Du-Moxibustion in a Mouse Model of Ankylosing Spondylitis
Summary
This paper describes in detail the operation procedures and precautions to be taken during Du-moxibustion in the treatment of ankylosing spondylitis in experimental mice.
Abstract
Ankylosing spondylitis (AS) is a progressively worsening and disabling form of arthritis that primarily affects the axial skeleton. This disease mainly involves the spine and the sacroiliac joint. Fusion of the spine and the sacroiliac joint may occur in the later stage of the disease, resulting in spinal stiffness and kyphosis, as well as difficulty in walking, which seriously affects the quality of work and daily living activities and imposes a heavy burden on the patient, the family, and society. Increasing attention has been paid to non-pharmacotherapy as an alternative therapy for AS. Moxibustion is an ancient therapeutic technique used in Traditional Chinese Medicine (TCM). Du-moxibustion therapy, a unique and innovative external treatment developed on the basis of ordinary moxibustion, has a definite therapeutic effect on AS. Du-moxibustion skillfully combines the compatible techniques of TCM to integrate meridians, acupoints, Chinese herbal medicine, and moxibustion. This paper describes the operation procedures and precautions to be taken during Du-moxibustion in experimental mice in detail to provide an experimental basis for the study of the mechanism of Du-moxibustion in the treatment of AS.
Introduction
Ankylosing spondylitis (AS) is a progressively worsening and disabling form of arthritis that primarily affects the axial skeleton. According to the latest epidemiological survey, ~0.01%-1.8% of people in the world suffer from this condition1, most of whom are young males (male to female ratio is ~2-3:1)2. The incidence of AS in the Chinese population is 0.2%-0.4%. This disease mainly involves the spine and the sacroiliac joint. Fusion of the spine and the sacroiliac joint may occur in the later stages of the disease, resulting in spinal stiffness and kyphosis, as well as difficulty in walking, which seriously affects the quality of work and daily living activities and imposes a heavy burden on the patient, family, and society1. Currently, there is no definitive solution for AS, and the provided treatments, both pharmacological and non-pharmacological, primarily focus on alleviating pain, slowing disease progression, and enhancing the quality of life. In recent years, because pharmacotherapeutic options have been very limited3, increasing attention has been paid to non-pharmacotherapy, which has become an alternative therapy for AS. In China, patients often prefer external treatment consisting of Traditional Chinese Medicine (TCM), which has minor side effects and great convenience during the treatment.
Moxibustion is an ancient therapeutic technique used in TCM. Du-moxibustion is an external treatment technique consisting of "medicated moxibustion with foam" on the spine segment of the Du meridian. It is mainly used in the treatment of AS and has shown safety and effectiveness. Compared with ordinary moxibustion, Du-moxibustion has the characteristics of a wide moxibustion area, a large moxa cone, strong firepower, and high temperature4. A systematic review and meta-analysis suggest that moxibustion is an effective complementary treatment for AS5. A recent study confirmed that the clinical symptoms and some inflammatory factors of AS patients were improved by moxibustion6. Studies have revealed that Du-moxibustion can increase the brain content of β-endorphin and exert a central analgesic effect7, downregulate the expression of the HLA-B27 gene8, and delay the recurrence rate of AS. Du-moxibustion also has an ameliorating effect on the progression of AS by decreasing the inflammatory index ESR (ESR) and levels of C-reactive protein (CRP), C-terminal peptide of type I collagen (CTX-I), and Dickkopf1 protein (DKK1)9,10.
Another clinical study showed that Du-moxibustion can further adjust the disordered T cell subsets, Ig, and complement C3 to balance the immune mechanism11. In terms of bone metabolism, moxibustion can inhibit the rise of serum alkaline phosphatase (ALP) and serum phosphorus (S-P), increase serum calcium (S-Ca), bone mineral density (BMD), and bone strength12. Moreover, Du-moxibustion can repair spinal function at multiple points to relieve fatigue symptoms in AS patients13. Some animal studies have revealed the potential mechanism of moxibustion in treating AS. A study indicated that moxibustion significantly improved the overall health status, reduced the levels of paw thickness, and decreased the levels of IL-1β, PGE2, IL-6, and TNF-α in the ligament tissue samples of the spine. Metabolic pathway analysis linked the identified metabolites to the TCA cycle, as well as lipid, amino acid, intestinal flora, and purine metabolism14. Another study showed that moxibustion suppressed the expression of pro-inflammatory cytokines, IL-1β, TNF-α, IL-17, and IL-6; reduced the mRNA levels of RANKL, RANK, ALP, and OCN; and improved the histopathological features in AS mice15.
While these studies suggest that moxibustion may be effective in the treatment of AS, more research is needed to confirm these findings and determine the optimal treatment protocol for moxibustion in patients with AS. Our team created and modified the moxibustion therapy based on the basic theory and practical operation of traditional Chinese moxibustion, which has been widely carried out and applied in Chinese clinical practice for over 35 years. Although there are few high-quality studies on the treatment of AS by Du-moxibustion, its indication in treating AS has been supported by evidence. However, the mechanism of treating AS with Du-moxibustion is worthy of further study. Animal study is an important method to explore the mechanism of Du-moxibustion in the treatment of AS. The clinical operation of Du-moxibustion is relatively mature but rarely used in the study of the mechanism in animal experiments. This paper describes in detail the operation and precautions to be taken during Du-moxibustion in the treatment of AS experimental mice.
Protocol
All animal experiments were approved by the Experimental Animal Welfare Ethics Review Committee of Shandong University of Traditional Chinese Medicine (No. SDUTCM20230831301).
