Purification of the Membrane Compartment for Endoplasmic Reticulum-associated Degradation of Exogenous Antigens in Cross-presentation
Summary
The method described here is a new vesicle isolation protocol, which allows for the purification of the cellular compartments where exogenous antigens are processed by endoplasmic reticulum-associated degradation in cross-presentation.
Abstract
Dendritic cells (DCs) are highly capable of processing and presenting internalized exogenous antigens upon major histocompatibility class (MHC) I molecules also known as cross-presentation (CP). CP plays an important role not only in the stimulation of naïve CD8+ T cells and memory CD8+ T cells for infectious and tumor immunity but also in the inactivation of self-acting naïve T cells by T cell anergy or T cell deletion. Although the critical molecular mechanism of CP remains to be elucidated, accumulating evidence indicates that exogenous antigens are processed through endoplasmic reticulum-associated degradation (ERAD) after export from non-classical endocytic compartments. Until recently, characterizations of these endocytic compartments were limited because there were no specific molecular markers other than exogenous antigens. The method described here is a new vesicle isolation protocol, which allows for the purification of these endocytic compartments. Using this purified microsome, we reconstituted the ERAD-like transport, ubiquitination, and processing of the exogenous antigen in vitro, suggesting that the ubiquitin-proteasome system processed the exogenous antigen after export from this cellular compartment. This protocol can be further applied to other cell types to clarify the molecular mechanism of CP.
Introduction
The MHC I molecules are expressed on the surface of all nucleated cells, with short antigenic peptides derived from endogenous antigens, which are processed by the ubiquitin-proteasome system in the cytosol1. After processing, antigenic peptides are transported into the endoplasmic reticulum (ER) lumen by the peptide transporter TAP. In the ER lumen, a series of specific chaperones assist the peptide loading and the correct folding of the MHC I complex. This series of molecules is called the peptide-loading complex (PLC), indicating that the ER is a central compartment for peptide loading upon MHC I2. After peptide loading, the MHC I molecules are transported to the cell surface and play a key role in the adaptive immune system as self-markers, and enables the CD8+ cytotoxic T lymphocytes (CTLs) to detect cancer cells or infectious agents by antigenic peptides from non-self proteins3.
In antigen presenting cells (APC), antigenic peptides from exogenous antigens are also presented upon MHC I4,5,6,7,8 via CP, which is mainly carried out by DCs9,10,11. CP is essential both for the activation of naïve CD8+ T cells and memory CD8+ T cells into anti-infectious and anti-tumoral CTLs12,13, and in the maintenance of immune tolerance by the inactivating of self-acting naïve T cells14,15. The CP plays many important roles in the adaptive immune system, however the molecular mechanisms of CP have yet to be described in detail. Previous studies of CP revealed that exogenous antigens were localized both in the ER and the endosome and were processed by ERAD, suggesting that exogenous antigens are transported from the endosome to the ER for ERAD-like processing and peptide loading16. However, accumulating evidence indicates that the peptide loading of CP is carried out not in the ER but rather in non-classical endocytic compartments, which also have distinctive features of the ER (Figure 1)17,18,19,20,21. To avoid degradation of the antigenic peptide precursors by the high activity of aminopeptidase22 in the cytosol, processing and peptide loading in CP occurs in the proximal area of these non-classical endocytic compartments (Figure 1). Though the characterizations of these endocytic compartments are controversial, there are no existing specific molecules other than exogenous antigens in this compartment.
ERAD is a cellular pathway, which specifically removes misfolded proteins from the ER. In the ERAD pathway, misfolded proteins are retrogradely transported through the ER membrane to the cytoplasm and processed by the ubiquitin-proteasome system23,24,25. When large molecules, such as proteins, are transported through the lipid bilayer, these molecules pass through a molecular apparatus called a translocon, such as the Sec61 complex and Derlin complex in the ER26, and the Tom complex and Tim complex in the mitochondria27. When exogenously-added antigens are transported through the ER membrane, they must penetrate the lipid bilayer in complex with translocons, such as the Sec61 complex. The method described here purified the targeted vesicle by utilizing these membrane-penetrating molecules as markers for the endocytic compartments.
