decontamination, are prepared before the experiment and placed inside the cabinet. In addition, plastic bags will be used for solid noninfectious waste. According to German rules, all work involving the handling and processing of Mtb cultures or tissue derived from Mtb infected mice must be carried out in a class 2 biosafety cabinet within a BSL3 lab. While handling mycobacteria, measures have to be taken to avoid the generation of aerosols at all times.
Female C57BL/6 mice (Charles River) aged between 6-8 weeks were used for all coinfection experiments and maintained under specific barrier conditions in BSL 3 facilities. Animal care and experimentation were performed in accordance with protocols approved by the Ethics Committee for Animal Experiments of the Ministry for Agriculture, Environment, and Rural Areas of the State of Schleswig-Holstein
For obtaining malarial mosquito stages, NMRI mice were purchased from Charles River Laboratory, Sulzfeld, Germany and kept under specific pathogen-free conditions within the University Heidelberg animal facility (IBF). All animal experiments were performed according to European regulations and approved by the state authorities of Baden-Württemberg (Regierungspräsidium Karlsruhe).
1. Aerosol Infection of Mice with Mtb using a Glas-Col Aerosol Chamber
The best way to standardize aerosol infection of experimental animals with Mtb is to use mycobacteria from frozen stocks with known CFU titers. To prepare stock cultures, culture Mtb in Middlebrook 7H9 broth supplemented with OADC (Oleic acid, Albumin, Dextrose, Catalase) enrichment medium and 0.05% Tween 80, a carbon source for mycobacteria and measure to avoid bacterial clumping at the same time. Cultures should have an OD ≤ 1 at the time of harvest. At higher OD, bacterial clumping increases and viability decreases. Store 1 ml aliquots at -80 °C. Determine the number of viable colony forming units (CFU) in frozen stocks by plating a series of 10-fold dilutions of three independent vials on 7H11 agar plates supplemented with 0.5% glycerol, 1 g/L asparagine, and OADC and enumerate colonies after 4 weeks of incubation at 37 °C. Perform aerosol infection as described below.
- Thaw Mtb stocks of known CFU titer and carefully mix the suspension five times to disperse bacterial clumps using a 1 ml syringe fitted with a 27 G needle. Avoid the production of aerosols.
- Depending on the desired infection dose, transfer the required volume of the mycobacterial stock into a 50 ml tube containing sterile PBS. The final volume is 6 ml. NOTE: By varying the number of microorganism in this suspension, the proportion of bacteria bearing aerosol droplets is varied. We usually aim at an uptake of 100 viable bacilli per lung (low-dose infection). In our experience, this requires around 1-2 x 106 Mtb/ml in a total volume of 6 ml of which 5.5 ml are nebulized (see below). It is recommended to do a series of experimental aerosol challenges with different concentrations of bacteria to find the ideal conditions for your infection. Whereas high dose infections with up to 5,000 mycobacteria can be done to speed up the infectious process, as few as 5 bacteria can be used to successfully infect mice by aerosol. The infection rate is commonly 100%.
- Remove sufficient volume (prepared in excess of final volume required) for plating to determine the inoculum titer; we usually remove 500 µl to plate technical triplicates.
- Place animals in a compartmented mesh basket (one mouse per basket) within the circular aerosol chamber and close the lid of the aerosol chamber. NOTE: Other models are equipped with a pie-shaped basket composed of five individual compartments, each of which can accommodate 20 mice.
- Attach the Venturi-nebulizer unit to the three stainless steel socket joints.
- Remove the mycobacterial suspension from the 50 ml tube with a 10 ml syringe fitted with an 18 G blunt needle and carry the syringe to the aerosol chamber in a closed transport box.
- Remove the screw cap of the nebulizer unit and carefully inject the mycobacteria suspension into the nebulizer. Avoid the generation of aerosols. Discard the syringe into a sharps container containing 2% Buraton. Seal the nebulizer with the screw cap.
- Switch on the main power switch and the UV lamp. The display on the control keypad shows “Glas-Col Apparatus Co”.
- Turn the program switch on. The display will show “Is the nebulizer ready?” “Is the basket loaded?” Press enter when ready”. Press enter.