1. Animal preparation
- Determine the experimental groups and the number of animals per group in advance.
- Choose male BALb/c mice, house them at a constant temperature of 22 ± 1 °C, constant humidity of 60 ± 5%, and 12 h diurnal cycle light environment.
2. Establishment of the AS mouse model
NOTE: The AS model was induced with cartilage proteoglycan (PG), which is a classic modeling method16,17,18,19,20,21. BALB/c strain mice were repeatedly immunized with PG to induce tendinitis and spondylitis. See the Table of Materials for details related to the materials, reagents, and instruments used in this protocol.
- Use a 200 µL pipette to draw 200 µL of PG and CFA (or IFA) into a 1.5 mL centrifuge tube. Shaking gently to mix the liquid in the centrifuge tube.
- Place the centrifuge tube in the tissue grinder for homogenization and then incubate on ice for 4 min. Repeat 5x. Set the following parameters in the tissue grinder: 60 Hz, grinding 30 s, interval 10 s for a total of 6 cycles.
- Extract 0.2 mL of emulsified PG protein from the centrifuge tube with a 1 mL syringe.
- Grab and fix the mouse with the left hand. Lay the mouse in a supine position, keeping the head lower than the tail to prevent damage to the abdominal organs when the syringe is inserted.
- Sterilize the abdomen with alcohol swabs using the right hand.
- With the syringe in the right hand, insert the needle subcutaneously slightly to the left or right of the linea alba ventralis in the lower half of the abdomen.
- Push the needle 3-5 mm subcutaneously parallel to the midabdominal line and then push the needle into the abdominal cavity at a 45° angle to the skin.
- Penetrate the peritoneum, withdraw the needle plug, and slowly inject the emulsified PG protein.
3. Du-moxibustion
NOTE: Prepare in advance the three most important raw materials for Du-moxibustion (Figure 1).
- Prepare moxa cones. Take the velvet in the hand, with two palms facing opposite directions exerting a twisting force to rub the velvet into a spindle shape (Figure 2)
- Cut the ginger into small pieces and puree it in a juicer (Figure 3). Filter the ginger puree through cotton gauze. Separate the ginger juice and ginger mud from the ginger puree to prepare for the next step.
- Prepare 10 g of ginger mud and 20 mL of ginger juice and place them in paper cups for later use.
- Weigh 0.1 g of TCM powder (a mixture of cinnamon powder, clove powder, notopterygium powder, antharidin powder, and artificial musk)22 and grind it in a mortar.
- Place the mice in the anesthesia induction box of the animal anesthesia machine and anesthetize them with a mixture of 2-3% isoflurane and oxygen at a flow rate of 2 L/min.
- Assess the depth of anesthesia by observing the absence of a startle reflex initially and verify the level of surgical anesthesia by checking for the absence of a pedal reflex in response to a gentle toe pinch.
- After the mouse has reached surgical plane anesthesia, take it out of the box and fix it on the operating table in a prone position. Align the mouse's mouth and nose with the outlet of the anesthetic.
- Administer anesthesia via a mask attached to the anesthetic machine, through which isoflurane and oxygen are delivered via a non-rebreathing circuit. Maintain the anesthesia and keep the mouse on a 37 °C heating pad during the entire operational procedure. Apply lubricant to the eyes to prevent desiccation.
- Locate the acupoints and identify the stimulation area (Figure 4).
- Shave the hair on the back of the mouse until the flesh-colored skin on the back of the mouse is seen. Ensure the width of hair removal is ~1.5 cm and the length is from the seventh cervical vertebra to the tail vertebra (Figure 5A).
- Apply the prepared ginger juice with a cotton gauze to the shaved area (Figure 3).
- Sprinkle the TCM powder evenly with a writing brush on the median line of the back of the mouse and cover it with mulberry paper (Figure 5B-D).
- Make a trapezoidal ginger column 6 cm long, 1.5 cm wide, and 3 mm thick with ginger mud. Fix the ginger column on the mulberry paper (Figure 5E).
- Make a groove above the midline of the ginger column (Figure 3), and place the moxa cone on the ginger column.
- Light the moxa cone, placing a new moxa cone after the previous one burns out. Check the firmness of the ginger column to prevent burning the mouse, burning a total of three cones (Figure 5F).
- After the burning of the moxa cones, wipe off the ginger and the TCM powder, and clean the back of the mouse. Discontinue the anesthesia at this point.
- Allow the mouse to regain consciousness in a new cage with unrestricted access to food and water. Place the cage on a heated pad to aid in the recovery process. Monitor the animal until it regains sufficient consciousness.
- Once the mouse has fully recovered, confirm the presence of the righting reflex before returning it to its regular housing.
NOTE: During the operation, it is necessary to monitor the mental state of the mice to avoid weak breathing. Prevent scalding, but keep the mice warm, and avoid bruising of the claws and tail.
4. Evaluation of arthritis index (AI)
- Evaluate the AI score three times: before modeling, after modeling, and after treatment. The assessment standards are as follows: 0, no redness or swelling; 1, slight redness or swelling in a few toes; 2, redness and swelling in most toe joints and toes; 3, serious redness and swelling in feet and below ankle joint; and 4, redness and swelling in feet and ankle joint23.
- Calculate the AI scores of all four paws, with a maximum value of 16.
5. Rotarod test
NOTE: The rotarod test is used to assess motor coordination and balance by recording the time the mice spend on the rotating drum. This experiment consisted of three trials separated by 20 min intervals. The official test data were recorded from the third trial, with the first two serving as training exercises.
