Quantification of Drosophila Grooming Behavior
Summary
This protocol describes a scalable individual grooming assay technique in Drosophila that yields robust, quantitative data to measure grooming behavior. The method is based on comparing the difference in dye accumulation on the bodies of un-groomed versus groomed animals over a set period of time.
Abstract
Drosophila grooming behavior is a complex multi-step locomotor program that requires coordinated movement of both forelegs and hindlegs. Here we present a grooming assay protocol and novel chamber design that is cost-efficient and scalable for either small or large-scale studies of Drosophila grooming. Flies are dusted all over their body with Brilliant Yellow dye and given time to remove the dye from their bodies within the chamber. Flies are then deposited in a set volume of ethanol to solubilize the dye. The relative spectral absorbance of dye-ethanol samples for groomed versus ungroomed animals are measured and recorded. The protocol yields quantitative data of dye accumulation for individual flies, which can be easily averaged and compared across samples. This allows experimental designs to easily evaluate grooming ability for mutant animal studies or circuit manipulations. This efficient procedure is both versatile and scalable. We show work-flow of the protocol and comparative data between WT animals and mutant animals for the Drosophila type I Dopamine Receptor (DopR).
Introduction
Grooming in Drosophila melanogaster (D. melanogaster ) is a robust innate behavior that involves the coordination of multiple independent motor programs1. Fruit flies clean their bodies of dust, microbes, and other pathogens which could inhibit normal physiological function such as vision and flight, or lead to significant immune challenges. In sensing and responding to both mechanical2 and immune activation3, flies repetitively rub their legs together or on a targeted body region until it is sufficiently clean and grooming progresses to another part of the body. Flies perform grooming movements in distinct bouts that largely occur in stereotyped patterns1,4. A behavioral hierarchy becomes apparent as grooming signals are prioritized. Circuits and patterns of activity have been identified in support of a model that grooming programs at the top of the hierarchy occur first and suppress parallel signals from areas of the body that are groomed subsequently5. Highest priority is given to the head, then the abdomen, wings, and finally the thorax5.
The grooming program in D. melanogaster is an ideal system for studying neural circuits, modulatory molecular signals, and neurotransmitters. For instance, compromise of neurofibromin function6, loss of Drosophila fragile X mental retardation protein (dfmr1)7, and exposure to bisphenol A (BPA)8 all cause excessive grooming and other behaviors that are analogous to discrete human symptoms of neurofibromatosis, fragile X syndrome, and aspects of autism spectrum disorders and Attention-deficit Hyperactivity Disorder (ADHD), respectively. Grooming behavior can also be habituated differentially across mutant strains2, lending this motor program to studies of behavioral plasticity. The breadth of neurological phenomena that can be modeled by Drosophila demands a novel comparative approach to measure the ability of flies to groom themselves.
The combined action of vesicular monoamine transporters and the relative abundance of dopamine and other biogenic amines in the body have been shown to mediate fruit fly grooming behavior 9,10. Octopamine and dopamine stimulate comparable hindleg grooming activity in decapitated flies, while tyramine, the precursor of octopamine, also triggers grooming to a lesser extent7. Four dopamine receptors have been identified in D. melanogaster11,12,13,14. By using the grooming assay method described in this protocol, we determined a role for the Type I family Dopamine Receptor DopR (DopR, dDA1, dumb) in hindleg grooming behavior15.
Grooming can be indirectly quantified by looking at the extent of cleanliness by which an animal can fully groom after dusting the entire body with a marker dye or fluorescent dust5,16. The remainder of dust left on the body can be used as a relative marker for the overall behavior. Dusty flies after being given sufficient time to groom may be manifesting a specific deficit in grooming behavior. As grooming investigations have become more extensive, protocols have incorporated such practices as decapitation to add pharmacological treatments onto the neck connective nerves10, tactile stimulation of bristles to elicit the grooming response2 , and video recording of behavior15. Direct observation of grooming can be easily studied by using visual observation and manually recording the frequency and duration of specific grooming events4.