- The display shows “Enter Preheat Time 900”; this means the preheat time for the incinerator (which decontaminates the exhaust air) is 900 sec. Press enter.
- The display will show “Enter Nebulizing time 1,800”; this means the nebulizing time is set to 1,800 sec as default. In order to extend the nebulizing time, enter “2,400” and press enter. NOTE: During the nebulizing cycle, air under pressure atomizes the suspension, thereby generating small aerosol droplets containing mycobacteria. With the main air flow, these droplets (approximately 2-5 µm in size) are carried into the aerosol chamber.
- The display will show “Enter C.D. Time 1,800”; this means that the decay cycle takes 1,800 sec. Set to “2,400” and press enter. NOTE: During this cycle, the cloud which has been built up in the aerosol chamber during the nebulizing cycle can decay. The small droplets are inhaled by the experimental animals.
- The display will show “Enter Dec Time 900”; this means that the UV light decontamination cycle will take 900 sec. Press enter. The machine will start cycling through preheat, nebulizing, cloud decay and UV decontamination.
CAUTION: The vacuum flow meter should indicate 60 cubic feet/hr (check when preheat cycle starts; adjust vacuum control valve if necessary) and the compressed air flow meter 10 cubic feet/hr (check when nebulizing cycle starts; adjust air control valve accordingly). - When the cycle is complete, the keypad will show “Process Complete – Remove Specimen”. Turn off the program, UV and main power switch.
- Check if the mycobacterial suspension has been nebulized completely and if not, record the remaining volume by carefully removing the suspension with an appropriate syringe fitted with an 18 G blunt needle. The remaining volume should not be more than 1 ml.
- Remove the nebulizer from the joints and place it in a pan containing 2% Buraton for a minimum of 2 hr, usually disinfection is done O/N. Afterwards, transfer the nebulizer to a fresh pan and rinse thoroughly with water. Leave nebulizer to air-dry.
- Open the aerosol chamber and return animals to their cages. Bag the baskets in autoclave bags and autoclave. Wipe clean the surfaces of the inside of the aerosol chamber with 2% Buraton. NOTE: For safety reasons, a powered air purifying respirator should be worn during this procedure.
- Using the remaining 500 µl of the mycobacterial suspension (see step 1.3) plate 10-fold dilutions of a technical triplicate (3 x 100 µl) on 7H11 agar plates.
2. Verify Uptake of Desired CFU in Lungs
One day after aerosol infection of experimental animals determine the bacterial load in the lungs of designated control animals in order to verify the uptake of the desired CFU. NOTE: We usually designate an extra group of 3-5 mice for day 1 CFU determination.
To monitor the course of Mtb infection over time, the mycobacterial tissue burden can be examined by plating serial dilutions of whole organ homogenates for CFU determination. The lung is the primary site for disease manifestation in tuberculosis however spleen, liver and lymph nodes are usually analyzed accordingly. The dilutions to be plated depend on the expected mycobacterial load in the organs, which depend on the initial inoculum (low dose vs. high dose), which gives rise to different bacterial loads in tissue, as well as on the organ and time point to be analyzed. Upon low dose infection, the bacterial load of Mtb H37Rv in the lung usually reaches a plateau between day 25-30.
- Place euthanized animals (Euthanasia: CO2 asphyxiation or terminal anesthesia) on absorbent paper on a dissecting board in a class II biosafety cabinet and disinfect mice with 70% ethanol. NOTE: 70% alcohol is for surface decontamination only and will not kill Mtb.
- Make a small incision in the middle of the abdomen and retract the mouse skin above the head.
- Open the abdomen and the thoracic cage with surgical scissors and remove the thoracic wall so that the lungs are accessible.
- Remove the lungs and transfer into a 15 ml tube with homogenization buffer (sterile water/1% v/v Tween 80/1% w/v albumin).
- Using the plunger of a 5 ml syringe strain lungs through 100 µm sieves into a small Petri dish.
- Flush sieves several times using a 1 ml pipette equipped with a barrier tip and distribute the lung homogenate among 8 agar plates (ca. 250 µl/plate; make sure they have a well dried surface).