- Place the mice on rotating drums that rotate under continuous acceleration from 4 to 40 rpm over 300 s24.
- Measure the latency time (the time until the mouse falls off the rod). Provide padding to prevent injury when it falls off.
6. Open field test (OFT)
NOTE: The OFT is used to evaluate the state of autonomic movement, aiming to identify pathological behavior. Four square open field arenas (50 cm x 50 cm x 40 cm) were placed together to form the apparatus. The bottom of the box was divided into nine equal squares.
- Control the background noise of the laboratory to below 65 dB.
- Wipe the entire apparatus with 75% ethanol before each trial.
- Place all mice in the testing room 1 h before the test to allow them to adapt to their environment.
- Put the mice in the square open field arenas and allow them to explore for 10 min.
- Record the total distance and movement speed for 10 min.
7. Euthanasia and specimen collection
- Place the mouse in the anesthesia induction box and anesthetize it with a mixture of 2-3% isoflurane and oxygen at a flow rate of 2 L/min. Once the mouse enters a state of deep anesthesia confirmed by a lack of pedal reflex, transfer it to the operating table for maintenance anesthesia.
- Grab and fix the mouse with the non-dominant hand.
- Lightly press on the eye area to make the eyeballs congested and protrude.
- Use scissors to trim the whiskers of the mouse to prevent hemolysis caused by hairs.
- Confirm the depth of anesthesia by checking for the absence of a pedal reflex in response to a toe pinch. Use forceps to grasp the eyeball and quickly remove it, allowing the blood to flow into a microcentrifuge tube from the eye socket.
NOTE: Alternative blood collection methods, such as cardiac puncture, can also be used. - When the blood dripping rate slows down, gently press on the mouse's heart area to speed up the blood pumping and obtain more blood. Then, euthanize the mouse using cervical dislocation.
- Hold the leg just above the ankle joint using forceps in the non-dominant hand. Then, cut the leg just above the ankle joint using scissors.
8. Histological analysis
- Immerse the ankle specimens in 4% paraformaldehyde for more than 24 h. Decalcify in 10% EDTA (pH = 7.4) for 1 month.
- Soak the specimens in 4% paraformaldehyde for 2 h.
- Then, soak the specimens in a gradient of increasing ethanol concentrations (70%, 80%, 90%, 100%, 100%, 100%). Soak each ethanol concentration for 1 h.
- Soak the specimens in xylene for 3 h.
- Immerse the ankle specimens in paraffin for 7 h. Set aside paraffin blocks in a 4 °C refrigerator.
- Slice paraffin blocks to a thickness of 6 µm using a semi-automatic paraffin sectioning machine.
- Soak the slices for 4 x 5 min in xylene to complete dewaxing.
- Soak the slices in a gradient of decreasing ethanol concentrations (100%, 100%, 95%, 75%) for 2 min each. Soak in distilled water for 2 min.
- Stain the slices with hematoxylin for 10 min and rinse with distilled water for 5 s.
- Soak the slices for 30 s in the differentiation fluid and rinse with distilled water for 5 s.
- Stain the slices with eosin for 1 min and rinse with distilled water for 3 min.
- Soak the slices in a gradient of increasing ethanol concentrations (80%, 95%, 100%, 100%) for 1 min each. Soak in xylene for 3 x 3 min and seal the slices25.
9. Enzyme-linked immunosorbent assay (ELISA)
- Measure the IL-17 and TNF-α concentrations in plasma using the respective kits according to the manufacturers' instructions.
10. Statistical analysis
- Express the data as mean ± standard error of the mean (SEM).
- Determine the significance through one-way analysis of variance (ANOVA) or two-way repeated measures ANOVA, followed by Bonferroni or Tamhane T2's post hoc tests.
- Consider P < 0.05 to be statistically significant.
Representative Results
PG-induced model mice developed symptoms of peripheral arthritis, characterized by redness and swelling of the extremities and toes, and gradually developed axial arthritis as early as week 14, which is very similar to the manifestation of AS. Therefore, the AS mouse model was considered successful if the arthritis index (AI) was over 3 points at 14 weeks after the last injection22. When the paws of the mice appeared swollen, the Du-moxibustion treatment was carried out once a week for a total of 3x. After treatment, the mice were euthanized by cervical dislocation under isoflurane deep anesthesia, their blood was collected, and the ankles were excised.
Effects of Du-moxibustion on motor coodination and autonomic movement
Bovine PG-induced AS was adopted as a model to observe the effect of Du-moxibustion at the Du meridian on motor coordination. Initially, the joint swelling score was used to assess whether the model was successful, and motor coordination and autonomic movement were evaluated using the rotarod test and OFT.
Evaluation of AI determined the severity of peripheral arthritis. After modeling, two-way repeated-measures ANOVA revealed that the joint swelling scores in the model and Du-moxibustion groups significantly increased compared with those in the control group before the Du-moxibustion (P < 0.01; Figure 6A). Du-moxibustion treatment decreased joint swelling scores compared with those of the model group after the Du-moxibustion (P < 0.05; Figure 6A).
The rotarod test was employed to evaluate motor coordination and balance. Two-way repeated-measures ANOVA revealed significant differences over time (F = 13.928, P = 0.000 < 0.05) and interaction effects of time and group (F = 12.583, P = 0.000 < 0.05). After modeling, the latency time of the control group was not different from that before modeling. However, after modeling, the latency times of the model and Du-moxibustion groups significantly decreased compared with those in the control group (P < 0.01; Figure 6B), and there were no significant differences between the model group and the Du-moxibustion group (P > 0.05; Figure 6B). After treatment, the latency time of the Du-moxibustion group significantly increased compared to that of the model group (P < 0.05; Figure 6B). These results indicated that Du-moxibustion was effective in improving motor coordination in AS mice.
