Analysis of Neural Crest Migration and Differentiation by Cross-species Transplantation
Summary
An approach for analyzing migration and eventual fate of avian neural crest cells in quail-chick chimeric embryos is described. This method is a simple and straightforward technique for tracing neural crest cells during migration and differentiation that are otherwise difficult to distinguish within an unmanipulated chick embryo.
Abstract
Avian embryos provide a unique platform for studying many vertebrate developmental processes, due to the easy access of the embryos within the egg. Chimeric avian embryos, in which quail donor tissue is transplanted into a chick embryo in ovo, combine the power of indelible genetic labeling of cell populations with the ease of manipulation presented by the avian embryo.
Quail-chick chimeras are a classical tool for tracing migratory neural crest cells (NCCs)1-3. NCCs are a transient migratory population of cells in the embryo, which originate in the dorsal region of the developing neural tube4. They undergo an epithelial to mesenchymal transition and subsequently migrate to other regions of the embryo, where they differentiate into various cell types including cartilage5-13, melanocytes11,14-20, neurons and glia21-32. NCCs are multipotent, and their ultimate fate is influenced by 1) the region of the neural tube in which they originate along the rostro-caudal axis of the embryo11,33-37, 2) signals from neighboring cells as they migrate38-44, and 3) the microenvironment of their ultimate destination within the embryo45,46. Tracing these cells from their point of origin at the neural tube, to their final position and fate within the embryo, provides important insight into the developmental processes that regulate patterning and organogenesis.
Transplantation of complementary regions of donor neural tube (homotopic grafting) or different regions of donor neural tube (heterotopic grafting) can reveal differences in pre-specification of NCCs along the rostro-caudal axis2,47. This technique can be further adapted to transplant a unilateral compartment of the neural tube, such that one side is derived from donor tissue, and the contralateral side remains unperturbed in the host embryo, yielding an internal control within the same sample2,47. It can also be adapted for transplantation of brain segments in later embryos, after HH10, when the anterior neural tube has closed47.
Here we report techniques for generating quail-chick chimeras via neural tube transplantation, which allow for tracing of migratory NCCs derived from a discrete segment of the neural tube. Species-specific labeling of the donor-derived cells with the quail-specific QCPN antibody48-56 allows the researcher to distinguish donor and host cells at the experimental end point. This technique is straightforward, inexpensive, and has many applications, including fate-mapping, cell lineage tracing, and identifying pre-patterning events along the rostro-caudal axis45. Because of the ease of access to the avian embryo, the quail-chick graft technique may be combined with other manipulations, including but not limited to lens ablation40, injection of inhibitory molecules57,58, or genetic manipulation via electroporation of expression plasmids59-61, to identify the response of particular migratory streams of NCCs to perturbations in the embryo’s developmental program. Furthermore, this grafting technique may also be used to generate other interspecific chimeric embryos such as quail-duck chimeras to study NCC contribution to craniofacial morphogenesis, or mouse-chick chimeras to combine the power of mouse genetics with the ease of manipulation of the avian embryo.62
Protocol
1. Incubate chick and quail eggs to the desired stage
For HH9 embryos, typical incubation times range from 29-33 hours at 38 °C.63
- Wash any debris from the eggs with tepid water.
- Arrange chicken eggs on tray horizontally. Mark the side facing up with pencil; this will correspond to the region where the embryo will be localized. Incubate quail eggs blunt end up.
- Place in 38 °C humidified incubator. Turn on rocking function.
2. Prepare eggs for windowing and dissection
- Remove eggs from incubator, and sterilize the tops with 70% ethanol. It is best to spray on the ethanol and wipe it off quickly with a paper towel or Kimwipe to avoid any absorption of ethanol through the eggshell.
- Place chicken egg onto an individual egg holder (we use a Petri dish lined with folded paper towels, e.g., “Kimwipes”). Using AA forceps, tap a small hole into the upper surface of the eggshell at the pointed end of the egg.
- Remove 1.5-3mL of light albumin from the chicken egg with an 18½ G hypodermic needle and 5 mL syringe. It is best to insert the needle vertically into the hole, with the bevel facing the pointy end of the egg (Figure 2A). This way, when albumin is withdrawn, there is little risk of accidentally damaging the yolk via suction. Discard the albumin. This step will lower the yolk and embryo within the egg to allow for a window to be opened in the eggshell.
- Wipe the hole with 70% ethanol as described above and seal it with Scotch tape. It is important that the eggshell surrounding the hole is completely devoid of albumin and dry or the adhesive will not seal.
- With the AA forceps, tap another hole into the marked upper surface of the horizontal egg, not quite at the apex. Take care not to let the forceps go too far through the eggshell to avoid damaging the yolk or embryo.