We designed a fifteen-well grooming chamber that can be constructed with a 3D printer or laser cutter, and the blueprint designs are available for reproduction15. The design uses two joined central plates with openings matched and separated by mesh and two additional sliding top and bottom plates, from which flies and/or dye is loaded, respectively. After allowing dusted flies time to groom, we deposit them in ethanol to solubilize the dye and measure the absorbance of this solution at the wavelength of the dye. A plate reader can be used for multiple parallel samples or a single-read spectrophotometer can be used for individual samples. This method minimizes the error induced by handling and allows for grooming assays to be run on a smaller, cost-efficient scale. This method is derived and modified from the methods pioneered by Julie Simpson and Andrew Seeds, who use larger grooming chambers with heating elements for temperature sensitive circuit manipulations5. The following protocol showcases the quantification of grooming of the whole body as well as showing alternate methods for quantitation of dye accumulation on individual body parts. We also present sample comparison data between WT and DopR mutants, as well as methods for calculating a simple performance index for grooming behavior.
Protocol
1. Preparation
- Prepare an aspirator for moving live Drosophila from a culture vial to the grooming chamber. Aspirators allow transfer of conscious animals to the behavioral chambers to ensure that anesthesia does not affect subsequent behavioral observation.
- Using scissors cut 1.5 feet of tygon tubing ID ⅛", OD ¼".Cut at least 1 inch off of the tip of a 1 mL disposable micropipette tip. Hold a 1 cm square piece of mesh (opening 0.196 inch) over the cut tip.
NOTE: Most 1mL tips have a gradation line where the tip sits in the package box, typically cut to that line. - Snugly place a fresh 1 mL micropipette tip over the mesh/cut tip. Observe the mesh form a tight barrier between the two tips. The inside of the new tip creates a holding chamber for flies.
- Cut the extreme tip of the new micropipette tip to widen the opening enough to allow passage of a single fly. The hole will roughly match the opening of the grooming chamber (approximately 1.5-2 mm in diameter).
- Snugly fit the nested tips onto the end of the tygon tubing. Push the tips into the tube to ensure a tight fit to allow for vacuum pressure.
- On the other end of the tubing, cut the tip of a 200 μL micropipette tip and fit the narrow cut end into the tubing. This is the "mouth" side of the aspirator.
- Using scissors cut 1.5 feet of tygon tubing ID ⅛", OD ¼".Cut at least 1 inch off of the tip of a 1 mL disposable micropipette tip. Hold a 1 cm square piece of mesh (opening 0.196 inch) over the cut tip.
- Prepare dust aliquot tubes. Weigh approximately 5 mg of Brilliant Yellow dye on tared weighing paper. Pour the dust into a 0.6 mL microcentrifuge tube and tightly close the cap.
NOTE: We advise the use of nitrile gloves during aliquot preparation, as some latex gloves are permeable to the dye. - Repeat step 1.2) for all samples in the experiment (one for every fly in each chamber).
- Prepare ethanol (EtOH) tubes: Pipet 1 mL of 100% EtOH into 1.5 mL microcentrifuge tubes. Label tubes for genotypes or conditions.
- Repeat step 1.4) for all samples in the experiment (one for every fly in each chamber). Cap and save the EtOH tubes for completion of the grooming assay. Also include at least one "Blank" sample which will only be 1 mL EtOH without a fly for negative controls.
- Assemble each grooming chamber by screwing the sliding top plate (with the smaller holes) but leaving the bottom plate (with the larger holes) removed for addition of dust.
- Place each necessary grooming chamber flat on a table with the top face down. Fly entry side is face-down.
- Load 5 mg Brilliant Yellow dye aliquot into each chamber by tapping the tube against the surface of the chamber above the desired well. Tap the chamber against the table to ensure that all of the dust falls through the mesh to the top plate.
- Screw the bottom plate onto each chamber such that the wider ends of the conical openings face outward and the holes do not rest over the wells. Secure the bottom plate tightly so that it does not slide and release dye.
- Flip the chamber over and knock it flat against a table a few times so that the dust falls through the mesh to rest on the bottom plate.
- Slide the top plate of the chamber into the "open position" so that the small holes align with the fifteen wells, allowing the introduction of flies into individual wells.
2. Fly-dusting and Grooming
- Load one fly into the mouth aspirator by gently sucking through one end of the aspirator as if using a straw. Deposit the flies by lightly blowing and directing the opening toward the target.