OFT was performed to detect autonomic movement. The parameters were collected and analyzed automatically using the software (Figure 6C–E); the total distance and average speed were mainly observed in this study. There was no significant difference in the total distance and average speed among the groups before modeling (P > 0.05; Figure 6C,D). After modeling, the total distance and average speed significantly reduced compared with the control group (P < 0.05; Figure 6C,D). After treatment, the total distance and average speed of the Du-moxibustion group significantly increased compared to those of the model group (P < 0.05; Figure 6C,D). These results indicated that Du-moxibustion was effective in improving autonomic movement in AS mice.
Effects of Du-moxibustion on ankle joint pathology
HE staining was used to observe the pathological changes in the ankle joint. In the control group, there was no damage to the synovium structure, no congestion in the synovium, and no obvious inflammatory cell infiltration and necrosis. However, in the model group, synovium hyperplasia and infiltration were observed in the joint cavity, in addition to inflammatory cell infiltration and tissue edema. Combined with AI evaluation, it was proved that the AS model was successfully established. Compared with the model group, the synovium of mice in the Du-moxibustion group was more intact, interstitial edema was reduced, and the articular cartilage was relatively intact (Figure 7).
Effects of Du-moxibustion on plasma IL-17 and TNF-α levels
ELISA was performed to measure IL-17 and TNF-α in the plasma. The plasma IL-17 and TNF-α levels of the model group were significantly increased compared with those of the control group (P < 0.01; Figure 6F,G). Du-moxibustion decreased plasma IL-17 and TNF-α levels (P < 0.05; Figure 6F,G).
Figure 1: The raw materials needed for Du-moxibustion. Please click here to view a larger version of this figure.

Figure 2: Making a moxa cone. Please click here to view a larger version of this figure.

Figure 3: Prepare ginger water and make a trapezoidal ginger column. Please click here to view a larger version of this figure.
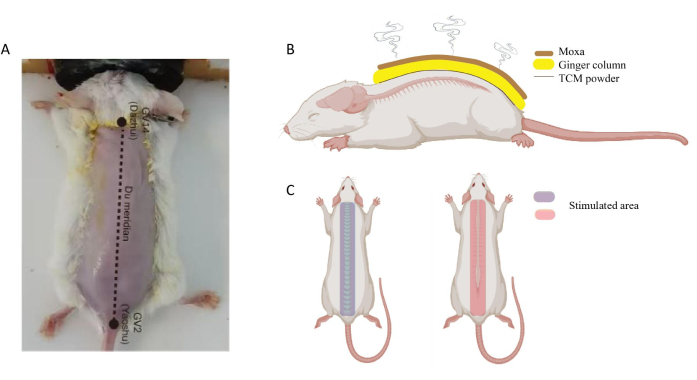
Figure 4: Location of GV14 and GV2 in mouse. (A) GV14 located at the subspinous process of the seventh cervical vertebra; GV2 located in the hiatus sacral canal. (B) Schematic diagram of Du moxibustion on the mouse. (C) Stimulated area of Du moxibustion. Please click here to view a larger version of this figure.
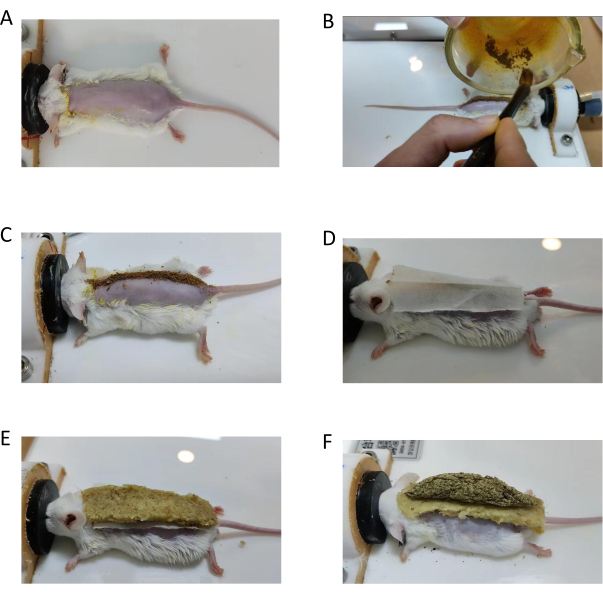
Figure 5: Operation procedure of Du-moxibustion in mouse. (A) Shave the hair and smear the ginger juice on the mouse in the prone position. (B,C) Sprinkle TCM powder evenly. (D) Cover the powder with mulberry paper. (E) Fix the ginger column on the mulberry paper firmly. (F) Put a moxa cone on the ginger column; then, light it. Please click here to view a larger version of this figure.