- Insert one side of the curved iris forceps into the hole parallel to the eggs shell. Pinching down on the shell, working in a circular motion to break a ~2 cm in diameter window in the chicken egg shell. Discard the removed eggshell. Alternatively, cover the upper surface of the egg with packing tape and use a pair of scissors to cut out the window. This minimizes the chance of eggshell debris falling into the egg. The embryo should appear as an opaque disc on top of the yolk. Discard any unfertilized eggs (identified by small white spot on surface of yolk, or absence of blastoderm).
- Inject a small amount of India ink (diluted 1:10 in sterile Ringer’s solution containing “Pen/Strep” antibiotic: final concentration 100 μg/mL penicillin and 100 μg/mL streptomycin) beneath the blastoderm to assist with staging the embryo. Use a 1 mL syringe and 26½ G hypodermic needle, bent into a 45° angle at the base of the needle, with the bevel facing up; alternatively, one may use a pulled glass Pasteur pipet and mouth pipetting apparatus (wear eye protection when bending hypodermic needle or breaking glass needle). Puncture the yolk membrane outside the perimeter of the blastoderm and slide the tip of the needle beneath the embryo, close to the surface of the yolk but not in the embryonic layers (Figure 2B). Inject just enough ink to outline the embryo, then carefully withdraw the needle (Figure 2C). Injecting too much ink can lead to embryo death. It is important not to inject any air bubbles beneath the embryo, as this can be a source for contamination. NB: All Ringer’s solution used in this procedure is sterilized and contains Pen/Strep as defined above. Sterile phosphate buffered saline (PBS) is also an acceptable alternative to Ringer’s solution in all steps of this protocol.
- Stage the embryo according to Hamburger and Hamilton63 and record the stage on the eggshell in permanent ink or pencil. Stages are best assessed under stereomicroscopy, with fiber optic “gooseneck” light sources which have a limited heat load.
- Apply 2-3 drops warm sterile Ringer’s solution to the surface of the embryo to prevent dehydration and contamination. Temporarily seal the window with parafilm stretched over the surface of the egg.
- Repeat steps 2.2-2.9 with all chick eggs before beginning the transplant experiments.
- Since quail embryos are only used for donor tissues, in ovo steps 2.2-2.9 may be omitted. For ex ovo preparation of donor embryos, quail eggs are incubated blunt side up and opened in this region with curved forceps to reveal the embryo. Using dissecting scissors, make four cuts in the shape of a square through the vitelline membrane and blastoderm outside the embryo (make sure that all cuts meet). Using a curved iris forceps, gently grasp one cut edge and lift the embryo from the egg and transfer it to Ringers solution in a Petri dish. Alternatively, one may use the curved iris forceps to lift the excised embryo from beneath, or an embryo spoon or plastic transfer pipette cut through the wider part of the cylinder, may be used to transfer the embryo out of the egg. Gently remove the vitelline membrane with #5 forceps. Collect the remaining quail embryos as above, and stage them under a dissecting microscope.
3. Prepare the host embryo to receive the graft
- Remove parafilm from the host (chick) embryo and using a sharpened tungsten needle or #5 forceps, tear a small hole in the vitelline membrane at the desired region of the neural tube (Figure 3A).
- Add a drop of Ringer’s solution to the embryo. Take care to keep the embryo completely submerged in Ringer’s solution during the entire procedure to prevent dehydration of the tissue. Keep adding drops of Ringer’s solution to the surface of the embryo via transfer pipette if necessary.
- Using a pulled glass needle, carefully make rostral and caudal transverse incisions corresponding to the length and region of interest in the dorsal neural tube. (Pulled glass needles are generated from silicon glass capillary tubes which have been pulled under heat with a needle pulling apparatus.) For unilateral grafts the incisions should only extend to the lumen of the dorsal neural tube, and for bilateral grafts, make the incisions across the entire dorsal neural tube. Then cut bilaterally between the dorsal neural tube and the paraxial mesoderm.
- Carefully separate the excised explant from the neural tube then remove it from the embryo by aspirating into a glass micropipette (Figure 3B).
4. Prepare the donor graft tissue
- Choose a stage-matched quail embryo. Hold the embryo down with AA forceps, remove the vitelline membrane, and excise a similar-sized region of neural tube as described above (3.3-3.4) (Figure 3A’-B’). Depending on the needs of your experiment, this region may be a complementary region of the host (chick) neural tube or from a different region along the rostro-caudal axis. (NB: for larger regional grafts, including complete neural tube grafts, researchers may wish to add a protease digestion at this step to remove any adherent mesenchymal tissue from the donor tissue prior to transplantation. However, for small dorsal neural tube grafts at early stages, as is detailed here, protease digestion is not necessary as the neural tube is easily separated from adjacent mesenchyme by strictly surgical techniques.)
5. Graft the tissue
- Aspirate the donor explant into a glass micropipette containing Ringer’s solution. Be careful not to introduce any bubbles into the pipette.
- Transfer the explant adjacent to the excised region of the chick host. Using pulled glass needle, orient the explant and gently guide it into the ablated region (Figure 3C).