The method described here is a new vesicle purification protocol using the DC-like cell line DC2.428 and biotinylated ovalbumin (bOVA) as an exogenous antigen. The endocytic compartments were purified by streptavidin (SA)-magnetic beads using the membrane-penetrating bOVA as a maker. In this purified microsome, some exogenously added bOVA was still preserved in membrane fractions but were transported to the outside of microsome, and then ubiquitinated and processed in vitro29. This purified microsome contained not only endocytic compartment-specific proteins but also ER-resident proteins for ERAD and the peptide loading complex; suggesting that the cellular compartment is the prospective endocytic compartment for CP29. This protocol is not dependent on the kind of exogenous antigens, and is also applicable for other DC subsets and other cell types, such as macrophages, B cells, and endothelial cells, to clarify the precise molecular mechanism of DCs for proficient CP.
Protocol
1. Growing Cells and Addition of Exogenous Antigens
- Prepare bOVA using a biotin-protein labeling kit following the manufacturer's protocol.
NOTE: Ordinarily, bOVA contains 2 M biotin per 1 M OVA on average. - Grow DC2.4 cells in RPMI-1640 supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 U/mL penicillin-streptomycin, 55 mM 2-mercaptoethanol, 10 mM HEPES (pH 7.5), and 10% fetal calf serum (hereafter RPMI) at 37 °C in 5% CO2 in a humidified incubator (hereafter without mention, cells are incubated at this condition). It is also possible to use DMEM supplemented with 10% fetal calf serum, 3.7 g/L NaHCO3 (hereafter DMEM) in place of RPMI.
- A day before purification, split the cells into RPMI to 1 x 105 cells/mL in a tissue culture plate. Avoid keeping the cells at a confluent state. DC2.4 cells can highly incorporate exogenous antigens until a semi-confluent state, but rapidly lose the ability after reaching an over-confluent state.
- Before the DC2.4 cells are semi-confluent, add 250 µg/mL of bOVA for 1 x 106 cells/mL and incubate for 2 – 4 h.
NOTE: During downstream experiments, all buffers and reagents are kept at 4 °C unless otherwise indicated.
2. Preparation of Microsomes
- Harvest the DC2.4 cells by gentle pipetting from the tissue plate into a new 50 mL conical tube.
- Centrifuge at 1,000 x g for 5 min, 4 °C and carefully remove the medium with bOVA by aspiration.
- Wash the DC2.4 cells twice with 40 mL of PBS buffer (1.37 mM NaCl, 8.1 mM Na2HPO4, 2.68 mM KCl, 1.47 mM KH2PO4) by centrifugation at 1,000 x g for 5 min, 4 °C and discard the supernatant each time by aspiration.
- Resuspend the pellet in step 2.3 in 1 – 2 mL (1/20-1/10 volume of culture medium) of homogenization medium (0.25 M sucrose, 1 mM EDTA, 10 mM HEPES-NaOH [pH 7.4]) with 1/1,000 volume of protease inhibitor cocktails and transfer the resuspended DC2.4 cells into an ice-cold Dounce homogenizer.
- Disrupt the resuspended DC2.4 cells gently by 10 – 20 strokes with the ice-cold Dounce homogenizer.
NOTE: During the disruption step, the Dounce homogenizer is cooled on ice. - Transfer the cell-disrupted suspension to a new 15 mL conical tube.
- Add 8 – 9 mL homogenization medium to 10 mL total and centrifuge the conical tube for 10 min at 2,000 x g and 4 °C.
- Transfer the supernatant to a new 15-mL conical tube to remove unbroken cells and nuclei, and centrifuge the conical tube again for 10 min at 2,000 x g and 4 °C.
- Transfer the supernatant into a new ultracentrifugation tube and centrifuge for 45 min at 100,000 x g and 4 °C.
- Aspirate the supernatant and resuspend the pellet carefully in 1 – 2 mL of homogenization medium with 1/1,000 volume of protease inhibitor cocktails by pipetting the solution up and down several times to make a microsome fraction for downstream experiments.
- Transfer the microsome fraction into a new 5-mL round bottom tube.
NOTE: After this step, it is possible to enrich objective microsomes by iodixanol density gradient centrifugation according to the manufacturer's protocol, to remove non-specific microsomes. The peak fractions for exogenous antigens are collected and then subjected to the next step of purification (step 3).
3. Purification of Microsomes with bOVA Undergoing ERAD
- Add 1/100 volume of fresh SA-magnetic beads to the microsome fraction of step 2.11 in the 5 mL round bottom tube.