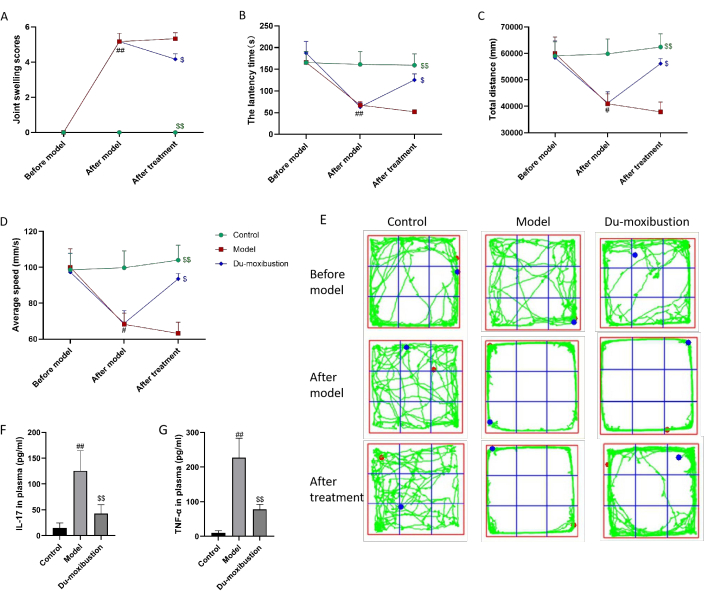
Figure 6: Effects of Du-moxibustion on motor coordination, autonomic movement, and IL-17 and TNF-α levels. (A) The joint swelling scores in mice. (B) The latency time in the rotarod test. (C–E) Total distance, average speed, and track diagram of OFT. (F) IL-17 levels in plasma. (G) TNF-α levels in plasma. Values are expressed as the mean ± SEM (n = 6). ##P < 0.01, #P < 0.05 versus the control group; $$P < 0.01, $P < 0.05 versus the model group. Abbreviations: IL = interleukin; TNF = tumor necrosis factor; OFT = open field test. Please click here to view a larger version of this figure.
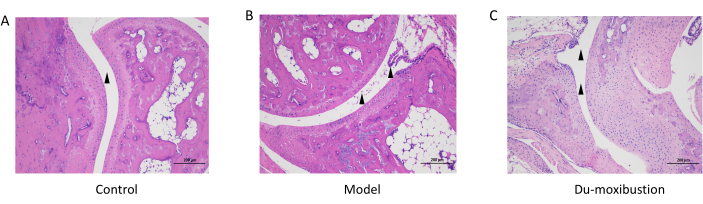
Figure 7: HE staining of the ankle joint. (A) The joint cavity was smooth without inflammatory infiltration in the control group. (B) In the model group, synovium hyperplasia and infiltration were observed in the joint cavity. (C) In Du-moxibustion group, the joint cavity of mice was smooth and edema was alleviated. Scale bars = 200 µm. Abbreviation: HE = hematoxylin-eosin. Please click here to view a larger version of this figure.
Discussion
AS is clinically manifested as back, lumbar, and joint swelling pain, and spinal deformity and joint rigidity may occur in severe cases26. Early diagnosis and treatment can improve the quality of life, reduce disability rates, and improve the prognoses of patients with AS. According to traditional Chinese medicine (TCM), AS is believed to be caused by a deficiency of Yang in the kidney and the Du meridian, as well as an accumulation of cold evil internally. TCM holds the view that moxibustion, based on the concept of meridians, can generate Qi that warms the body's surface. This Qi then travels downwards along the meridians and acupoints. In The Inner Canon of Huangdi (a very classic and important TCM masterwork), it is recorded that "the diseased Du meridian is treated." As AS is located in the Du meridian, stimulating the Du meridian is used as a therapeutic point. The Du meridian is considered to be the ocean of Yang channels where Yang Qi reaches its peak. Moxibustion on the Du meridian can supplement Yang qi, activate the effect of the Du meridian, and adjust the warming effect of kidney Yang, that is, enhance the immune regulation function of Western medicine.
From the perspective of Western medicine, the spinal cord plays an important role in transmitting neural information. Du moxibustion, which acts on the entrance and exit of spinal nerve roots, can affect the function and regulatory mechanism of the nervous system through the regulation of neurotransmitters. In the 1990s, it was demonstrated that Du-moxibustion increased the content of β-endorphins in the hypothalamus of rats, thus having a central analgesic effect7. In addition, enthesitis is a common complication of AS, characterized by persistent pain and muscle stiffness, which can affect joint and body movement when severe. As a TCM treatment method that has been clinically validated, Du-moxibustion can effectively alleviate inflammation, promote tissue repair, and improve enthesitis in treating AS.
Specifically, the effects of acupuncture and moxibustion are mainly reflected in the following aspects. First, they promote blood circulation and the absorption of inflammation. Du-moxibustion stimulates blood circulation and metabolism in the tissues surrounding the spine through the warming effect and promotes the absorption of inflammation and tissue repair, thereby reducing the degree of inflammation and pain of tendon attachment inflammation. Second, they improve muscle and ligament tension. The stimulation effect of Du-moxibustion can improve the muscle tension around the spine, relax the hardened soft tissues, promote the activity of spinal joints, and reduce the pain and stiffness caused by muscle tension and ligament stretching. In summary, Du-moxibustion, as an adjunctive therapy, can comprehensively regulate the activities of the body's nervous, immune, and circulatory systems by acting on the spinal region, making the body more balanced as a whole.
Inflammation is the main manifestation of AS27. TNF-α is a multi-effector cytokine and inflammatory factor produced under the stimulation of mononuclear macrophages28. The activity of ALP and collagen synthesis can be inhibited by TNF-α, resulting in bone loss and cartilage destruction29. In recent years, studies have found that IL-17 plays an important role in the pathogenesis of AS, which has been fully demonstrated in a review30. IL-17 can directly act on osteoblasts, promote the expression of nuclear transcription factor-κB (NF-κB) receptor activator ligand (RANKL), and promote the robust generation of osteoclasts. At the same time, IL-17 also affects mesenchymal stem cells (MSCs), inducing their proliferation and promoting their differentiation into osteoblasts31. Kenna32 et al. found that the level of IL-17 in the peripheral blood of AS patients increased by almost two-fold compared with healthy controls. The study suggests that IL-17 antagonists may be a new option for AS patients who do not respond well to TNF-α antagonists33. A clinical study showed that a dosing regimen of IL-17 antagonists was superior to a placebo for improving radiographic axial spondyloarthritis signs and symptoms in patients34. The results of this study showed that the plasma levels of TNF-α and IL-17 in the model group were significantly higher than those in the control group, and the plasma levels of TNF-α and IL-17 were significantly decreased after Du-moxibustion treatment.