- Carefully add a few drops of Ringer’s solution to the embryo to prevent dehydration. Take care to avoid dropping liquid directly on the graft site as this may dislodge the graft.
- Seal the window with packing tape. Make sure that the tape seals the entire region of the windowed egg. This prevents dehydration and contamination of the embryo during subsequent incubation.
- Return the egg to the incubator until the desired stage. Make sure that the “rocking” function is turned off while incubating the chimeras. Eggs may be gently turned by hand two times a day as this may increase their viability if later stages of development are targeted.
6. Prepare chimeric embryos for sectioning
- At the experimental endpoint, cut away the tape covering the window using fine scissors.
- Grasp the edge of the blastoderm with curved iris forceps (easiest to do with serrated tips), then cut the embryo away from the yolk with 4 large cuts outside the boundaries of the blastoderm.
- Transfer the embryo to a Petri dish containing Ringer’s solution. Take care to minimize exposure of the embryo to air in order to avoid dehydration of the tissue.
- Under stereomicroscopy, gently separate the vitelline membrane from the blastoderm using #5 forceps.
- Carefully arrange the embryo in the dish so that the embryo is in the same orientation as it was in the egg, with surrounding membranes laid out flat.
- Use dissecting needles made from tungsten wires64 to cleanly remove the surrounding blastoderm from the embryo by arranging the needle at the boundary of the embryo and extraembryonic tissues, with the length of the tungsten wire parallel to the bottom of the dish. Use a gentle sawing motion to cut through the tissues.
- Carefully remove the amnion with #5 forceps. At this point you may, wish to either further dissect the region of your interest, or leave the embryo whole.
- Transfer the embryo to ice cold 4% paraformaldehyde and rock overnight at 4 °C.
- Prepare samples and embed for cryo- or paraffin sectioning.
Trace the grafted tissue within the host embryo. There are several techniques for identifying quail tissue within a chick embryo, including detection of quail nucleoli by hematoxylin staining (quail nuclei have very large, darker staining inclusions than chick nuclei), the Feulgen-Rossenbeck reaction, acridine-orange or biz-benzamide stain combined with electron microscopy, or immunolabeling for quail-specific antigens3,47,65,66. Here we use QCPN antigen and standard whole-mount or section immunofluorescence techniques to identify quail neural crest cells in quail-chick chimeras (Figure 4). This technique provides the most flexibility in experimental design, as the QCPN immunofluorescence may also be combined with other antibodies to identify differentiated cells derived from the donor (quail) tissue. Use standard whole-mount or section immunofluorescence techniques to label quail-derived cells in chimeras.
7. Representative Results
A representative image of the grafted region of the neural tube after 6h of re-incubation (to HH11) shows expected incorporation of the grafted donor (quail) tissue into the host (chick) neural tube (Figure 4A). Embryos showing incomplete integration of the graft, or asymmetric development of the cranial region or somites after reincubation should be discarded.
Cross section through the grafted region at HH11 show NCCs labeled with HNK-1 migrating laterally away from the neural tube. Quail cells contributing to the NCC migratory stream, and to the neural tube, are clearly labeled with QCPN (Figure 4B).
At later stages, quail NCC-derived cells can be traced to their final target tissue. QCPN labeled cells are interspersed within the chick embryo mesenchyme of the maxillary process at E5 (Figure 4C).
The QCPN antibody can be easily combined with other antibodies to examine the differentiation of quail-derived NCCs in the host environment. Quail NCC-derived trigeminal sensory neurons are labeled by QCPN and Tuj1 antibodies (Figure 4D).
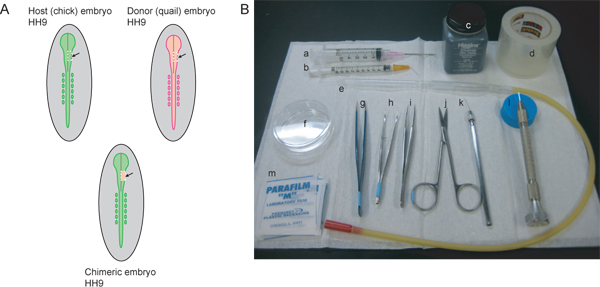
Figure 1. Overview of experimental procedure and necessary instruments. A) Host (chick) and donor (quail) embryos should be stage matched. To label the NCC stream that contributes to the trigeminal ganglion and maxillo-mandibular region, HH9 is ideal. At HH9, the neural tube is beginning to close in the rostral region, but is not yet completely sealed. The gray line in the diagrams represents the midline of the closing neural tube. The dotted white lines indicate the cut lines for excising a midbrain region of the right side of the neural tube from host and donor embryos. The excised region of the host embryo is discarded and the donor tissue transplanted in to generate the chimeric embryo. B) Necessary instruments include: a) 5mL syringe with 18½ G hypodermic needle, b) 1 mL syringe with 26½ G hypodermic needle bent at 45° angle, c) India ink, d) clear packing tape, e) glass pipette with mouth pipetting apparatus, f) 60 mm-Petri dish, g) AA forceps, h) curved iris forceps with serrated tips, i) #5 forceps, j) fine scissors, k) sharpened tungsten wire, l) pulled glass needle, m) parafilm squares.