NOTE: Before this step, it is possible to pre-clear the microsome fraction by control-magnetic beads to reduce contaminations of non-specific microsomes. - Gently mix well and rotate the round bottom tube slowly for 30 min at 4 °C.
- Add 2-3 mL of homogenization medium to 4 mL total into the round bottom tube and gently mix well.
- Place the round bottom tube on a magnetic stand and incubate for 10 min at 4 °C. Since the beads bound to the vesicles are attracted to the magnet, brown micro-beads will gradually accumulate to the tube wall closest to the magnet.
- With the tube remaining in the magnetic stand, carefully discard the supernatants by aspiration to remove the unbound vesicles.
- Wash the magnetic beads bound to the vesicles twice with 5 mL of homogenization medium by the magnetic stand for 10 min at 4 °C and discard the supernatant each time by carefully aspiration.
NOTE: Without the magnetic stand, purification by centrifugations at 2,000 x g for 10 min, 4 °C is also available. - Resuspend the magnetic beads bound to the vesicles carefully in 100 µL homogenization medium by pipetting the solution up and down several times.
- Transfer the resuspended vesicles into a new microtube as the purified microsome for downstream experiments.
NOTE: Typically, 50 µL microsome fraction containing 5 – 20 µg proteins from 1 x 107 cells can be isolated.
4. Analysis of the Purified Microsomes
- Resuspend the magnetic beads bound to the vesicles from step 3.8 by 100 – 200 µL of TNE buffer (20 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.5 M EDTA, 1% Nonidet P-40) with 1/1,000 volume of protease inhibitor cocktails instead of the homogenization medium.
- Transfer the lysate from step 4.1 to a new 1.5-mL microtube.
- Determine the protein concentration of step 4.2 by using a BCA assay kit per the manufacturer's protocol.
NOTE: Typically, 5 – 10 µL lysate from step 4.2 is enough to determine the protein concentration. - Transfer the lysate from step 4.2 (2 µg of protein for silver staining and 10 µg of protein for Western blotting) into new microtubes.
- Put the microtubes on a heat block at 95 °C and boil proteins in 1x SDS gel-loading buffer (100 mM Tris-HCl pH 6.8, 200 mM dithiothreitol, 4% SDS, 0.2% bromophenol blue, 20% glycerol) for 5 min.
- Centrifuge the microtubes for 10 min at 21,500 x g and 4 °C. Collect the supernatants into new microtubes to remove the insoluble fractions.
- Analyze the resolved proteins in step 4.6 (2 µg) by standard SD-PAGE.
- Visualize the protein bands by the silver staining using silver staining kit following the manufacturer's protocol.
- Analyze the resolved proteins in step 4.6 (10 µg) by standard SD-PAGE and Western-blotting. Use the reagent (SA-HRP) per the manufacturer's protocol and visualize by fluorography.
5. In Vitro Reconstitution of ERAD Ubiquitination of bOVA Using Purified Microsomes
- Transfer the purified microsomes (5 – 10 µg of protein) from step 3.8 with a 50% volume of reticulocyte lysate (RL), in 1x reaction buffer (50 mM Tris pH 7.4, 3 mM ATP, 0.5 mM MgCl2), and 0.2 pM Flag-tagged ubiquitin in a new microtube. The final volume of this experiment is 20 – 40 µL.
- Incubate the microtube for 2 h at 37 °C.
NOTE: If the amount of ubiquitinated bOVA in step 5.12 is too small to detect by Western blotting, add 10 µM of MG132 or 2 µM of lactacystin to inhibit the processing of the ubiquitinated bOVA by proteasomes. - Stop the reaction by placing the microtube on ice.
- Resolve the microsomes by adding 500 µL TNE buffer with 1/1,000 volume of protease inhibitor cocktails.
- Centrifuge the microtube for 10 min at 21,500 x g and 4 °C. Collect the supernatants into a new microtube to remove the insoluble fractions, which contain ubiquitinated bOVA in DC2.4 before the in vitro ubiquitination assay.
- Transfer the supernatant to a new microtube and add 1/100 volume of new SA-magnetic beads (usually 5 µL for 500 µL of supernatants).
- Gently mix well and rotate the microtube slowly for 30 min at 4 °C.
- Centrifuge the microtube for 10 min at 21,500 x g and 4 °C. Discard the supernatant by aspiration to recover the bOVA and ubiquitinated bOVA bound with the SA-magnetic beads.