Du-moxibustion therapy skillfully uses the compatible techniques of TCM to integrate meridians, acupoints, Chinese herbal medicine, and moxibustion. The raw materials of Du-moxibustion therapy mainly included moxa velvet, ginger, and TCM powder. From the perspective of TCM, moxa velvet, a processed product of mugwort, has the function of activating collaterals, dispelling cold, and relieving pain. Ginger plays a role in relieving external symptoms and dispelling cold. Modern research also shows that ginger has anti-inflammatory and analgesic properties35. Wiping the skin with ginger juice before treatment helps open the pores and promote transdermal permeation36, in addition to helping the TCM powder adhere to the skin. The trapezoidal ginger column is firmly fixed on the back to provide a solid base for the moxa cone. After moxa cone burning, the effects of firepower, ginger, and TCM powder work together on the Du meridian. In view of the pathogenesis of Yang deficiency in this disease, Du-moxibustion directly acts on the site of the disease, warming the meridians, dispersing cold, supplementing Yang Qi and essence, promoting blood circulation, and balancing Yin and Yang to treat the root of disease.
When performing Du-moxibustion, several details should be considered. First, the operation site of Du-moxibustion is fixed, from the Dazhui point (GV14) to the Yaoshu point (GV2) (anatomically from the 7th cervical spine spinous process to the tail vertebra, including the entire spine), which is consistent with the incidence site of AS (Figure 4). Second, the humidity and thickness of ginger are important. The excess ginger juice must be squeezed out after the ginger is processed into ginger mud so that the ginger mud has no granular feeling and its thickness is appropriate to be made into the trapezoidal ginger column. High humidity and moisture in the ginger mud can easily hinder the penetration of moxibustion fire and reduce the amount of moxibustion stimulation. If the ginger raw material is too dry and the particles are too large, it is difficult to make the trapezoidal ginger column, leading to rapid heat penetration and scalding of the skin. Third, the size and firmness of the moxa cone should be considered. Moxa cones used for moxibustion in mice are ~4 cm long and 0.5 cm in diameter and should be tight at the time of preparation. A test for the tightness is when the cone does not unravel even after being dropped on a surface. If the moxa cone is not compact enough, it will lead to a reduction in the amount of moxa velvet, a shorter burning time, and a reduction in firepower. Fourth, when igniting the moxa cone, the head, middle, and tail of the moxa cone should be lit with fine incense to make the moxa cone burn slowly to achieve the nourishing effect of ebb and flow. Fifth, musk is an important ingredient in the medicinal powder of moxibustion. The second day after moxibustion, there should be crystal clear blisters like small pearls on the Du meridian.
A large number of clinical studies have confirmed the definite efficacy of Du-moxibustion in the treatment of AS4,5,6. However, research on its mechanism of action in animal experiments progresses slowly. Through research and innovation, years of experience in the operation of Du-moxibustion have led us to investigate its use in mice experiments. This paper describes in detail the operation procedures and precautions of Du-moxibustion in the treatment of AS in experimental mice. In addition, the effect of moxibustion on motor coordination and the plasma levels of TNF-α and IL-17 in AS mice were observed. This study provided the most basic experimental basis for the study of the mechanism of Du-moxibustion in the treatment of AS. There are still some limitations to this study. For example, in future studies, we will pay attention to the evaluation of different parameters such as placebo moxibustion, dose, range, and treatment time to obtain the most effective combination schemes. In later studies, imaging methods can be used to evaluate the treatment results in a more intuitive way.
The application prospect of Du-moxibustion therapy is promising. The Du meridian, known as the Governor Vessel, plays a crucial role in regulating the flow of Qi and blood throughout the body. Moxibustion, a traditional Chinese therapy, involves the burning of mugwort on specific acupoints to stimulate the meridians and promote the body's self-healing mechanism. Compared with acupuncture, Du-moxibustion is a combination of various techniques that act on the body surface, which has the characteristics of comfort and a strong and wide range of action. Moreover, Du-moxibustion therapy focuses on the back and spinal area, targeting the Du meridian's pathway. It can enhance the body's immune system, improve blood circulation, and alleviate various physical and mental conditions. The therapy has demonstrated its effectiveness in treating diseases such as musculoskeletal disorders, neurological conditions, gynecological disorders, and mental health issues. With advances in research and technology, the application of Du-moxibustion therapy is expected to expand further. Future studies may explore its potential in areas such as pain management, stress reduction, digestive disorders, and respiratory conditions. The combination of traditional knowledge and modern scientific understanding could lead to better integration of Du-moxibustion therapy into mainstream healthcare practices. In conclusion, Du-moxibustion therapy holds great promise in its application and integration into various healthcare fields. Further research, clinical trials, and collaboration between traditional Chinese medicine practitioners and modern healthcare professionals are essential to unlock its full potential.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the General Program of the National Natural Science Foundation of China (No.82174491) and the Qilu School of Traditional Chinese Medicine Academic School Inheritance Project (No. Lu Weihan [2020]132). Thanks to the Laboratory Animal Center of Shandong University of Traditional Chinese Medicine for providing us with experimental conditions.