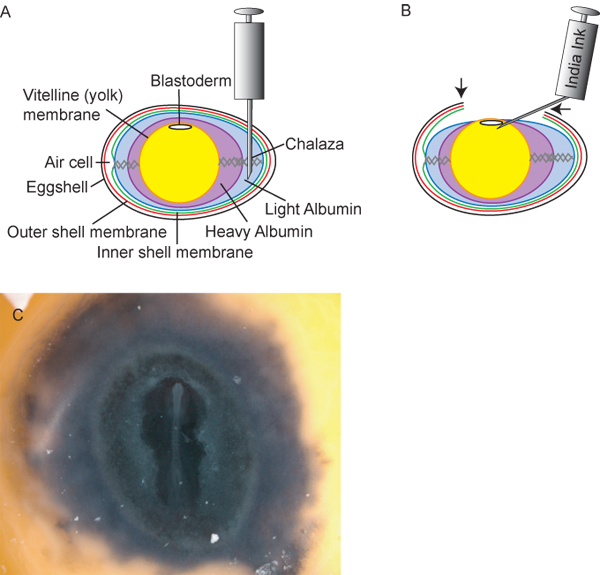
Figure 2. Preparation of eggs and embryos. A) Cross-sectional diagram of the egg, including ideal insertion point of 18½ G hypodermic needle for withdrawal of light albumin. B) After withdrawing ~3mL of light albumin, the yolk and embryo lowers within the egg, allowing the researchers to cut a “window” (arrows) in the eggshell to access the embryo. India ink, diluted 1:10 in sterile Ringer’s solution, can then be injected beneath the blastoderm to provide contrast for easy staging of the embryos. C) Chick embryo after inking.
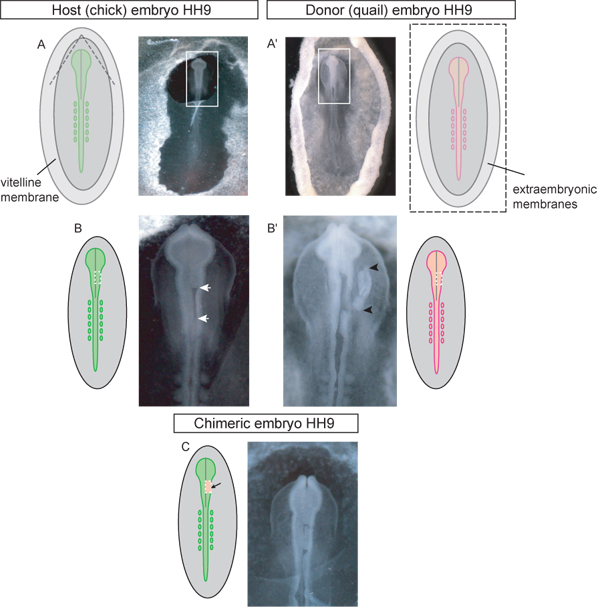
Figure 3. Schematic and examples of HH stage 9 donor and host embryos at each step of the grafting procedure. A) Chick host embryo. Dashed lines in diagram indicate where the vitelline membrane should be torn to access the cranial region for grafting. Once torn the triangular flap of vitelline membrane should be peeled caudally. Box in image indicates inset used in (B-C). A’) Quail donor embryo. Image is of donor embryo excised from egg and placed into Petri dish for dissection of the graft tissue. Box in image indicates inset used in (B’). Dashed lines in schematic diagram (A’) indicate where the vitelline and yolk membranes should be cut in order to remove the embryo from the egg. B) Host embryo with unilateral midbrain region of neural tube excised (white arrows) awaiting graft. Dotted line in schematic diagram indicates where cuts should be made to excise the neural tube in the midbrain region. B’) Donor embryo with dorsal neural tube graft tissue excised (graft tissue indicated by black arrowheads). Dotted line in diagram indicates where cuts should be made in the quail embryo to excise the donor tissue for unilateral grafting of the midbrain region of the neural tube. C) Chimeric embryo after grafting of quail dorsal neural tube explant into the chick midbrain region.