- Wash twice the collected SA-magnetic beads with 1 mL of TNE buffer by centrifugation for 10 min at 21,500 x g and 4 °C. Discard the supernatant each time by aspiration.
- Boil the SA-magnetic beads in 1x SDS gel loading buffer for 5 min at 95 °C on a heat-block to resolve the purified proteins by the SA-magnetic beads.
- Centrifuge the microtube for 10 min at 21,500 x g and 4 °C. Collect the supernatants into a new microtube to remove the insoluble fractions.
- Analyze the SA-magnetic beads bound to the proteins by standard SD-PAGE and Western-blotting. The use of antibodies (anti-Flag, anti-multi-Ub, and anti-mouse IgG-HRP) and the reagent (SA-HRP) is per the manufacturer's protocol and visualized by fluorography.
Representative Results
To elucidate the molecular mechanism of CP, it is necessary to identify the cellular compartments, where exogenous antigens undergo ERAD-like transport and processing. While observations by immunofluorescent microscopy or by electron microscopy identified the cellular compartment where exogenous antigens accumulated16,17,18,19,30,31,32,33, the cellular compartments for ERAD-like processing of exogenous antigens are not clearly defined. Recently it was shown that non-classical endosomes with ER resident molecules were responsible for CP34, but these cellular compartments were unpurified. The difficulty in isolating and purifying the cellular compartments can be attributed to the fact that exogenous antigens are localized both in the endosome and ER-like compartments and that there is no identifying molecule for these endocytic compartments other than exogenous antigens. However, the condition of exogenous antigens undergoing ERAD-like transport is different from the steady state; in the transport across lipid bimolecular membrane, exogenous antigens penetrate the membrane via translocons, such as Sec61 (Figure 2A). Thus, when using bOVA as an exogenous antigen, bOVA should be associated with Sec61. Since membrane-associated bOVA specifically bound with SA, the microsome prepared from DC2.4, which was pretreated with bOVA, could be isolated distinctively by SA-magnetic beads (Figure 2A). Then equivalent amounts of proteins from purified microsomes with or without pre-incubation of bOVA were resolved by SDS-PAGE followed by silver staining and Western blotting with SA-HRP (Figure 2B, 2C). As shown in Figure 2B and Figure 2C, the isolated microsomes contained several unique proteins that were purified dependent upon exogenously added bOVA and SA-magnetic beads. In addition to these unique proteins, isolated microsomes also contained nonspecific proteins, which bound to SA-magnetic beads with or without bOVA. Treatment of the microsome with trypsin before purification by the magnet prevented the purification of microsomes (Figure 2D), indicating that the purification methods depended on the presence of membrane-penetrating bOVA.
The purified microsomes showed the ability to ubiquitinate the incorporated bOVA in vitro, under the presence of RLs (Figure 3A). The amounts of bOVA and poly-ubiquitinated bOVA were augmented in the presence of MG132 (Figure 3B), indicating that the incorporated bOVA was processed by the ERAD system and that our purified microsomes contained ERAD machinery proteins.
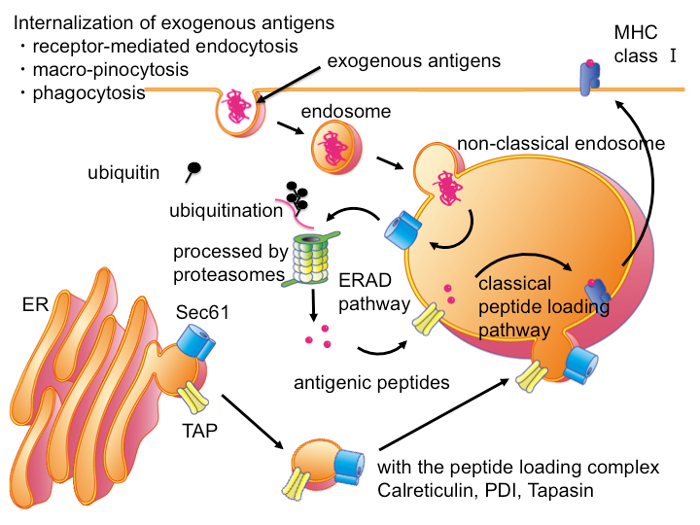
Figure 1: Intracellular Pathways for CP in DCs. In DCs, exogenous antigens are transported into non-classical endocytic compartments, which also contain ER-resident molecules in addition to molecules of the classical late endosome. In this compartment, exogenous antigens are exported into cytosol through translocons such as Sec61. In the cytosol, exogenous antigens are processed by the ubiquitin-proteasome system into antigenic peptides as ERAD substrates. Antigenic peptides are transported into same or adjacent non-classical endocytic compartments, or adjacent ER through TAP transporter and then loaded on the MHC I molecules by PLC. Please click here to view a larger version of this figure.