- Aspirate one fly into each well used for the experiment. After loading a well, slide the top plate so that the fly is trapped in the chamber and place tape over the opening of that well during loading of the whole chamber to prevent the fly's escape while loading other wells. If multiple flies are aspirated into a single well, leave that opening uncovered until only one fly remains.
- After all of the necessary wells are filled with flies, flip the chamber such that the bottom plate is upward so that the dust has the potential to coat the flies by falling through the mesh.
- Tightly secure the top and bottom plates by tightening the screws, and vortex the chamber to dust the flies for 4 s.
- Knock the chamber (top plate facedown) against a table twice so that dye falls through to the other side. Then, flip the chamber over (top plate face-up) and knock the chamber twice to ensure dye settles on the floor of the bottom chamber away from the fly held in the top chamber.
- For control flies which are not allowed to groom, immediately proceed to Step 3 of the protocol. For flies that will complete the assay, proceed to Step 2.7.
- Rest the chamber flat on a table or countertop with the top plate upward for thirty minutes to allow the flies to groom. Place the chamber in a noise and vibration-free space to keep your environment constant for all grooming experiments (lighting levels, humidity, and temperature). A tabletop incubator at RT or 25 °C with humidity controls is ideal.
3. Preparation of Samples and Absorbance Analysis
- Keeping the chamber level with the top plate upward, gently unscrew the bottom plate and remove it from the chamber, taking care not to lose dye.
- Place the chamber on a Fly CO2 pad with low airflow until the flies are anaesthetized.
- Unscrew the top plate from the chamber. Carefully use forceps to grab a leg and move one dusted fly from each chamber and deposit it in a 1.5 mL microcentrifuge tube containing EtOH. The transfer time for 15 chambers each containing one fly is approximately 3-5 min. Experimenters should factor in transfer times if/when staggering experiments with multiple chambers to ensure efficient staging of experiments.
- Once each microcentrifuge tube contains 1 fly, close the cap and invert the tube three times to agitate and mix the solution of dye and ethanol.
- Incubate tubes containing ethanol and flies for 5 h at RT to ensure full removal of the dye from the fly into solution. After the 5 h, briefly vortex each tube (2 s) to ensure clearance of the dye from the flies.
- Aliquot 50 µL of the experimental 1.5 mL tube into a well of a 96-well plate if available, taking note of the location of each sample on the plate. Add 200 µL of EtOH to dilute the sample 5x. The dilution avoids ceiling effects of heavily dusted flies or large grooming defects.
- Use a plate reader to analyze each sample at 397 nm. Alternatively, measure absorbance for individual samples and blanks on a standard spectrophotometer in the visible spectrum if a plate reader is unavailable.
- Read and record the sample through the plate reader and save the spreadsheet with the recorded samples on the plate.
4. Quantification of Results
- Compile results for all samples and measure the variance for each genotype or condition.
NOTE: Dye accumulation at time 0 min and at time 30 min are regarded as separate conditions using statistical analysis by one-way ANOVA and Bonferroni correction or other corrections for multiple parallel comparisons. - Calculate grooming percentage difference for each genotype/condition using the following equation: [(dye accumulation mean value at time 0' – dye accumulation mean value at time 30') / dye accumulation mean value at time 0] x 100.
- Express the percentage for each genotype and condition in bar plots and compare across genotypes and conditions.
Representative Results
The grooming assay yields quantitative data to assess behavioral performance based on the relative remainder of accumulated dye left on the bodies of flies after a set time of measurement for grooming (30 min). Sample images of the sliding grooming chamber design and major steps of the assay are highlighted in Figure 1. Flies aggregate a significant amount of dye from immediate dusting by vortexing in the presence of dye (Figure 2d, 2e). Dusted flies can retain a range of dye accumulation post-assay (Figure 2f, 2g). Therefore, solubilizing dye accumulation in EtOH and measuring absorbance of individual samples provides a reproducible and highly quantitative assessment of grooming ability.
In this study of DopR in regulation of hindleg grooming, we compared the grooming performance of a strong hypomorph (Figure 3a, 3b) and null DopR mutant to a WT strain (Figure 3c, 3d). The DopR mutants retained significantly more dye than their WT or rescued counterparts, indicating that the DopR mutants were less efficient at grooming behavior (Figure 3a, 3c). To refine the understanding of the role of DopR, we performed a parallel experiment with rutabaga flies, carrying a strong hypomorphic mutation of calcium-dependent adenylate cyclase, which functions downstream of G-Protein Coupled Receptors in Drosophila. These mutants displayed higher dye accumulation levels than DopR mutants (Figure 3e, 3f). The data suggest a significant role for DopR in grooming behavior and a possible contribution by other GPCRs working in parallel or in different locomotor programs for grooming behaviors.