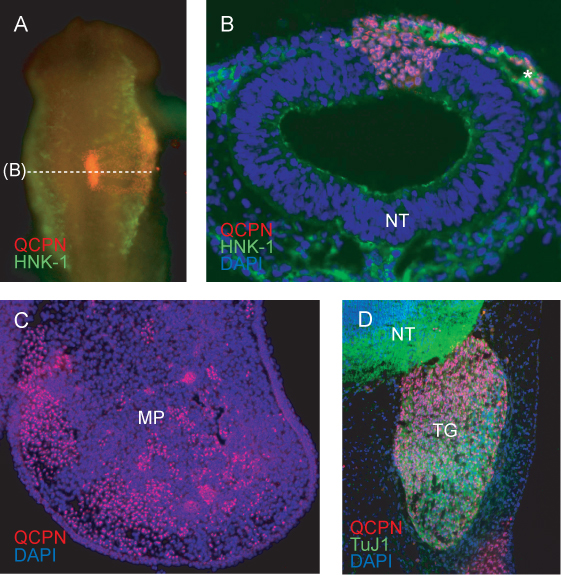
Figure 4. Immunofluorescence detecting quail-specific nuclear antigen QCPN in donor-derived cells in the chimeric embryo. A) Whole-mount image of HH11 chimera (grafted at HH9) showing QCPN staining in red, and HNK-1 staining (a marker of migrating NCCs) in green. 10X magnification, dorsal view. B) Cross section through embryo in (A), showing QCPN-positive quail derived cells in the neural tube and migratory NCC co-stained with HNK-1 (green). 40X magnification. C) Cross-section through maxillary process of E5 chimera (incubated for 3 days post graft), showing QCPN-positive NCC-derived cells (red) which have migrated from the grafted region of the neural tube to their final location in the embryo. 10X magnification. D) Section through E5 chimera showing contribution of quail derived NCC to the trigeminal ganglion (red) and differentiation into TuJ1-positive neurons (green). 40X magnification. *, NCCs; MP, maxillary process; NT, neural tube; TG, trigeminal ganglion.
Discussion
The grafting of quail neural tube into host chick embryos described here is a straightforward and inexpensive technique for tracing specific subpopulations of migrating NCCs emanating from different regions along the rostro-caudal axis21,67-69. This technique takes advantage of the ease of access to avian embryos (as compared to mammalian embryos) and may be combined with other techniques, such as tissue ablation, injection of inhibitory molecules, or genetic manipulation via electroporation of expression plasmids, to experimentally examine the response of specific migratory NCC populations to different developmental cues within the embryos47,69.
Quail-chick chimeras are a powerful tool for examining NCC migration and differentiation, and this technique may be adapted to include transplantation of other tissues besides the neural tube. Grafting of quail tissue into the chick embryo is a well-established technique13,21,70-76, but is best learned by visual demonstration, as the microdissection of small regions of neural tubes require very fine motor skills.
Because quail-derived cells can be easily distinguished from host chick cells via immunostaining with the quail-specific antibody QCPN, the developmental potential of NCCs arising from specific regions of the neural tube can be inferred from differentiation of donor (quail) cells at the experimental end point; for instance, the presence of QCPN positive cells in the periocular region and cornea, after grafting quail neural tube into the midbrain region of chick embryos at HH9 indicates that migratory neural crest from this region give rise to corneal endothelium and stroma77. Neural tube grafts at HH9-10 reveal NCC contribution to the trigeminal ganglion and branchial arches. Also, quail-derived sensory neurons can be tracked using another quail-neuron specific (QN) antibody78,79.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank members of the Lwigale laboratory for critique of the manuscript. SLG is supported by a Ruth L. Kirschstein NRSA Fellowship from the National Eye Institute (F32 EY02167301). PYL is supported by the National Eye Institute (EY018050).
Materials
| Reagent | Company | Catalog number |
| Chick eggs | Various – we use Texas A&M University’s Poultry Science Department, TX. | |
| Quail eggs | Various – we use Ozarks Egg Company, MO. | |
| Egg incubator (Digital Readout 1502 Sportsman Incubator w/Humidity 110-120 Volt AC) | www.poultrysupply.com | 1502 |
| Dumont AA forceps, Inox Epoxy-coated | Fine Science Tools | 11210-10 |
| Scotch tape | Any office supply store | |
| Curved Iris forceps | Fine Science Tools | 11065-07 |
| India ink | Any art supply store | |
| Pen/Strep (Penicillin, Streptomycin) Solution | VWR International | 101447-068 |
| Clear Packing tape | Any office supply store | |
| Needle pulling apparatus | Narashige, Japan | PE-21 |
| Pulled glass needle, made from 1.5-1.8 x 100mm borosilicate glass capillary tube | Kimble chase | 34500 99 |
| Pulled glass pipette, made from 5¾” Pasteur pipette | Fisher Scientific | 13-678-6A |
| Mouth pipette apparatus (aspirator tube assembly for calibrated microcapillary pipette) | Sigma-Aldrich | A5177-52A |
| Dumont #5 forceps | Fine Science Tools | 11251-30 |
| Tungsten wire, 0.1mm diameter | VWR International | AA10404-H2 |
| Needle holders (Nickel-plated pin holder) | Fine Science Tools | 26018-17 |
| QCPN antiserum | Developmental Studies Hybridoma Bank, University of Iowa | QCPN |
| Alexa Fluor secondary antibody (e.g., Alexa Fluor 594 goat anti-mouse IgG1) | Invitrogen | A21125 |
Ringer’s Solution (2L):
|
All reagents from Fisher Scientific |
|
References
- Le Douarin, G., Renaud, D. Morphologic and physiologic study of the differentiation in vitro of quail embryo precardial mesoderm. Bull. Biol. Fr. Belg. 103 (3), 453-468 (1969).