- Carefully plate the samples using disposable spreaders which are discarded into 2% Buraton.
- Leave agar plates to dry inside the cabinet. Seal every single plate with parafilm, wrap in aluminum foil and incubate upright at 37 °C for at least 4 weeks.
3. Construction of Escape-proof Mosquito Cages
Rodent Plasmodium strains are not harmful to humans. However, since Mtb-infected mice are the recipients of the parasite and work is done under BSL 3 conditions, precautions are necessary to prevent the mosquitoes from escaping their cages. Depending on the planned infection regimen, autoclavable and reusable metal-frame cages or self-made disposable cages can be used. The latter ones are recommended if by bite infection of experimental animals is required to be carried out by a defined number of mosquitoes per mouse. If self-made cages are required, prepare cages to exact specifications as the health of the mosquitoes and the safety of the laboratory depends upon their sound construction (Figure 2).
- Take a carton such as a half-pint ice cream carton or paper coffee cup (Figure 2).
- Cut a small opening into the side of the carton and cover with a double layer of latex with a slit cut in each piece to create a secure entrance for the mosquitoes. NOTE: The opening has an overall size of 2 cm x 2 cm, the tubing (aspirator device) that we use to bring in and/or take-out mosquitoes is a modified 15 ml Falcon tube attached to a vacuum pump and hence serves as an aspirator with a diameter of 1.5 cm.
- Tape a piece of filter paper on the inside bottom of the carton to soak up drips.
- Close off ice cream cups with netting (double layer of Nylon mesh, size 1 mm x 1 mm) and fix to the carton by tape and elastic band.
- For infection experiments where defined numbers of mosquitoes per mouse are required, prepare cages with 10-15 infected mosquitoes, each of which ideally contains an average of 10,000 wild-type salivary gland P. berghei sporozoites.
- Ideally, starve mosquitoes for 24 hr prior to performing the blood meal.
4. Malaria Infection of Mice by Mosquito Bite
The rodent malaria parasite used herein is P. berghei however any other rodent Plasmodium strain of interest can be used. To obtain infectious mosquitoes for sporozoite transmission the whole life cycle of the parasite is maintained in both the vertebrate host (mouse) and the mosquito vector. Parasite maintenance is carried out in an insectary. For natural transmission experiments, the minimum number of sporozoites per salivary gland should be no less than 10,000. For detailed protocols on parasite maintenance we refer to Methods in Malaria Research by Moll et al., 2013.
- Anaesthetize naïve mice or animals preinfected for 40 days with Mtb with Ketamine (100 mg/kg) and Xylazine (7 mg/kg) solution by intraperitoneal injection (200 µl/mouse). NOTE: The time between Mtb and Plasmodium infection can be adjusted depending on the underlying research question. Likewise, the order of pathogen challenges can be reversed, i.e. mice can be exposed to infectious mosquito bite prior to Mtb infection.
- Place anaesthetized mice onto the netting of the mosquito cages (one mouse per mosquito cage for defined mosquito to mouse ratio) and allow the mosquitoes to feed through the membrane for 10-15 min. NOTE: The presence of blood in the mosquito guts implies feeding and thus, sporozoite transmission.
- Transfer mice back into their cages and supervise constantly until they wake up from anesthesia. NOTE: Place mice on paper towel and not on bedding (risk of suffocation) and keep warm (place closely together and cover bodies with paper towel).
- Kill the mosquitoes by spraying them with 70% ethanol or other disinfectants through the netting of the cage. Autoclave cages and discard if disposable ones were used.
5. Monitoring Parasitemia
- Puncture tail veins at the very end with a needle and collect one drop of blood at each end of a slide.
- Use another slide to pull one drop of blood over half of the slide's length. Flip over the spreader to use the other edge for the second smear. Leave slides to air dry.
- Giemsa stain
- Place slides in slide holders and fix the blood smears by brief immersion in absolute methanol. NOTE: Because the use of glass ware should be kept at minimum in the BSL 3 we use polypropylene cuvettes for all solutions.
- Immerse the smears in Giemsa (1:10 in deionized water). After 10 min, dip the slides in and out of staining cuvettes filled with deionized water a few times to rinse away the excess stain.