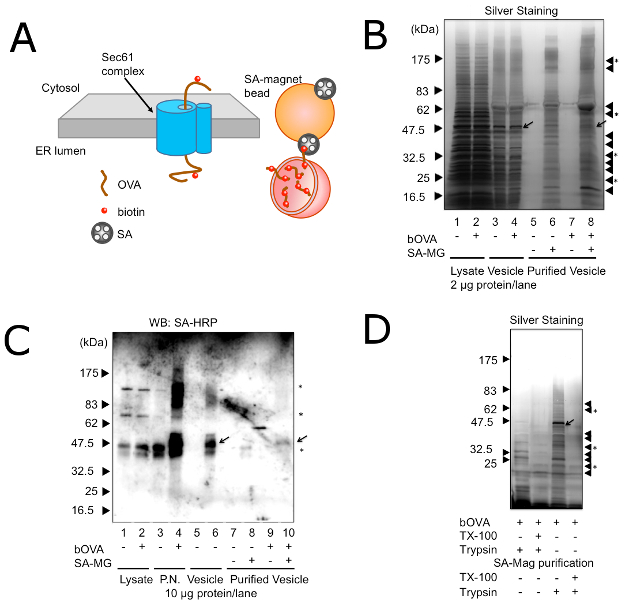
Figure 2: Purification of Microsomes with bOVA Undergoing ERAD. (A) A schematic model of purification of microsomes with bOVA undergoing ERAD. bOVA is associated with the membrane through the Sec61 translocon and targeted with SA-magnetic beads. (B) Microsomes with (+) or without (-) prior addition of bOVA were purified with (+) or without (-) SA-magnetic beads. Proteins (2 µg) or corresponding volumes of purified proteins were resolved on 7.5 – 15% SDS-PAGE, and silver staining was used to visualize protein bands. Triangles on the right side indicate nonspecific proteins binding to the SA-magnetic beads. Triangles with asterisks indicate unique proteins found only in the presence of exogenously added bOVA and SA-magnetic beads. The arrow shows bOVA. (C) Microsomes with (+) or without (-) prior addition of bOVA were purified with (+) or without (-) SA-magnetic beads. Proteins (10 µg) or corresponding volumes of purified proteins were resolved on 7.5 – 15% SDS-PAGE, and subjected to Western blotting with SA-HRP. P.N.: post-nuclear fraction. Asterisks in the right indicate non-specific bands with SA-HRP. Equivalent results were attained by at least three independent assays. (D) Microsomes with prior addition of bOVA were purified with SA-magnetic beads. Microsomes were treated with (+) or without (-) trypsin and TX-100 before purification (left two lanes) or after purification (right two lanes). Proteins (2 µg) or corresponding volumes of purified proteins were resolved on 7.5 – 15% SDS-PAGE, and silver staining was used to visualize protein bands. Triangles on the right side indicate nonspecific proteins binding to the SA-magnetic beads. Triangles with asterisks indicate unique proteins found only in the presence of exogenously added bOVA and SA-magnetic beads. Equivalent results were attained by at least three independent assays. Reprinted with permission from reference28. Please click here to view a larger version of this figure.