- Teillet, M. A., Ziller, C., Le Douarin, N. M. Quail-chick chimeras. Methods. Mol. Biol. 461, 337-350 (2008).
- Le Douarin, N. A biological cell labeling technique and its use in expermental embryology. Dev. Biol. 30 (1), 217-222 (1973).
- Noden, D. M. An analysis of migratory behavior of avian cephalic neural crest cells. Dev. Biol. 42 (1), 106-130 (1975).
- Johnston, M. C. A radioautographic study of the migration and fate of cranial neural crest cells in the chick embryo. Anat. Rec. 156 (2), 143-155 (1966).
- Noden, D. M. The control of avian cephalic neural crest cytodifferentiation. I. Skeletal and connective tissues. Dev. Biol. 67 (2), 296-312 (1978).
- Oka, K. The role of TGF-beta signaling in regulating chondrogenesis and osteogenesis during mandibular development. Dev. Biol. 303 (1), 391-404 (2007).
- Chai, Y. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 127 (8), 1671-1679 (2000).
- Lengele, B., Schowing, J., Dhem, A. Embryonic origin and fate of chondroid tissue and secondary cartilages in the avian skull. Anat. Rec. 246 (3), 377-393 (1996).
- Le Douarin, N. M., Ziller, C., Couly, G. F. Patterning of neural crest derivatives in the avian embryo: in vivo and in vitro studies. Dev. Biol. 159 (1), 24-49 (1993).
- Lallier, T. E. Cell lineage and cell migration in the neural crest. Ann. N.Y. Acad. Sci. 615, 158-171 (1991).
- Nakamura, H. Mesenchymal derivatives from the neural crest. Arch. Histol. Jpn. 45 (2), 127-138 (1982).
- Le Lievre, C. S., Le Douarin, N. M. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J. Embryol. Exp. Morphol. 34 (1), 125-154 (1975).
- Rawles, M. E. The Development of Melanophores from Embryonic Mouse Tissues Grown in the Coelom of Chick Embryos. Proc. Natl. Acad. Sci. U.S.A. 26 (12), 673-680 (1940).
- Rawles, M. E. The Pigment-Forming Potency of Early Chick Blastoderms. Proc. Natl. Acad. Sci. U.S.A. 26 (1), 86-94 (1940).
- Mosher, J. T. Intrinsic differences among spatially distinct neural crest stem cells in terms of migratory properties, fate determination, and ability to colonize the enteric nervous system. Dev. Biol. 303 (1), 1-15 (2007).
- Dupin, E., Le Douarin, N. M. Development of melanocyte precursors from the vertebrate neural crest. Oncogene. 22 (20), 3016-3023 (2003).
- Faraco, C. D., Vaz, S. A., Pastor, M. V., Erickson, C. A. Hyperpigmentation in the Silkie fowl correlates with abnormal migration of fate-restricted melanoblasts and loss of environmental barrier molecules. Dev. Dyn. 220 (3), 212-225 (2001).
- Selleck, M. A., Bronner-Fraser, M. Avian neural crest cell fate decisions: a diffusible signal mediates induction of neural crest by the ectoderm. Int. J. Dev. Neurosci. 18 (7), 621-627 (2000).
- Stocker, K. M., Sherman, L., Rees, S., Ciment, G. Basic FGF and TGF-beta 1 influence commitment to melanogenesis in neural crest-derived cells of avian embryos. Development. 111 (2), 635-645 (1991).
- Le Douarin, N. M., Teillet, M. A. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev. Biol. 41 (1), 162-184 (1974).
- Noden, D. M. The control of avian cephalic neural crest cytodifferentiation. II. Neural tissues. Dev. Biol. 67 (2), 313-329 (1978).
- Barraud, P. Neural crest origin of olfactory ensheathing glia. Proc. Natl. Acad. Sci. U.S.A. 107 (49), 21040-21045 (2010).
- Li, H. Y., Say, E. H., Zhou, X. F. Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells. 25 (8), 2053-2065 (2007).
- Carney, T. J. A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development. 133 (23), 4619-4630 (2006).
- Maro, G. S. Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat. Neurosci. 7 (9), 930-938 (2004).
- Bronner-Fraser, M. Molecular analysis of neural crest formation. J. Physiol. Paris. 96 (1-2), 3-8 (2002).
- Paratore, C., Goerich, D. E., Suter, U., Wegner, M., Sommer, L. Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development. 128 (20), 3949-3961 (2001).
- Britsch, S. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 15 (1), 66-78 (2001).
- Bronner-Fraser, M. Origin of the avian neural crest. Stem Cells. 13 (6), 640-646 (1995).
- Jessen, K. R., Mirsky, R. Neural development. Fate diverted. Curr. Biol. 4 (9), 824-827 (1994).
