Summary
Evaluation of colonic motility in the guinea pig distal colon with the Gastrointestinal Motility Monitor (GIMM) is a straightforward and simple to learn approach to quantitatively evaluate propulsive motility in the gastrointestinal tract.
Abstract
The Gastrointestinal Motility Monitor (GIMM; Catamount Research and Development; St. Albans, VT) is an in vitro system that monitors propulsive motility in isolated segments of guinea pig distal colon. The complete system consists of a computer, video camera, illuminated organ bath, peristaltic and heated water bath circulating pumps, and custom GIMM software to record and analyze data. Compared with traditional methods of monitoring colonic peristalsis, the GIMM system allows for continuous, quantitative evaluation of motility. The guinea pig distal colon is bathed in warmed, oxygenated Krebs solution, and fecal pellets inserted in the oral end are propelled along the segment of colon at a rate of about 2 mm/sec. Movies of the fecal pellet proceeding along the segment are captured, and the GIMM software can be used track the progress of the fecal pellet. Rates of propulsive motility can be obtained for the entire segment or for any particular region of interest. In addition to analysis of bolus-induced motility patterns, spatiotemporal maps can be constructed from captured video segments to assess spontaneous motor activity patterns. Applications of this system include pharmacological evaluation of the effects of receptor agonists and antagonists on propulsive motility, as well as assessment of changes that result from pathophysiological conditions, such as inflammation or stress. The guinea pig distal colon propulsive motility assay, using the GIMM system, is straightforward and simple to learn, and it provides a reliable and reproducible method of assessing propulsive motility.
Protocol
1. Preparation of Colon Tissue for GIMM
- To prepare a segment of distal colon for the Gastrointestinal Motility Monitor (GIMM), first place the isolated colon in ice-cold Krebs solution (121 mM NaCl, 5.9 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 25 mM NaHCO3, 1.2 mM NaH2PO4, and 8 mM glucose; aerated with 95% O2/5%CO2). Clear away remaining mesentery from the outer wall and make a small incision in the oral end so it can be distinguished when placed into the organ bath. Note: tissue may remain in iced Krebs solution for up to 2 hours prior to experimentation.
- Next, position the inflow and outflow conduits in the organ bath so they are outside of the camera field to prevent interference with the image acquisition. Continuously perfuse the organ bath with prewarmed (37°C) oxygenated (95%, 5% CO2) Kreb’s solution at a flow rate of 10 ml/min.
- Keeping track of the oral vs. anal ends, pin a segment of distal colon (at least 5 cm) on either end in the organ bath, allowing a small degree of laxity so that the segment can move freely up to 1 cm in the middle. The oral end should be positioned towards the researcher for ease of placing the fecal pellet. Colonic segments should be pinned in the same manner by the same researcher for every experiment within a given set of experiments because the length and tension of the segment affects the rate of propulsive motility, with longitudinal stretch decreasing the rate of transit (Dickson et al., 2007).
- Allow the preparation to equilibrate for at least 30 min.
2. Setting up GIMM and Data Acquisition
- In the GIMM system, the colonic segment in the perfusion chamber is illuminated from beneath. A digital video camera interfaced with a computer is positioned above the chamber. Ensure that both the light illumination source and GIMM software are turned on.
- After setting up a new experiment in the GIMM software application, begin the first trial by inserting an epoxy-coated fecal pellet into the oral end of the colonic segment to initiate peristalsis. Click on the camera toggle switch on the computer to turn on the camera and click on the record button to start recording. The movement of the pellet in the anal direction is recorded by the video camera and the digital movies are stored on a PC for later analysis. When the pellet has reached the end of the colonic segment, click the record button to stop recording.
- To obtain a control value for the rate of propulsion, start with a colonic segment from a healthy animal and without applying drugs. Conduct 3-5 trials in a single preparation, with a recovery period of 5 min between each run.
- To determine the effects of certain conditions or drugs on colonic motility, perform 3-5 trials/preparation for each experimental condition. In addition, perform each experiment on at least five different colons from at least five different animals.
- In the analysis of the digital movies, the rate of fecal pellet propulsion is calculated as the time it takes for a pellet to traverse X cm of the colonic segment.
3. Construction of Spatiotemporal Maps
- The digital videos acquired from individual GIMM runs can be converted to spatiotemporal maps using the custom GIMM software.
- On the horizontal axis, changes in colonic diameter are plotted over time by converting the image of the colon in each video frame to a silhouette, and calculating and converting the diameter along the entire length into grey-scale. The end result is that the pellet and areas of relaxation appear black, while areas of contraction appear white.
- The distance traveled by the pellet through the colon segment is represented on the vertical axis.
4. Representative Spatiotemporal Maps
- Shown here are representative spatiotemporal maps showing pellet motility in colonic segments under various experimental conditions. The y (vertical) axis represents pellet position over time, while the x (horizontal) axis represents the distance that the pellet progressed through the colon segment.