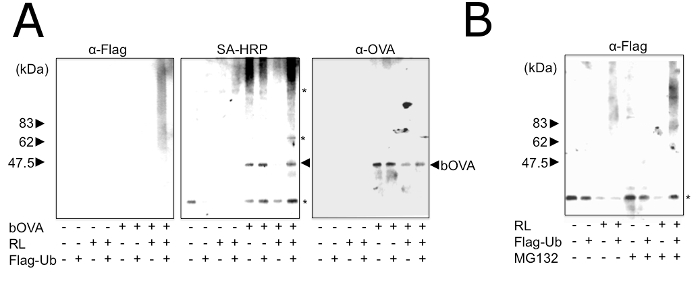
Figure 3: In Vitro Reconstitution of Processing and Ubiquitination using OVA in Purified Microsomes. (A) Purified microsomes with (+) or without (-) prior addition of bOVA were treated with (+) or without (-) RL and Flag-Ub for 1 h and were solubilized using TNE. bOVA was purified with SA-magnetic beads and subjected to Western blotting with the indicated antibodies. Asterisks in the right indicate non-specific bands with SA-HRP. Equivalent results were attained by at least three independent assays. (B) Purified microsomes were treated with (+) or without (-) RL, Flag-Ub, and MG132 for 1 h and were then solubilized using TNE. bOVA was purified with SA-magnetic beads and subjected to Western blotting with SA-Flag. Asterisks in the right indicate non-specific bands with SA-HRP. Equivalent results were attained by at least three independent assays. Reprinted with permission from reference28. Please click here to view a larger version of this figure.
Discussion
In previous studies of CP, the incorporated exogenous antigens accumulated in the restricted area of the late endosome or ER by immunofluorescent microscopy16,30,31,32. It is estimated that ERAD-like transport and processing of exogenous antigens are carried out in these specialized areas of the ER or late endosome, as the cellular compartment was identified by sucrose or iodixanol density gradient centrifugation using ubiquitinated bOVA as an ERAD-like processing marker. After stimulating their innate immunity, CP efficiency significantly increased, and the peak fraction for bOVA together with ubiquitinated bOVA migrated to a higher density fraction (our unpublished results). The aims of these experiments were to identify specific molecular markers for the ER or late endosome, which migrated together with bOVA or ubiquitinated bOVA. But in the results, both ER resident molecules and late endosome-resident molecules migrated together with the bOVA and ubiquitinated bOVA, indicating that these experiments were unsuccessful in ascertaining the cellular compartment for ERAD-like transport and processing as the classical ER or late endosome. In these experiments, there were no specific molecules in the endocytotic compartments except bOVA or ubiquitinated bOVA. However, while the exogenous antigen underwent ERAD-like transport, these molecules penetrated the lipid bilayer membrane of ER through translocons, such as Sec61 complex (Figure 1, Figure 2A). The membrane sticking bOVA was selected as a marker of the endocytic compartments, and the microsomes were purified by SA-magnetic beads (Figure 2A, 2B). In these purified microsomes, accumulated bOVA came across ERAD-like ubiquitination and processing under the presence of RL and ATP in vitro (Figure 3A, 3B). Since RL contains all cytosolic molecular apparati, such as ubiquitination-related molecules and proteasomes in addition to molecules for translation and transcription, the addition of RL enables ubiquitination of bOVA in purified microsome. These results indicate that purified microsomes were the objective cellular compartments for ERAD-like transport and processing of exogenous antigens, both containing endosome-specific molecules and ER-resident molecules at the same time; Lamp1 showed both precursor and mature forms in the purified microsome.
This protocol is dependent upon the amount of membrane-penetrating exogenous antigens; it is important to incorporate enough of the exogenous antigens to DC2.4 cells, which shows a high ability to incorporate exogenous antigens in the semi-confluent state. Elongating the 6 – 12 h incubation time for DC2.4 with bOVA increases the amount of incorporated bOVA. The addition of inhibitors for proteasomes, MG132 or lactacystin, moderately increases the amounts of purified microsomes. The preparation of the microsome is one of the most critical steps for this protocol. The free bOVA, which is not incorporated into DC2.4 cells, should be removed by washing the cells with PBS, to prevent non-specific binding of the free bOVA to the microsome fraction. To reduce damages upon membrane compartments, cells are carefully disrupted by the Dounce homogenizer in ice water. Then unbroken cells and nuclei were removed by centrifugation as the microsomes were prepared from the post-nuclear fraction. After preparation of the microsomes, one-step purification by SA-magnetic beads is carried out. In this step, careful washing of the magnet bound microsome is necessary to remove the non-specific binding of other cellular compartments and to not lose the specifically bound microsome.