To investigate the differences between grooming abilities of foreleg vs hindleg grooming programs, we directly assessed dye accumulation on separable body parts during grooming (Figure 4). By dissecting the heads, wings, or "body" (abdomen/thorax/legs) of individual flies post-assay, we were able to reliably quantitate differences and similarities between genotypes. The findings supported an interpretation that no differences were present for foreleg grooming programs, as no significant differences were observed for heads of WT Vs. DopR mutants. However, significant differences were observed for both wing and body measurements between genotypes. This supplemental technique to perform the primary assay on body parts instead of whole flies allows a simple method to initially distinguish foreleg versus hindleg grooming programs. These results were further extended and supported by careful behavioral observations and video recording or tracking of individual components of the foreleg or hindleg behaviors. All results from Figures 2-4 are reproduced with permission from Genes, Brain, and Behavior15.
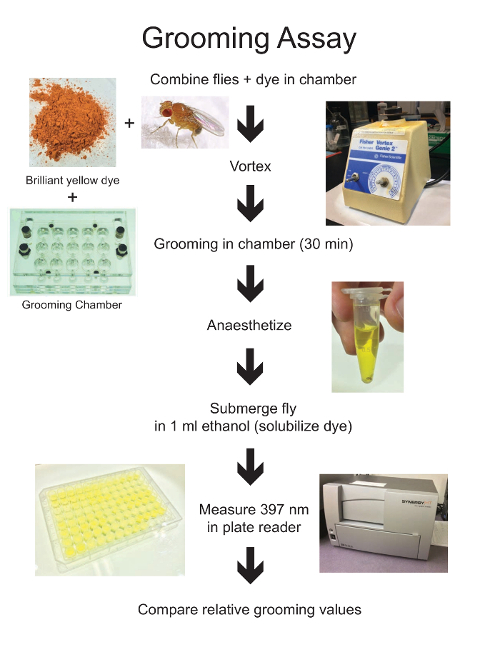
Figure 1: Drosophila Grooming Assay Workflow. This simplified flowchart outlines the major steps in the grooming protocol, highlighting some of the necessary materials and equipment. Please click here to view a larger version of this figure.
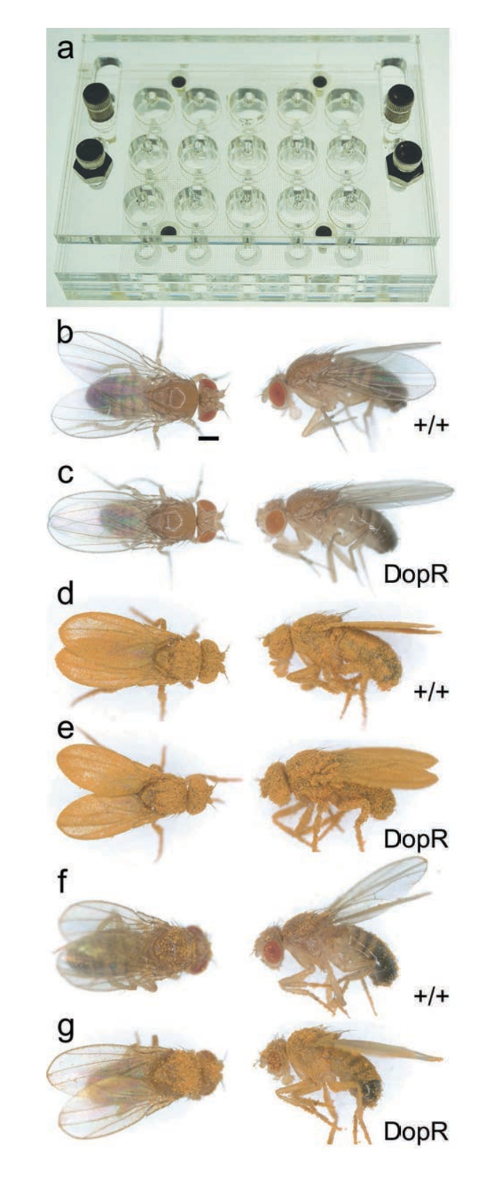
Figure 2: Quantification of Grooming based-on Dye Accumulation. (a) Grooming chamber. Individual flies and Brilliant Yellow dye are placed in individual wells (1 fly : 1 well). Dimensions and blueprints for production in supplemental data. (b, d, f) WT adult male Drosophila (+/+). (c, e, g) DopRf02676/DopRf02676 adult male Drosophila. (b, c) Flies before dusting. (d, e) Flies immediately after dusting by vortexing chamber, prior to grooming (time: 0 min). (f, g) Flies after grooming (time: 30 min). Scale bar = 400 µm. Reprinted with permission from Genes, Brain, and Behavior15. Please click here to view a larger version of this figure.