- Finally, air dry slides in a vertical position.
- ALTERNATIVE STAIN: Wright´s stain
- Dissolve Wright´s stain at a concentration of 1 mg/ml in methanol.
- Filter through folded filter prior to use.
- Immerse the blood smears in staining solution and incubate for 4 min (NOTE: methanol fixation of smears is not necessary as the staining solution is methanol based).
- Wash by rinsing slides in deionized water as described above and leave slides to air dry.
- Once Giemsa/Wright´s stain is complete, gently transfer all reagents used into a Buraton-containing disposal pot.
- Blood smear analysis
- Analyze stained blood smears by light microscopy at a 100-fold magnification with oil immersion.
- Count uninfected and infected erythrocytes in an area of the blood smear where erythrocytes are arranged in a monolayer.
- In order to achieve a well-defined parasitemia, count at least 10 different fields of view displaying an erythrocyte monolayer.
- Calculate the relative parasitemia (in %) by dividing the number of parasitized erythrocytes by the number of erythrocytes and multiplying by 100.
Representative Results
Infection of mice with Mtb and P. berghei via the natural route, i.e. aerosol and mosquito bite, respectively, results in consistent and reproducible infection of all animals (Table 1)1. We can monitor successful infection and the course of both malaria and tuberculosis by determining the initial occurrence (prepatency) and number of parasites in the blood (P. berghei parasitemia) and the burden of Mtb in different organs, respectively. An example of successful malaria parasite transmission by infectious mosquito bite is shown in Table 1 and Figure 3. Feeding of 10 sporozoite-infected mosquitoes on each mouse resulted in a 100% infection rate as confirmed by the presence of blood-stage parasites 4-5 days later (Table 1 and Figure 3A). Importantly, we find similar parasite numbers in the blood of individual mice of the same experimental group, indicating that sporozoite transmission by mosquito bite results in a consistent and reproducible infection. By comparing the prepatency in naïve control mice with that in Mtb-coinfected animals, we can see that prepatency is slightly delayed in mice that had been preinfected with Mtb (Table 1, Figure 3B)1. As we have reported previously1, we can determine the impact of Mtb coinfection on the course of malaria by daily monitoring parasitemia in Giemsa-stained blood smears as shown in Figure 3B.
Infection of mice with Mtb by the use of an inhalation exposure system results in successful deposition of bacteria in the lungs with relatively low variability between individual mice (Figure 4A). We can follow mycobacterial loads in lungs and other organs of interest over time by CFU determination. An example for the mycobacterial load in lungs of Mtb infected C57BL/6 mice in the absence or presence of P. berghei is shown in Figure 4B.
Table 1. Prepatency after sporozoite transmission by mosquito bite. All mice developed blood-stage infections after natural transmission of P. berghei sporozoites by mosquito bite. Adapted from1 PLoS ONE, 7(10), e48110 with permission.
| Experimental mouse group (C57BL/6) | Challenge by 10 infectious mosquito bites | No. of blood-stage positive animals / No. of animals per group | Mean prepatency [d] |
| naïve | P. berghei | 7/7 | 4,4 |
| Mtb infected | P. berghei | 7/7 | 5,5 |
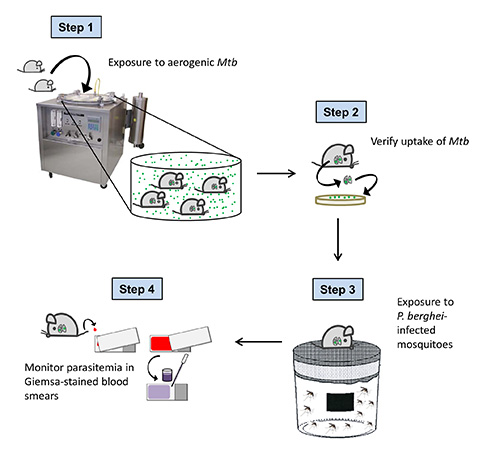
Figure 1. Overall scheme of the experimental procedure. C57BL/6 mice are exposed to Mtb-containing aerosol droplets in a Glas-Col aerosol chamber. One day after aerosol infection, the uptake of Mtb is verified by CFU analysis of the lung. 40 days after Mtb challenge (when tuberculosis has become chronic in infected mice), animals are exposed to P. berghei infected mosquitoes. To confirm successful sporozoite transmission and to follow the course of Plasmodium infection, parasitemia is monitored daily in Giemsa-stained blood smears starting 3 days after mosquito bite.