- Le Douarin, N., Dulac, C., Dupin, E., Cameron-Curry, P. Glial cell lineages in the neural crest. Glia. 4 (2), 175-184 (1991).
- Chan, W. Y., Cheung, C. S., Yung, K. M., Copp, A. J. Cardiac neural crest of the mouse embryo: axial level of origin, migratory pathway and cell autonomy of the splotch (Sp2H) mutant effect. Development. 131 (14), 3367-3379 (2004).
- Bronner-Fraser, M. Segregation of cell lineage in the neural crest. Curr. Opin. Genet. Dev. 3 (4), 641-647 (1993).
- Peters-vander Sanden, M. J., Luider, T. M., vander Kamp, A. W., Tibboel, D., Meijers, C. Regional differences between various axial segments of the avian neural crest regarding the formation of enteric ganglia. Differentiation. 53 (1), 17-24 (1993).
- Kuratani, S., Bockman, D. E. Capacity of neural crest cells from various axial levels to participate in thymic development. Cell Tissue Res. 263 (1), 99-105 (1991).
- Leblanc, G. G., Epstein, M. L., Bronner-Fraser, M. E. Differential development of cholinergic neurons from cranial and trunk neural crest cells in vitro. 137 (2), 318-330 (1990).
- Golding, J. P., Trainor, P., Krumlauf, R., Gassmann, M. Defects in pathfinding by cranial neural crest cells in mice lacking the neuregulin receptor ErbB4. Nat. Cell. Biol. 2 (2), 103-109 (2000).
- Kulesa, P. M., Bailey, C. M., Kasemeier-Kulesa, J. C., McLennan, R. Cranial neural crest migration: new rules for an old road. Dev. Biol. 344 (2), 543-554 (2009).
- Lwigale, P. Y., Bronner-Fraser, M. Semaphorin3A/neuropilin-1 signaling acts as a molecular switch regulating neural crest migration during cornea development. Dev. Biol. 336 (2), 257-265 (2009).
- Killian, O. l. e. s. n. i. c. k. y., Birkholz, E. C., A, D., Artinger, K. B. A role for chemokine signaling in neural crest cell migration and craniofacial. Dev. Biol. 333 (1), 161-172 (2009).
- Gammill, L. S., Gonzalez, C., Bronner-Fraser, M. Neuropilin 2/semaphorin 3F signaling is essential for cranial neural crest migration and trigeminal ganglion condensation. Dev. Neurobiol. 67 (1), 47-56 (2007).
- Osborne, N. J., Begbie, J., Chilton, J. K., Schmidt, H., Eickholt, B. J. Semaphorin/neuropilin signaling influences the positioning of migratory neural crest cells within the hindbrain region of the chick. Dev. Dyn. 232 (4), 939-949 (2005).
- Kanzler, B., Foreman, R. K., Labosky, P. A., Mallo, M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development. 127 (5), 1095-1104 (2000).
- Garcia-Lopez, R., Pombero, A., Martinez, S. Fate map of the chick embryo neural tube. Dev. Growth Differ. 51 (3), 145-165 (2009).
- Goldstein, A. M., Nagy, N. A bird’s eye view of enteric nervous system development: lessons from the avian embryo. Pediatr. Res. 64 (4), 326-333 (2008).
- Le Douarin, N., Dieterlen-Lievre, F., Creuzet, S., Teillet, M. A. Quail-chick transplantations. Methods Cell. Biol. 87, 19-58 (2008).
- Wingate, R. J., Lumsden, A. Persistence of rhombomeric organisation in the postsegmental hindbrain. Development. 122 (7), 2143-2152 (1996).
- Karagenc, L., Sandikci, M. Tissue distribution of cells derived from the area opaca in heterospecific quail-chick blastodermal chimeras. J. Anat. 216 (1), 16-22 (2010).
- Teague, W. J., Jayanthi, N. V., Lear, P. V., Johnson, P. R. Foregut mesenchyme contributes cells to pancreatic acini during embryonic development in a chick-quail chimera model. Pediatr. Surg. Int. 21 (3), 138-142 (2005).
- Borue, X., Noden, D. M. Normal and aberrant craniofacial myogenesis by grafted trunk somitic and segmental plate mesoderm. Development. 131 (16), 3967-3980 (2004).
- He, L. Three different fates of cells migrating from somites into the limb bud. Anat. Embryol. (Berl). 207 (1), 29-34 (2003).
- Huang, R., Zhi, Q., Christ, B. The relationship between limb muscle and endothelial cells migrating from single somite. Anat. Embryol. (Berl). 206 (4), 283-289 (2003).
- Hidalgo-Sanchez, M., Simeone, A., Alvarado-Mallart, R. M. Fgf8 and Gbx2 induction concomitant with Otx2 repression is correlated with midbrain-hindbrain fate of caudal prosencephalon. Development. 126 (14), 3191-3203 (1999).