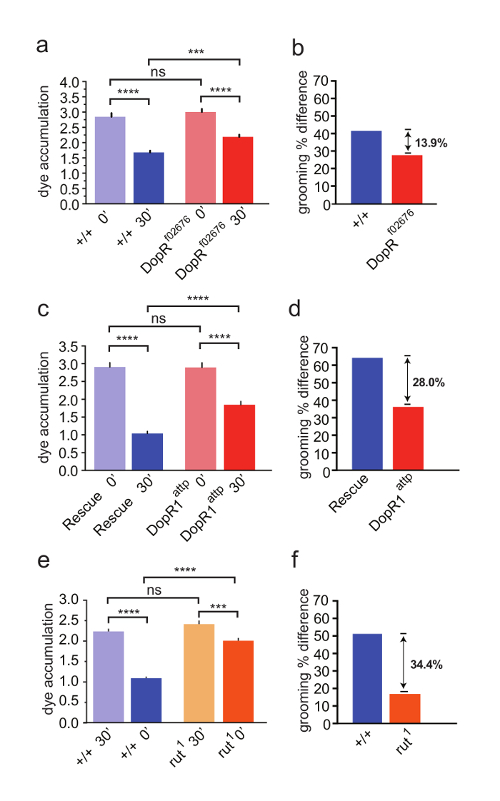
Figure 3: Dopamine Receptor (DopR/dDA1/dumb) is Required for Modulation of Grooming Behavior. (a, c, e) Grooming of wildtype (blue) and mutant flies (red/orange) measured at 0 min or 30 min after dusting. SEM is measured for each genotype and condition. (a, b) n = 43 flies per genotype and condition. (a) WT and DopRf02676 flies both exhibit grooming behavior (+/+ 30' compared to +/+ 0' = p value <0.0001, DopRf02676 30' compared to DopRf02676 0' = p value <0.0001). DopR flies fail to groom as well as WT animals (DopRf02676 30' compared to +/+ 30' = p value <0.001). Acute dusting of each genotype at 0' results in equivalent accumulation of dust (ns = not significant). (b, d, f) Grooming percent difference is calculated for each genotype (dye accum. at 0' – dye accum. at 30' / dye accum. 0' x 100) providing a relative value for comparing grooming behaviors. (c) DopRattp/DopRattp nullflies display a grooming deficit (DopRattp 30' compared to +/+ 30' p value <0.0001). (d) Grooming percent difference for DopRattp null. (c,d) n = 45 flies for each genotype or condition. e) rut1homozygous flies display a grooming deficit (rut1 30' compared to +/+ 30', p value = <0.0001). f) grooming index for rut1 flies. (e, f) n = 30 flies for each genotype or condition. Statistical analyses by One Way ANOVA and Bonferonni Correction. Reprinted with permission from Genes, Brain, and Behavior 15. Please click here to view a larger version of this figure.