Materials
| 10% EDTA | Shanghai Macklin Biochemical Co., Ltd.,Shanghai, China | ||
| alcohol pads | HYNAUT, Qingdao Hainuo Biological Engineering Co., LTD, Qingdao, China | ||
| anesthesia machine | Medical Supplies & Services INT. LTD, Keighley, UK | ||
| centrifuge tubes | Axygen, Corning, NewYork, UAS | ||
| complete Frech's adjuvant (CFA) | aladdin,Shanghai, China | F393378 | |
| cotton ball | Henan RUIKE MEDICAL Equipment Co., Ltd.,Xinxiang, China | ||
| cotton gauze | Henan RUIKE MEDICAL Equipment Co., Ltd.,Xinxiang, China | ||
| cutting board | self-preparation | ||
| decorin from bovine articular cartilage | Sigma-Aldrich, MO, USA | D8428 | |
| depilatory cream | Veet, Reckitt Benckiser (China) Investment Co. LTD, Shanghai, China | ||
| electronic scale | Shanghai Yajin Electronic Technology Co., Ltd.,Shanghai, China | ||
| Eppendorf tube | Axygen, Corning, NewYork, UAS | ||
| eye lubricant | Beijing Shuangji Pharmaceutical Co., LTD., Beijing, China | ||
| ginger | self-preparation | ||
| GraphPad Prism 7 software | GraphPad Software,Boston, USA | ||
| hair clipper | Super human Group CO LTD, Jinhua, China | ||
| heating pads | Shenzhen Leshuo Tech Co., Ltd.,Shenzhen, China | ||
| incomplete Freund’s adjuvant (IFA) | aladdin,Shanghai, China | F393371 | |
| injection syringe | Shandong Xinhua Ande Medical Supplies Co., LTD, Zibo, China | ||
| isoflurane | Shenzhen Rayward Life Technology Co., LTD, Shenzhen, China | R510-22-10 | |
| joss stick | Shijiazhuang Lidu Fragrant Industry Co., LTD.,Shijiazhuang, China | ||
| juicer | Braun (Shanghai) Co., Ltd.,Shanghai, China | ||
| knife | self-preparation | ||
| lighter | Zhejiang tiger-lighter Co. LTD | ||
| mortar | Luoyang Yinai Ceramic Technology Co., LTD.,Luoyang, China | ||
| Mouse IL-17 ELISA Kit | absin, Shanghai, China | abs520009-96T | |
| Mouse TNF-α ELISA Kit | Wuhan Sanying, Wuhan, China | KE10002 | |
| mulberry paper | Yishui County Mulinsang paper Co., LTD, Linyi, China | ||
| OFT | Xinruan,Shanghai, China | XR-XZ301 | |
| paper cup | self-preparation | ||
| pipettes | OXFORD BIO INSTRUMENTS INC.,Oxford, UK | ||
| refined moxa velvet | self-preparation | ||
| rotarod | Xinruan,Shanghai, China | XR-6C | |
| scientz-48L cryogenic high throughput tissue grinder | Ningbo Xinzhi Biotechnology Co., LTD | ||
| scissors | Shandong Jiaren Medical Supplies Co., Ltd., Zibo, China | ||
| semi-automatic paraffin sectioning machine | Leica Camera AG, Watznach, Germany | ||
| SPSS 25.0 software | International Business Machines Corporation, NewYork, UAS | ||
| TCM powder | self-preparation | ||
| tips | Biosharp, Labgic, Beijing, China | ||
| writing brush | Yishui County Mulinsang paper Co., LTD, Linyi, China |
References
- Sieper, J., Poddubnyy, D. Axial spondyloarthritis. Lancet. 390 (10089), 73-84 (2017).
- Lee, W., Reveille, J. D., Weisman, M. H. Women with ankylosing spondylitis: a review. Arthritis Rheum. 59 (3), 449-454 (2008).
- Sari, &. #. 3. 0. 4. ;., Öztürk, M. A., Akkoç, N. N. of ankylosing spondylitis. Turk J Med Sci. 45 (2), 416-430 (2015).
- Zhang, L., et al. Governor vessel moxibustion: Ancient Chinese medical technology with new vitality. Chin J Integr Med. 23 (5), 396-400 (2017).
- Hu, J., et al. Moxibustion for the treatment of ankylosing spondylitis: a systematic review and meta-analysis. Ann Palliat Med. 9 (3), 709-720 (2020).
- Liu, Y., Wang, P., Sun, Y. Y., Qu, J., Li, M. Efficacy of thunder-fire moxibustion in treating ankylosing spondylitis of kidney deficiency and governor meridian cold and its influence on TNF-α and RANKL: study protocol for a prospective, nonblinded, single-center, randomized controlled trial. Trials. 23 (1), 344 (2022).
- Chong, G. Q., Ma, Y., Sun, H. S., Zhang, T., Peng, W. Study on clinical and analgesic mechanism of moxibustion in the treatment of ankylosing spondylitis. J Clin Acupunct Moxib. (06), 48-49 (1999).
- Liu, W. Q., Lu, X. H. Clinical observation of Melilotus extract tablets combined with Dujiu for treating ankylosing spondylitis. Pharm Res. 35 (03), 182-184 (2016).