In this one step purification method, a significant percentage of the target microsome is required. If the ratio of the objective microsome is too small, the amount of purified target microsome decreases by competition with non-specific binding of other cellular compartments. Consequently, under those conditions, the enrichments of the target microsome and the clearance of the non-specific microsome would be required. It is also possible to introduce additional steps before purification by the SA-magnetic beads. One such step is the sucrose or iodixanol density gradient centrifugation of the microsome. Since the prepared microsomes contain all membrane products, it might be possible to enrich the target microsome before SA-magnetic beads purification by selecting the antigen rich fractions for purification. Another possible step is the pre-clearance of the microsome by control-magnetic beads without SA, to reduce the non-specific background associations of microsomes against SA-magnetic beads. In these experiments, both steps improved the purity of the target microsome effectively, but diminished the total amount of purified products, indicating these additional steps are selected as means of further experiments.
It is well known that high-speed centrifugation has been shown to cause the fusion or clotting of intracellular vesicles. These fusions or clottings will produce artificial microsomes, which are derived from the non-specific interaction among different kinds of organelle-derived vesicles. These artificial microsomes statistically contain every membrane molecule such as the mitochondrial resident molecule, Golgi apparatus resident molecule, etc. These purified microsomes did not include the caveosome resident proteins, Golgi apparatus resident proteins, or early endosome-resident proteins, such as caveolin1, GM130, and EEA1, indicating that the SA-purified microsomes are not artificial products derived from the fusion among different kinds of vesicles. Without the high-speed centrifugation, bOVA-sticking vesicles were isolated, but the control experiments from vesicles without bOVA showed higher amounts of non-specific molecules. This indicates that this preliminary method is insufficient for successive experiments and that the high-speed centrifugation step is necessary for this purification protocol.
This protocol applies to other cell types, such as different DC subsets, or other APCs. It is also possible to use the various types of exogenous antigens in different conditions, such as fluorescent proteins, antigen-antibody complex, beads bound antigens, etc. Furthermore, we can purify target microsomes using specific antibodies against the exogenous antigens. Although several additional steps or modifications might be required to apply this protocol to other cells and antigens, it can elucidate the molecular mechanisms of the proteasome-dependent CP by comparing the purified molecules among different experiments. Thus, the described protocol has room for modification and improvement.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work is supported by the Takasaki University of Health and Welfare.
Materials
| RPMI 1640 | gibco by life technologies | 11875-093 | |
| Fetal bovine serum | Equitech bio | SFB30 | |
| Sodium pyruvate | gibco by life technologies | 11360-070 | |
| MEM non-essential amino acids | gibco by life technologies | 11140-050 | |
| HEPES | gibco by life technologies | 15630-080 | |
| 2-mercaptoethanol | gibco by life technologies | 21985-023 | |
| L-glutamine | gibco by life technologies | 25030-164 | |
| Penisicillin-Sreptomycin | gibco by life technologies | 15140-122 | |
| DMEM | gibco by life technologies | 12100-46 | |
| OVA | SIGMA | A5503 | |
| Biotin-protein labelling kit | Thermo Fisher Scientific | F6347 | |
| MG-132 | Santa Cruz Biotechnology | 201270 | |
| lactacystin | SIGMA | L6785 | |
| Dounce homogenizer | IUCHI | 131703 | |
| protease inhibitor cocktails | SIGMA | P8340 | |
| iodixanol | Cosmo bio | 1114542 | |
| SA-magnetic beads | New England Biolabs | 201270 | |
| control magnetic beads | Chemagen | M-PVA012 | |
| magnetic stand | BD Biosciences | 552311 | |
| BCA protein assay kit | Thermo Fisher Scientific | 23225 | |
| silver staining kits | Cosmo bio | 423413 | |
| Reticulocyte Lysate | Promega | 1730714 | |
| Flag-tagged ubiquitin | SIGMA | U5382 | |
| anti-ovalbumin (OVA,mouse) | Antibody Shop | HYB 094-06 | |
| ant-multi-ubiquitin (mouse) | MBL | D058−3 | |
| anti-Flag (mouse) | SIGMA | F3165 | |
| trypsin | SIGMA | 85450C |
References
- van Endert, P. Providing ligands for MHC class I molecules. Cell Mol Life Sci. 68 (9), 1467-1469 (2011).
- Janeway, C., Travers, P., Walport, M., Shlomchik, M. . Immunobiology: The Immune System in Health and Disease. , (2001).
- McDevitt, H. O. Discovering the role of the major histocompatibility complex in the immune response. Annu Rev Immunol. 18 (1), 1-17 (2000).
- Bedoui, S., et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 10 (5), 488-495 (2009).