- Verberne, M. E., Gittenberger-de Groot, A. C., Poelmann, R. E. Lineage and development of the parasympathetic nervous system of the embryonic chick heart. Anat. Embryol. (Berl). 198 (3), 171-184 (1998).
- Burns, A. J., Douarin, N. M. The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 125 (21), 4335-4347 (1998).
- Debby-Brafman, A., Burstyn-Cohen, T., Klar, A., Kalcheim, C. F-Spondin, expressed in somite regions avoided by neural crest cells, mediates inhibition of distinct somite domains to neural crest migration. Neuron. 22 (3), 475-488 (1999).
- Lwigale, P. Y., Bronner-Fraser, M. Lens-derived Semaphorin3A regulates sensory innervation of the cornea. Dev. Biol. 306 (2), 750-759 (2007).
- Nakamura, H., Funahashi, J. Introduction of DNA into chick embryos by in ovo electroporation. Methods. 24 (1), 43-48 (2001).
- Chen, Y. X., Krull, C. E., Reneker, L. W. Targeted gene expression in the chicken eye by in ovo electroporation. Mol. Vis. 10, 874-883 (2004).
- Sato, F., Nakagawa, T., Ito, M., Kitagawa, Y., Hattori, M. A. Application of RNA interference to chicken embryos using small interfering RNA. J. Exp. Zool. A. Comp. Exp. Biol. 301 (10), 820-827 (2004).
- Lwigale, P. Y., Schneider, R. A. Other chimeras: quail-duck and mouse-chick. Methods Cell. Biol. 87, 59-74 (2008).
- Hamburger, V., Hamilton, H. L. A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 195 (4), 231-272 (1992).
- Brady, J. A simple technique for making very fine, durable dissecting needles by sharpening tungsten wire electrolytically. Bull. World Health Organ. 32 (1), 143-144 (1965).
- Le Douarin, N. M. A Feulgen-positive nucleolus. Exp. Cell. Res. 77 (1), 459-468 (1973).
- Feulgen, R., Rossenbeck, H. Mikroskopisch-chemischer Nachweis einer Nucleinsaure vom typus der Thymonucleinsiiure und die darauf beruhende elektive Faibung von Zellkemen in mikroskopischen Praparaten. Hoppe-Seyler’s Z. Physiol. Chem. 135, 203-252 (1924).
- Lwigale, P. Y., Conrad, G. W., Bronner-Fraser, M. Graded potential of neural crest to form cornea, sensory neurons and cartilage along the rostrocaudal axis. Development. 131 (9), 1979-1991 (2004).
- Weston, J. A. A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev. Biol. 6, 279-310 (1963).
- Le Douarin, N. M., Kalcheim, C. . The Neural Crest. , (2009).
- Le Douarin, N. M., Teillet, M. A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J. Embryol. Exp. Morphol. 30 (1), 31-48 (1973).
- Douarin, N. M. L. e., Jotereau, F. V. Tracing of cells of the avian thymus through embryonic life in interspecific chimeras. J. Exp. Med. 142 (1), 17-40 (1975).
- Le Douarin, N. M., Renaud, D., Teillet, M. A., Le Douarin, G. H. Cholinergic differentiation of presumptive adrenergic neuroblasts in interspecific chimeras after heterotopic transplantations. Proc. Natl. Acad. Sci. U. S. A. 72 (2), 728-732 (1975).
- Houssaint, E., Belo, M., Le Douarin, N. M. Investigations on cell lineage and tissue interactions in the developing bursa of Fabricius through interspecific chimeras. Dev. Biol. 53 (2), 250-264 (1976).
- Le Douarin, N. M., Jotereau, F. V., Houssaint, E., Belo, M. Ontogeny of the avian thymus and bursa of Fabricius studied in interspecific chimeras. Ann. Immunol. (Paris). 127 (6), 849-856 (1976).
- Fontaine, J., Le Douarin, N. M. Analysis of endoderm formation in the avian blastoderm by the use of quail-chick chimaeras. The problem of the neurectodermal origin of the cells of the APUD series. J. Embryol. Exp. Morphol. 41, 209-222 (1977).
- Narayanan, C. H., Narayanan, Y. On the origin of the ciliary ganglion in birds studied by the method of interspecific transplantation of embryonic brain regions between quail and chick. J. Embryol. Exp. Morphol. 47, 137-148 (1978).
- Lwigale, P. Y., Cressy, P. A., Bronner-Fraser, M. Corneal keratocytes retain neural crest progenitor cell properties. Dev. Biol. 288 (1), 284-293 (2005).
- Lwigale, P. Y. Embryonic origin of avian corneal sensory nerves. Dev. Biol. 239 (2), 323-337 (2001).
- Tanaka, H., Kinutani, M., Agata, A., Takashima, Y., Obata, K. Pathfinding during spinal tract formation in the chick-quail chimera analysed by species-specific monoclonal antibodies. Development. 110 (2), 565-571 (1990).