- Zhang, Y. L., Lin, J. H., Zhou, Y. Y., Zhao, G. Q., He, Y. T. Clinical observation of Bushen Qiangdu Zhilou decoction combined Du-moxibustion in treating ankylosing spondylitis. Chin J Exp Tradit Med Formulae. 21 (10), 190-194 (2015).
- Guo, L. J., et al. Clinical effect of moxibustion at governor vessel combined with Bushen Quhan Huashi prescription for ankylosing spondylitis and its effect on levels of CTX-I and DKK1 in serum. New Chin Med. 53 (10), 117-121 (2021).
- Deng, Y. X. Governor moxibustion treatment of ankylosing spondylitis the adjustment of the T-cell subsets observed. J Practical Tradi Chin Inter Med. 26 (18), 77-78 (2012).
- Wang, Y. Q. Mechanism of exercise combined with moxibustion in the treatment of osteoporosis in castrated rats. Chin J Gerontol. 37 (04), 839-840 (2017).
- Ren, J. Y., Li, F., Yan, J., Wan, C., Cai, L. Clinical study of Du-moxibustion combined with needling Huatuo Jiaji acupoint in the treatment of AS of kidney-Yang deficiency. J Clin Acupunct Moxib. 35 (06), 41-44 (2019).
- Xu, X., et al. Metabolomic analysis of biochemical changes in the tissue and urine of proteoglycan-induced spondylitis in mice after treatment with moxibustion. Integr Med Res. 10 (1), 100428 (2021).
- Xu, X., et al. Moxibustion attenuates inflammation and alleviates axial spondyloarthritis in mice: Possible role of APOE in the inhibition of the Wnt pathway. J Tradit Complement Med. 12 (5), 518-528 (2022).
- Li, X., et al. Inflammation intensity-dependent expression of osteoinductive wnt proteins is critical for ectopic new bone formation in ankylosing spondylitis. Arthritis Rheum. 70, 1056-1070 (2018).
- Cui, H., et al. CXCL12/CXCR4-Rac1-mediated migration of osteogenic precursor cells contributes to pathological new bone formation in ankylosing spondylitis. Sci Adv. 8 (14), eabl8054 (2022).
- Braem, K., Lories, R. J. Insights into the pathophysiology of ankylosing spondylitis: contributions from animal models. Joint Bone Spine. 79 (3), 243-248 (2012).
- Mikecz, K., Glant, T. T., Poole, A. R. Immunity to cartilage proteoglycans in BALB/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis Rheum. 30, 306-318 (1987).
- Li, Z., et al. Tenascin-C-mediated suppression of extracellular matrix adhesion force promotes entheseal new bone formation through activation of Hippo signalling in ankylosing spondylitis. Ann Rheum Dis. 80 (7), 891-902 (2021).
- Glant, T. T., Cs-Szabo´, G., Nagase, H., Jacobs, J. J., Mikecz, K. Progressive polyarthritis induced in BALB/c mice by aggrecan from human osteoarthritis cartilage. Arthritis Rheum. 41, 1007-1018 (1998).
- Hu, B., Li, Y., Tan, J. Research progress of moxibustion in the treatment of ankylosing spondylitis. Rheum & Arthritis. 11 (05), 77-80 (2022).
- Yang, Y., Dong, Q., Li, R. Matrine induces the apoptosis of fibroblast-like synoviocytes derived from rats with collagen-induced arthritis by suppressing the activation of the JAK/STAT signaling pathway. Int J Mol Med. 39 (2), 307-316 (2017).
- Shiotsuki, H., et al. A rotarod test for evaluation of motor skill learning. J Neurosci Methods. 189 (2), 180-185 (2010).
- Liu, P., et al. A mouse model of ankle-subtalar joint complex instability induced post-traumatic osteoarthritis. J Orthop Surg Res. 16 (1), 541 (2021).
- Taurog, J. D., Chhabra, A., Colbert, R. A. Ankylosing spondylitis and axial spondyloarthritis. N. Engl. J. Med. 374, 2563-2574 (2016).
- Schett, G., et al. Enthesitis: From pathophysiology to treatment. Nat. Rev. Rheumatol. 13, 731-741 (2017).
- Duan, W. X., et al. Effect of different concentrations of Moxa-smoke on lung function and TNF-α and IL-1 β levels in serum and lung tissues in normal rats. Zhen Ci Yan Jiu [Acupuncture Research]. 43 (2), 98-103 (2018).
- Callhoff, J., Sieper, J., Weiß, A., Zink, A., Listing, J. Efficacy of TNFα blockers in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a meta-analysis. Ann Rheum Dis. 74 (6), 1241-1248 (2015).
- Schinocca, C., et al. Role of the IL-23/IL-17 pathway in rheumatic diseases: an overview. Front Immunol. 12, 637829 (2021).
- Gravallese, E. M., Schett, G. Effects of the IL-23-IL-17 pathway on bone in spondyloarthritis. Nat Rev Rheumatol. 14 (11), 631-640 (2018).
- Kenna, T. J., et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 64 (5), 1420-1429 (2012).
- Poddubnyy, D., Sieper, J. Treatment of axial spondyloarthritis: what does the future hold. Curr Rheumatol Rep. 22 (9), 47 (2020).
- van der, H. D., et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled. Lancet. 392 (10163), 2441-2451 (2018).
- Fu, Y. S., et al. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed Pharmacother. 141, 111888 (2021).
- Hassan, A. S., Hofni, A., Abourehab, M. A. S., Abdel-Rahman, I. A. M. Ginger extract-loaded transethosomes for effective transdermal permeation and anti-inflammation in rat model. Int J Nanomedicine. 18, 1259-1280 (2023).