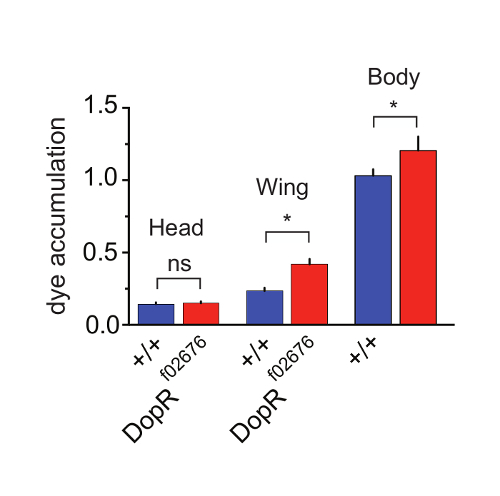
Figure 4: Dopamine Receptor Function Potentiates Hindleg Grooming. (a) Grooming of individual regions of WT (blue) and DopRf02676/DopRf02676 (red) measured at 30 min after dusting and subsequent dissection. p value for wing grooming = 0.0204. p value for body = 0.0302. n = 33-35 flies for all conditions. SEM is measured for each genotype and condition. Statistical analysis by One Way ANOVA and Bonferonni Correction. Reprinted with permission from Genes, Brain, and Behavior15. Please click here to view a larger version of this figure.
Supplemental Figure: Alternate Method for Visual Quantification of Grooming Behavior using NIH Image J Pixel Intensity Software. (A) Visualization of wildtype Drosophila under dissecting scope. Oval defines dorsal abdomen as the region of analysis for pixel intensity. (B) Visualization of dorsal abdomen after dusting with Ultra Green V10 fluorescent paint pigment. Standard filter for Green fluorescence captures the blue-green pigment. (C) WT animal after coating with UGV10 pigment (pregrooming). (E) WT animal after coating with UGV10 pigment (postgrooming). (D) DopRf02676 homozygous mutant after coating with UGV10 pigment (pregrooming). (F) DopRf02676 homozygous mutant after coating with UGV10 pigment (post-grooming). (G) Quantification of pixel intensity for all conditions. n = 15 flies for each genotype or condition. Statistical analyses by One Way ANOVA and Bonferonni Correction. ns = nonsignificant difference. *** represents a p <0.001. Please click here to download this file.
Discussion
The grooming assay is relatively straightforward, but we would caution experimenters to pay special attention to the following issues. Maintaining a tight seal by tightening the screws on the top and bottom plates after introducing flies and dye is essential for reproducible results. The Brilliant Yellow dye is very fine and loose joints will allow losses of dye from the edges of the chamber. The irregularity in dye content for each well could easily throw off grooming quantification as non-uniform dusting will increase variance and false-positive rates for grooming events. Additionally we encourage experimenters to be aware of the environment in which they choose to hold the grooming chamber during the 30 min grooming period. Environmental constancy for temperature, lighting, time of day, and humidity are essential for consistent performance for Drosophila behavior assays. Holding the grooming chambers within a small incubator to minimize distracting or variable lab environments is ideal for maximal reproducibility of results, but other spatial arrangements can also be successful.
With regards to dye accumulation measurements, we have observed that the dye is permeable to latex gloves, so nitrile gloves are preferable for handling and weighing dust. Take care not to spread dust widely across lab surfaces, as it will easily stain clothing and potentially contaminate surfaces or equipment. Isolating one area and one fly-station/CO2 pad for experiments is preferable to contain dye exposure. We also encourage experimenters to be sure to use UV-transparent 96-well plates. The 397nm absorbance peak for Brilliant Yellow dye is near the UV spectrum, and standard 96 well plates may give weak or inaccurate measures for dye dilutions. The dye is also soluble in water as an alternative to EtOH. However, Drosophila do not readily sink in water without significant vortexing and small air bubbles along the body of dyed animals show lower consistency and solubility of dye. Ethanol shows consistently better results and lower variance in direct comparisons.