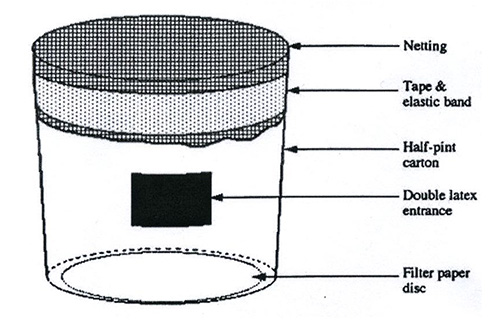
Figure 2. Sketch of a mosquito cage. Infectious mosquitoes are contained inside a carton such as a half-pint ice cream carton. A small opening is cut into the side of the carton and covered with a double layer of latex with a slit cut in each piece to create a secure entrance for the mosquitoes. A piece of filter paper is taped on the inside bottom of the carton to soak up drips. Ice cream cups are closed off with netting (Nylon mesh) which is fixed to the carton by tape and elastic band.
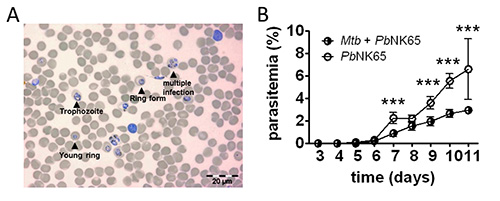
Figure 3. Blood parasitemia. A) Example of a Wright`s stained blood smear showing parasitized erythrocytes (Scale bar, 20 µm). B) Parasitemia in mouse blood was monitored by analyzing daily Giemsa-stained blood smears. Coinfected mice have a reduced parasitemia compared to Plasmodium single infected mice (n=10); results are shown as means ± SD; ***p<0,001. Reprinted from 1 PLoS ONE, 7(10), e48110 with permission.
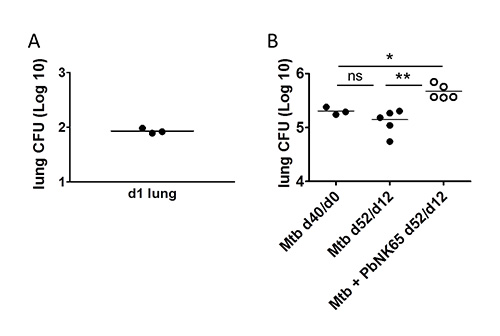
Figure 4. Detection of Mtb in the lung. C57BL/6 mice were aerosol challenged with 100 CFU of Mtb and 40 days later, coinfected with P. berghei by mosquito bite. A) One day after aerosol infection the uptake of the desired number of Mtb was verified by plating whole lung homogenates for CFU determination. B) Bacterial loads at the time of coinfection were determined in lung lysates of 3 mice via CFU analysis (Mtb d40/d0). 12 days after coinfection, lung lysates were plated for CFU analysis to determine the impact of Plasmodium coinfection on Mtb control. Note, that Mtb numbers in lungs increased during coinfection with P. berghei NK65. X-axis labeling: time after Mtb-infection/time after coinfection. Adapted from1 PLoS ONE, 7(10), e48110 with permission.
Discussion
We described how mice can be productively infected with Mtb and P. berghei via their natural routes of infection. We apply established infection protocols to study experimental tuberculosis13 or malaria12 and recently adopted them to study coinfection between Mtb and Plasmodium in the mouse model1.