- Segura, E., Villadangos, J. A. Antigen presentation by dendritic cells in vivo. Curr Opin Immunol. 21 (1), 105-110 (2009).
- Cheong, C., et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 143 (3), 416-429 (2010).
- Henri, S., et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 207 (2), 189-206 (2010).
- Shortman, K., Heath, W. R. The CD8+ dendritic cell subset. Immunol. Rev. 234 (1), 18-31 (2010).
- Carbone, F. R., Kurts, C., Bennett, S. R., Miller, J. F., Heath, W. R. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol Today. 19 (8), 368-373 (1998).
- Nair-Gupta, P., Blander, J. M. An updated view of the intracellular mechanisms regulating cross-presentation. Front Immunol. , (2013).
- Joffre, O. P., Segura, E., Savina, A., Amigorena, S. Cross-presentation by dendritic cells. Nat Rev Immunol. 12 (8), 557-569 (2012).
- Kurts, C., et al. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 184 (3), 923-930 (1996).
- Kurts, C., Kosaka, H., Carbone, F. R., Miller, J. F., Heath, W. R. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 186 (2), 239-245 (1997).
- Bonifaz, L., et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 196 (12), 1627-1638 (2002).
- Heath, W. R., Carbone, F. R. Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol. 1 (2), 126-134 (2001).
- Imai, J., Hasegawa, H., Maruya, M., Koyasu, S., Yahara, I. Exogenous antigens are processed through the endoplasmic reticulum-associated degradation (ERAD) in cross-presentation by dendritic cells. Int Immunol. 17 (1), 45-53 (2005).
- Houde, M., et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 425 (6956), 402-406 (2003).
- Guermonprez, P., et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 425 (6956), 397-402 (2003).
- Burgdorf, S., Scholz, C., Kautz, A., Tampe, R., Kurts, C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 9 (5), 558-566 (2008).
- Lizée, G., et al. Control of dendritic cell cross-presentation by the major histocompatibility complex class I cytoplasmic domain. Nat Immunol. 4 (11), 1065-1073 (2003).
- Basha, G., et al. A CD74-dependent MHC class I endolysosomal cross-presentation pathway. Nat Immunol. 13 (3), 237-245 (2012).
- Saveanu, L., Carroll, O., Hassainya, Y., van Endert, P. Complexity, contradictions, and conundrums: studying post-proteasomal proteolysis in HLA class I antigen presentation. Immunol Rev. 207 (1), 42-59 (2005).
- Wiertz, E. J., et al. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 384 (6608), 432-438 (1996).
- Hampton, R. Y. ER-associated degradation in protein quality control and cellular regulation. Curr Opin Cell Biol. 14 (4), 476-482 (2002).
- Tsai, B., Ye, Y., Rapoport, T. A. Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat Rev Mol Cell Biol. 3 (4), 246-255 (2002).
- Nyathi, Y., Wilkinson, B. M., Pool, M. R. Co-translational targeting and translocation of proteins to the endoplasmic reticulum. Biochim Biophys Acta. 1833 (11), 2392-2402 (2013).
- Varabyova, A., Stojanovski, D., Chacinska, A. Mitochondrial protein homeostasis. IUBMB Life. 65 (3), 191-201 (2013).
- Shen, Z., Reznikoff, G., Dranoff, G., Rock, K. L. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 158 (6), 2723-2730 (1997).
- Imai, J., Otani, M., Sakai, T., Hatta, S. Purification of the subcellular compartment in which exogenous antigens undergo endoplasmic reticulum-associated degradation from dendritic cells. Heliyon. , (2016).
- Kovacsovics-Bankowski, M., Rock, K. L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 267 (5195), 243-246 (1995).
- Ackerman, A. L., Giodini, A., Cresswell, P. A role for the endoplasmic reticulum protein retro translocation machinery during cross presentation by dendritic cells. Immunity. 25 (4), 607-617 (2006).
- Ackerman, A. L., Kyritsis, C., Tampé, R., Cresswell, P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci U S A. 100 (22), 12889-12894 (2003).
- Saveanu, L., et al. IRAP identifies an endosomal compartment required for MHC class I cross-presentation. Science. 325 (5937), 213-217 (2009).
- Zehner, M., et al. The translocon protein Sec61 mediates antigen transport from endosomes in the cytosol for cross-presentation to CD8(+) T cells. Immunity. 42 (5), 850-863 (2015).