Our assay can be easily modified for supplementary techniques and more refined studies, such as dissection of the fly body post-assay (Figure 4). Such supplements may be necessary to understand which grooming steps are affected, as the dye accumulation only broadly points to behavioral shifts without directly addressing the fundamental biological or neural cause. Once differences are observed, parallel experiments using video recording or direct-observation methods are essential in understanding the behavioral root of any differences in grooming efficiency. This can be investigated further by visual quantification of dust particle accumulation on specific body parts using pixel intensity measures through the NIH ImageJ software program, along with direct tracking of foreleg and hindleg behaviors by scoring video footage. With regards to alternate methods for visual quantification of fluorescent dust, early experiments in the lab utilized a fluorescent paint pigment derived from Alkaline Rare Earth Metal Silicate-Aluminate Oxide Europium. After dusting the flies with the fluorescent paint pigment and anaesthetizing or decapitating the animals after grooming, the bodies can be recorded and analyzed by digital microscopy using a standard fluorescent filter for GFP on a dissecting scope (Supplemental Data). While this method does allow for accurate quantification of pixel intensity that parallels the results observed using Brilliant Yellow dye, we found that the throughput of this method is relatively slow and the pigment particles vary slightly in size and are less uniform than the coating observed for Brilliant Yellow dye. However for some experimental applications this alternate method is appropriate, and there is significant variety in available fluorescent colors that could be useful for drosophila and nondrosophila applications or iterative dusting experiments where quantification of separate events are essential. It should be noted that the paint pigment itself is water-soluble but rapidly loses its fluorescence in water, severely handicapping the utility of this pigment for standard absorbance measures.
These kinds of methods targeting specific foreleg or hindleg grooming events are essential for understanding the precise nature of a phenotype and may highlight potential neural circuits or locomotor programs to investigate further. Additionally, for parallel control experiments, it is essential to rule out potential non-specific behavioral reasons that grooming could be affected. If animals display broad motor deficits or abnormalities, it is possible that grooming deficits are secondary to the broad locomotor phenotypes. Simple video-tracking of WT animals versus the mutant or circuit manipulated conditions can easily discriminate between large shifts in speed or total distance traveled, independent of grooming deficits.
The grooming assay is a versatile method suitable for both small and large-scale studies of this complex multistep locomotor program; the designs are easily modified to increase chamber dimension or number15. The method yields robust quantitative data that is ideal for comparative assessment of flies' grooming ability. Production of the chambers is cost-efficient, and can be made using many different machines (3D printers, laser cutters, CNC mills) depending on available resources. The technique allows for intermediate throughput of individual samples that could be easily expanded for genetic screens and functional circuit-mapping studies.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We wish to thank Brian Shepherd, Tat Udomritthiruj, Aaron Willey, Ruby Froom, Elise Pitmon, and Rose Hedreen for early work in testing and establishing this methodology and chamber designs. We thank Kelly Tellez and Graham Buchan for reading and editing the manuscript. We thank Andrew Seeds and Julie Simpson for their pioneering work and their advice and support in suggesting the use of Brilliant Yellow Dye (Sigma). This work is supported in part by the Mary E. Groff Surgical and Medical Research and Education Charitable Trust, the Bronfman Science Center, and the Hellman Fellows Program.
Materials
| High-Flex Tygon PVC Clear Tubing | McMaster-Carr | 5229K54 | ID 1/8", OD 1/4", used with micropipettor tips and mesh to construct mouth aspirators |
| Micropipette tips (1ml and 200ul) | Genesee Scientific | 24-165, 24-150R | |
| Nylon Mesh Screen, 2" x 2.6" | McMaster-Carr | 9318T44 | Used to construct grooming chamber and mouth aspirators |
| Dumont #5 Forceps | Roboz Surgical Instrument | RS-5050 | |
| Brilliant Yellow Dye | Sigma-Aldrich | 201375-25G | we recommend use of nitrile gloves while handling this product |
| Vortexer | Fisher Scientific | 12-812 | set to "touch" |
| Ethanol | Carolina Biological Supply | 86-1282 | |
| 1.5 ml microcentrifuge tubes | VWR International | 10025-726 | |
| 0.65 ml microcentrifuge tubes | VWR International | 20170-293 | tubes can be reused with successive assays |
| UV 96 well plate | Corning | 26014017 | |
| BioTek Synergy HTX Platereader | BioTek | need to download catalog to access product number | http://www.biotek.com/products/microplate_detection/synergy_htx_multimode_microplate_reader.html?tab=overview |
| Gen5 Microplate Reader and Imager Software | BioTek | ||
| Microsoft Excel | Microsoft | https://www.microsoftstore.com/store/msusa/en_US/pdp/Excel-2016/productID.323021400?tduid=(65d098c0e83b86c952bdff5b0719c83f)(256380)(2459594)(SRi0yYDlqd0-LI..ql4M2LoZBEhcBljvIA)() | |
| Drosophila Incubator | Tritech | DT2-CIRC-TK | |
| 1/4" acrylic plastic | McMaster-Carr | 8473K341 | |
| 8-32 nuts | McMaster-Carr | 90257A009 | |
| 8-32 x 1" hex cap screws | McMaster-Carr | 92185A199 | the bottom plate needs to be tapped for this size screw |
| 8-32 x 1/2" hex cap screws | McMaster-Carr | 92185A194 | the second plate from the top needs to be tapped |
| 2-56 3/8" flat head phillips machine screws | McMaster-Carr | 91500A088 | these hold the two middle plates together |
| 0.175" ID, 1/4" OD, 0.34" aluminum pipe | McMaster-Carr | 92510A044 | Manufactured in-house; product listed is approximately the same dimensions and should work for size 8 screws. These act as sheaths for the 1" screws and set the hex cap up slightly from the surface of the top plate |
References
- Szebenyi, A. L. Cleaning Behaviour in Drosophila-Melanogaster. Animal. Behaviour. 17, (1969).