The most critical steps for successful infections are the transmission of both pathogens at the desired numbers. Mycobacterial stocks should not be older than 2 years because they will lose viability over time, and the originally determined titer of the stocks will most likely no longer be accurate. As a result, infection doses would be much lower than expected. Therefore, CFU titers of stock cultures should be determined every few months. Equally important is the standardized cultivation and generation of Mtb stocks in the first place. Culture conditions such as medium, supplements, time, and volume should be standardized in order to be able to compare data from experiments using different stocks of Mtb. Mycobacteria tend to clump during culture, therefore it is important to keep culture conditions standardized, harvest bacteria at the same growth phase and resuspend frequently during aliquoting. Otherwise there can be a wide variation in CFU titers and infection outcome.
The generation of homogeneously infected mosquito batches is another critical step. Since different mosquitoes will feed on each individual mouse, it is important that all of the mosquitoes used for one infection experiment come from the same batch and that only those batches where more than 90% of mosquitoes are infected are used.
The current model can be used to dissect immune responses and immune modulations in a coinfected host by comparing it to the immune responses in single infected hosts. We can apply various well established methodologies such as histology, immunohistochemistry, cytometry analysis of immune cell populations, or PCR and ELISA for cytokine and chemokine detection to the tissue or body fluids of interest. It should be noted, that such mouse models have certain limitations. Whereas the majority of people infected with Mtb develop a latent infection with no signs of clinical symptoms, mice develop a chronic disease with progressive lung pathology. Still, C57BL/6 mice are relatively resistant to Mtb infection and do not develop classical granulomas as observed in human tuberculosis patients. As a result, immunopathological reactions in the mouse do reflect neither latent nor active human tuberculosis disease appropriately. Despite the discrepancy between latent and chronic disease in man and mouse, mice have been extensively used to identify and study host factors that control Mtb infection and are valuable and indispensable tools to investigate immune responses in infectious diseases. Importantly, susceptibility to Mtb infection varies considerably between commonly used inbred mouse strains and more susceptible strains such as DBA or C3H can be included in co- and single-infection studies. Moreover, apart from the pathogen species described herein, any other Mycobacterium species of interest (e.g. clinical isolates) could be used. Likewise, while no single malaria model replicates all aspects of human disease different mouse models do closely resemble different aspects of naturally acquired human malaria infection. For instance, P. berghei ANKA and P. yoelii YM/17XL are widely studied models of human (P. falciparum-induced) severe malaria, with P. berghei ANKA being regarded as an excellent model for human cerebral malaris14,15. P. berghei NK65 is an excellent model for hyperparasitaemia and acute malarial anemia, and has recently been described as an experimental model for malaria-associated acute respiratory distress syndrome (MA-ARDS16). Using the described coinfection model, we could recently show that concurrent P. berghei NK65 infection exacerbates chronic tuberculosis1.
Apart from using different rodent malaria parasites, the order and timing of infection events may be adapted depending on the underlying research question. In the field, concomitant Plasmodium infections may be present at the time of Mtb infection or acquired subsequently. In both scenarios the immune responses to Mtb and Plasmodium may be affected differently. Therefore, mice can be infected with Plasmodium sporozoites either before or after Mtb infection. Furthermore, mice can be challenged at different times post Mtb infection to assess the impact of the Plasmodium-induced immune response on acute versus chronic tuberculosis. Importantly, when choosing a malaria parasite for studying malaria-tuberculosis coinfection, one should be aware that some rodent Plasmodium strains cause acute disease in certain mouse strains and succumb to infection within only days or weeks while Mtb causes chronic and long-lasting infections in immunocompetent mice. Hence, timing of coinfection is crucial in order to avoid premature death of experimental animals.
The modulation of host immunity by simultaneous infection with multiple pathogen species remains poorly understood. Our experimental coinfection model provides a powerful tool to investigate Mycobacterium–Plasmodium interaction with the immune system of a coinfected host and will contribute to our understanding how coinfections can affect pathogenesis, disease outcome, vaccination, immunodiagnostics, and therapy.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Miriam Ester for mosquito breeding. This work was supported by in-house funding from the Research Center Borstel and joined funding by the Leibniz Center Infection.