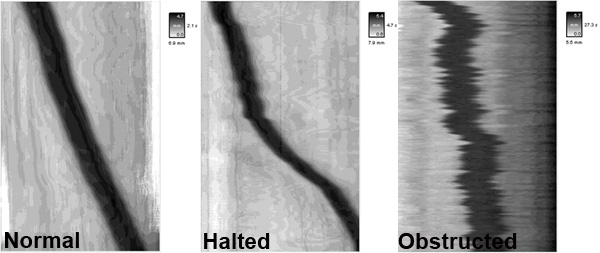
- In the spatiotemporal map from a control colon (untreated, normal), the pellet will progress linearly at a rate of ~2 mm/sec. In contrast, the administration of certain drugs or inflammation in the colon can result in disrupted motility patterns such as halted motility and obstructed motility.
- Results from GIMM can also be shown as graphs, in which the y-axis represents the distance in millimeters traveled by a fecal pellet and the x-axis represents time in seconds. For example, administration of DAMGO (D-Ala2, N-Me-Phe4, Gly-ol5), an opiate μ-receptor agonist, causes a decrease in propulsive motility in the colon.
Discussion
Nerve-mediated propulsive motility in the gastrointestinal tract was first described over one century ago by Bayliss and Starling (Bayliss and Starling, 1899). This observation led to the designation of the nerves of the gut as the enteric nervous system (ENS), a distinct division of the autonomic nervous system (Langley, 1921). Neurogenic intestinal peristalsis involves stretch and/or mucosal stimulation at a given point and reflex mediated contractions above, or oral to, the level of stimulus and relaxation in the aboral direction. The result is the generation of a pressure gradient that propels the luminal contents in an oral to aboral direction. In the small intestines of a variety of species, and in the rat large intestine, peristaltic waves of contractions can be activated by infusion of fluid into the lumen of segments of bowel. In the guinea pig distal colon, natural or artificial fecal pellets can be inserted into the oral end of the colon and their progress along the segment of colon can be easily monitored. Thus, the guinea pig distal colon provides a simple and useful assay for investigating propulsive motility in the bowel.
Peristalsis is a complex neural reflex that involves a number of neuroactive compounds and receptors. As a result, compounds that affect neurotransmission in the ENS affect the rate of propulsive motility. Serotonin, released primarily from enterochromaffin cells in the intestinal mucosa, is involved in the initiation of the peristaltic reflex. Using the guinea pig distal colon, Grider and colleagues demonstrated that propulsive motility is enhanced by intralumenal administration of 5-HT4 receptor agonists (Foxx-Orenstein et al., 1998; Grider et al., 1998), while bath of application of 5-HT3 and 5-HT4 receptor antagonists decreases the rate of propulsion (Kadowaki et al., 1996; Linden et al., 2003b). Furthermore, inhibition of the serotonin transporter with serotonin selective reuptake inhibitors (SSRIs) increases peristalsis at low concentrations, yet decreases peristalsis at higher concentrations, likely due to desensitization of 5-HT receptors (Kadowaki et al., 1996; Wade et al., 1996). Other signaling molecules that contribute to guinea pig propulsive motility include acetylcholine acting at nicotinic receptors, which is the primary mediator of interneuronal signaling in the intestines. Blocking synaptic transmission with the nicotinic receptor antagonist hexamethonium decreases pellet propulsion at high concentrations, while lesser concentrations do not affect the rate of propulsion (Kadowaki et al., 1996). Opiates, and opioid receptor agonists, which have long been known to have inhibitory effects on gastrointestinal motility, decrease pellet propulsion in the guinea pig propulsive motility model (Foxx-Orenstein et al., 1998; Wood et al., 2009). Interestingly, daikenchuto, a traditional Japanese herbal medicine used clinically to treat vomiting, stomachache, and disordered motility, decreases the rate of pellet propulsion when administered with the opioid antagonist naloxone, and when administered alone, results in reverse peristalsis (Wood et al., 2009). These findings illustrate the usefulness of the guinea pig propulsive motility model to assess the contributions of various compounds and their receptors on colonic motility patterns.