- Corfas, G., Dudai, Y. Habituation and dishabituation of a cleaning reflex in normal and mutant Drosophila. J Neurosci. 9 (1), 56-62 (1989).
- Yanagawa, A., Guigue, A. M. A., Marion-Poll, F. Hygienic grooming is induced by contact chemicals in Drosophila melanogaster. Frontiers in Behavioral Neuroscience. 8, (2014).
- Dawkins, R., Dawkins, M. Hierarchical Organization and Postural Facilitation – Rules for Grooming in Flies. Animal Behaviour. 24 (Nov), 739-755 (1976).
- Seeds, A. M., et al. A suppression hierarchy among competing motor programs drives sequential grooming in Drosophila. Elife. 3, e02951 (2014).
- King, L. B., et al. Neurofibromin Loss of Function Drives Excessive Grooming in Drosophila. G3-Genes Genomes Genetics. 6 (4), 1083-1093 (2016).
- Tauber, J. M., Vanlandingham, P. A., Zhang, B. Elevated Levels of the Vesicular Monoamine Transporter and a Novel Repetitive Behavior in the Drosophila Model of Fragile X Syndrome. Plos One. 6 (11), e27100 (2011).
- Kaur, K., Simon, A. F., Chauhan, V., Chauhan, A. Effect of bisphenol A on Drosophila melanogaster behavior–a new model for the studies on neurodevelopmental disorders. Behav Brain Res. 284, 77-84 (2015).
- Chang, H. Y., et al. Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Molecular Psychiatry. 11 (1), 99-113 (2006).
- Yellman, C., Tao, H., He, B., Hirsh, J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 94 (8), 4131-4136 (1997).
- Feng, G. P., et al. Cloning and functional characterization of a novel dopamine receptor from Drosophila melanogaster. Journal of Neuroscience. 16 (12), 3925-3933 (1996).
- Gotzes, F., Balfanz, S., Baumann, A. Primary Structure and Functional-Characterization of a Drosophila Dopamine-Receptor with High Homology to Human D(1/5). Receptors. Receptors & Channels. 2 (2), 131-141 (1994).
- Han, K. A., Millar, N. S., Grotewiel, M. S., Davis, R. L. DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron. 16 (6), 1127-1135 (1996).
- Sugamori, K. S., Demchyshyn, L. L., Mcconkey, F., Forte, M. A., Niznik, H. B. A Primordial Dopamine D1-Like Adenylyl Cyclase-Linked Receptor from Drosophila-Melanogaster Displaying Poor Affinity for Benzazepines. Febs Letters. 362 (2), 131-138 (1995).
- Pitmon, E., et al. The D1 family dopamine receptor, DopR, potentiates hind leg grooming behavior in Drosophila. Genes Brain and Behavior. 15 (3), 327-334 (2016).
- Phillis, R. W., et al. Isolation of mutations affecting neural circuitry required for grooming behavior in Drosophila melanogaster. Genetics. 133 (3), 581-592 (1993).
- Hampel, S., Franconville, R., Simpson, J. H., Seeds, A. M. A neural command circuit for grooming movement control. Elife. 4, e08758 (2015).
- Kays, I., Cvetkovska, V., Chen, B. E. Structural and functional analysis of single neurons to correlate synaptic connectivity with grooming behavior. Nature Protocols. 9 (1), 1-10 (2014).