Materials
| Buraton | Schülke | active ingredients: aldehyds (formaldehyde, glutaraldehyde, oxalaldehyde, ethyl hexanal) | |
| Middlebrook 7H9 | Sigma | M0178 | For Mtb broth cultures |
| Middlebrook 7H11 | BD Biosciences | 283810 | Agar medium for Mtb culture |
| Middlebrook OADC enrichment medium | BD Biosciences | 212240 | Add to 7H9 and 7H11 for Mtb culture |
| Staining Dish | Science Services | E62542-12 | |
| 24-Slide Holder w/Handle | Science Services | E62543-06 | |
| Giemsas Azur-Eosin-Methylene blue solution | Merck Millipore | 109204 | |
| Wright´s stain | Sigma | W0625 | |
| Inhalation Exposure System | Glas-Col | ||
| Nebulizer-Venturi | Glas-Col | ||
| Ice cream cups | Häagen-Dazs | Used as mosquito cages | |
| Metal-frame mosquito cages | BioQuip Products | 1450A |
References
- Mueller, A. -. K., et al. Natural Transmission of Plasmodium berghei Exacerbates Chronic Tuberculosis in an Experimental Co-Infection Model. PLoS ONE. 7, e48110 (2012).
- Korbel, D. S., Schneider, B. E., Schaible, U. E. Innate immunity in tuberculosis: myths and truth. Microbes Infect. 10, 995-1004 (2008).
- Aly, A. S., Vaughan, A. M., Kappe, S. H. Malaria parasite development in the mosquito and infection of the mammalian host. Annu. Rev. Microbiol. 63, 195-221 (2009).
- Cirimotich, C. M., Dong, Y., Garver, L. S., Sim, S., Dimopoulos, G. Mosquito immune defenses against Plasmodium infection. Dev. Compar. Immuno. 34, 387-395 (2010).
- Page, K. R., et al. Mycobacterium-induced potentiation of type 1 immune responses and protection against malaria are host specific. Infect. Immun. 73, 8369-8380 (2005).
- Hawkes, M., et al. Malaria exacerbates experimental mycobacterial infection in vitro and in vivo. Microbes Infect. 12, 864-874 (2010).
- Scott, C. P., Kumar, N., Bishai, W. R., Manabe, Y. C. Short report: modulation of Mycobacterium tuberculosis infection by Plasmodium in the murine model. Am. J. Trop. Med. Hyg. 70, 144-148 (2004).
- Hoffman, S. L., Doolan, D. L. Malaria vaccines-targeting infected hepatocytes. Nat. Med. 6, 1218-1219 (2000).
- Mueller, A. K., et al. Genetically attenuated Plasmodium berghei liver stages persist and elicit sterile protection primarily via CD8 T cells. Am. J. Pathol. 171, 107-115 (2007).
- Mueller, A. K., Labaied, M., Kappe, S. H., Matuschewski, K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 433, 164-167 (2005).
- Nussenzweig, R. S., Vanderberg, J., Most, H., Orton, C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 216, 160-162 (1967).
- Vaughan, J. A., Scheller, L. F., Wirtz, R. A., Azad, A. F. Infectivity of Plasmodium berghei sporozoites delivered by intravenous inoculation versus mosquito bite: implications for sporozoite vaccine trials. Infect. Immun. 67, 4285-4289 (1999).
- Coleman, J., Juhn, J., James, A. A. Dissection of midgut and salivary glands from Ae. aegypti mosquitoes. J. Vis. Exp. , e228 (2007).
- Franke-Fayard, B., et al. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol. Biochem. Parasitol. 137, 23-33 (2004).
- Lin, J. W., et al. Loss-of-function analyses defines vital and redundant functions of the Plasmodium rhomboid protease family. Mol. Microbiol. 88, 318-338 (2013).
- Bancroft, J. D. G., M, . Theory and Practice of Histological Techniques. , (2007).
- Schneider, B. E., et al. A role for IL-18 in protective immunity against Mycobacterium tuberculosis. Eur. J. Immunol. 40, 396-405 (2010).
- Craig, A. G., et al. The role of animal models for research on severe malaria. PLoS Pathog. 8, e1002401 (2012).
- de Souza, J. B., Hafalla, J. C., Riley, E. M., Couper, K. N. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology. 137, 755-772 (2010).
- Van den Steen, P. E., et al. Immunopathology and dexamethasone therapy in a new model for malaria-associated acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 181, 957-968 (2010).