A number of studies have demonstrated that inflammation leads to changes in the electrical and synaptic properties of colonic neurons (Linden et al., 2003a; Lomax et al., 2005), and that these changes can persist for weeks following recovery from inflammation (Krauter et al., 2007; Lomax et al., 2007). The impact of colitis-induced neuroplastic changes on propulsive motility can be investigated in the guinea pig distal colon using the GIMM system. This system has several advantages over the traditional method of monitoring colonic motility, which assesses propulsive motility by simply measuring the time a fecal pellet travels a given distance. Previous studies of inflammation-induced neural plasticity in the colon have indicated there is a decrease in the velocity of pellet propulsion (Linden et al., 2003a; Linden et al., 2005), yet more recent investigations using the GIMM system have revealed more complex changes (Strong et al., 2010). For example, the rate of pellet propulsion is decreased in ulcerated regions of the inflamed distal colon, yet motility is accelerated in adjacent regions, a phenomenon that was not recognized by the earlier “stopwatch” method. Other studies have shown that dysmotility during the active phase of inflammation can be restored with inhibition of COX-2 (Linden et al., 2004), yet altered motility that persists beyond the resolution of inflammation is COX-2 insensitive (Krauter et al., 2007). Furthermore, in these later studies, spatiotemporal maps generated by the GIMM system revealed a stepwise pattern of colonic motility that was observed in post-inflammatory animals that contributes to the decreased rates of propulsion.
In conclusion, the guinea pig distal colon propulsive motility assay represents a high throughput means of measuring the effects of test compounds and pathological conditions on colonic motility, Furthermore, the GIMM system provides a simple and straightforward approach to assess colonic propulsive motility in vitro. It allows for continuous measurements of pellet propulsion, as well as the generation of spatiotemporal maps to study spontaneous activity patterns. As compared to traditional methods, it can also yield more complex phenomenon that can be quantitated and reanalyzed multiple ways as digital files are saved to the system and are easily accessible.
Disclosures
The authors have nothing to disclose.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Krebs’ Solution | ||||
| Isoflurane | Anesthesia | Webster Veterinary | ||
| Epoxy-coated fecal pellet | native guinea pig pellet dried and epoxy (black nail polish) coated | |||
| Forceps | Fine Science Tools | |||
| Micro Scissors | Fine Science Tools | |||
| Stainless Steel Pins | ||||
| Beakers | 500 mL | |||
| Gas Tank | 95% O2/5% CO2 | |||
| Timer | ||||
| Gastrointestinal Motility Monitor | Catamount Research and Development | http://www.catamountresearch.com/products/gimm.htm |
References
- Bayliss, W. M., Starling, E. H. The movements and innervation of the small intestine. J of Physiol (Lond). 24, 99-143 .
- Dickson, E. J., Spencer, N. J., Hennig, G. W., Bayguinov, P. O., Ren, J., Heredia, D. J., Smith, T. K. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology. 132, 1912-1924 (2007).
- Foxx-Orenstein, A. E., Jin, J. G., Grider, J. R. 5-HT4 receptor agonists and delta-opioid receptor antagonists act synergistically to stimulate colonic propulsion. Am J Physiol. 275, 979-983 (1998).
- Grider, J. R., Foxx-Orenstein, A. E., Jin, J. G. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 115, 370-380 (1998).
- Kadowaki, M., Wade, P. R., Gershon, Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. Am J Physiol. 271, 849-857 (1996).
- Krauter, E. M., Strong, D. S., Brooks, E. M., Linden, D. R., Sharkey, K. A., Mawe, G. M. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil. 19, 990-1000 (2007).
- Langley, J. N. . The Autonomic Nervous System. , (1921).
- Linden, D. R., Sharkey, K. A., Mawe, G. M. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J Physiol. 547, 589-601 (2003).
- Linden, D. R., Sharkey, K. A., Ho, W., Mawe, G. M. Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon. J Physiol. 557, 191-205 (2004).
- Linden, D. R., Chen, J. X., Gershon, M. D., Sharkey, K. A., Mawe, G. M. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 285, 207-216 (2003).
- Linden, D. R., Couvrette, J. M., Ciolino, A., McQuoid, C., Blaszyk, H., Sharkey, K. A., Mawe, G. M. Indiscriminate loss of myenteric neurones in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterol Motil. 17, 751-760 (2005).
- Lomax, A. E., Mawe, G. M., Sharkey, K. A. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J Physiol. 564, 863-875 (2005).
- Lomax, A. E., O’Hara, J. R., Hyland, N. P., Mawe, G. M., Sharkey, K. A. Persistent alterations to enteric neural signaling in the guinea pig colon following the resolution of colitis. Am J Physiol Gastrointest Liver Physiol. 292, 482-491 (2007).
- Strong, D. S., Cornbrooks, C. F., Roberts, J. A., Hoffman, J. M., Sharkey, K. A., Mawe, G. M. Purinergic neuromuscular transmission is selectively attenuated in ulcerated regions of inflamed guinea pig distal colon. J Physiol. 588, 847-859 (2010).
- Wade, P. R., Chen, J., Jaffe, B., Kassem, I. S., Blakely, R. D., Gershon, Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 16, 2352-2364 (1996).
- Wood, M. J., Hyman, N. H., Mawe, G. M. The Effects of Daikenchuto (DKT) on Propulsive Motility in the Colon. J Surg Res. , (2009).

