An In Vivo Method to Study Mouse Blood-Testis Barrier Integrity
Summary
Here, we present a protocol to assess the blood-testis barrier integrity by injecting inulin-FITC into testes. This is an efficient in vivo method to study blood-testis barrier integrity that can be compromised by genetic and environmental elements.
Abstract
Spermatogenesis is the development of spermatogonia into mature spermatozoa in the seminiferous tubules of the testis. This process is supported by Sertoli cell junctions at the blood-testis barrier (BTB), which is the tightest tissue barrier in the mammalian body and segregates the seminiferous epithelium into two compartments, a basal and an adluminal. The BTB creates a unique microenvironment for germ cells in meiosis I/II and for the development of postmeiotic spermatids into spermatozoa via spermiogenesis. Here, we describe a reliable assay to monitor BTB integrity of mouse testis in vivo. An intact BTB blocks the diffusion of FITC-conjugated inulin from the basal to the apical compartment of the seminiferous tubules. This technique is suitable for studying gene candidates, viruses, or environmental toxicants that may affect BTB function or integrity, with an easy procedure and a minimal requirement of surgical skills compared to alternative methods.
Introduction
Mammalian spermatogenesis is considered a highly structured process that encompasses spermatogonial self-renewal and differentiation through spermatocytes into haploid spermatozoa via mitosis, meiosis, and spermiogenesis, during which dramatic biochemical and morphological changes occur. Developing germ cells are progressively transported from the base of the seminiferous tubule toward the lumen. This process is regulated by cell-cell contacts between germ cells and Sertoli cells1,2. Adjacent Sertoli cells form the BTB that is located near the base of the seminiferous tubule. The BTB physically divides the epithelium into a basal and an adluminal compartment. During stages VIII – IX of the epithelial cycle, preleptotene/leptotene spermatocytes from the basal compartments migrate across the BTB, entering the adluminal compartments3. Therefore, the function of the BTB is to provide an immunoprivileged microenvironment for the completion of meiosis and spermiogenesis4,5,6. Unlike other blood-tissue barriers (e.g., blood-brain barrier) that are only composed of tight junctions (TJs), the BTB is formed by four different junctions (TJs, ectoplasmic specializations, gap junctions, and intermediate filament-based desmosomes) between Sertoli cells1,7.
Many studies have used genetically-modified mice, virus infections, and environmental toxicants to investigate mechanisms of BTB integrity7,8,9. The BTB disruption induces impaired spermatogenesis and subfertility or infertility. Since the BTB formation and integrity have been confirmed to be affected by contacts between Sertoli cells8, an in vitro model based on primary culture of isolated Sertoli cells has been used for BTB study. However, this model cannot accurately mimic BTB dynamics in vivo. Moreover, no such co-culture of germ cells with Sertoli cells has been established as capable of reflecting all relevant structural and functional components of the BTB10,11.
In general, in vivo BTB integrity assays are typically based on small molecules, such as EZ-Link Sulfo-NHS-LC-Biotin and FITC-conjugated inulin (inulin-FITC). Normally, the diffusion of biotin or inulin-FITC from the basal compartment is blocked by BTB structure. Therefore, we are able to use this method to assess the extent of BTB damage compared with control groups. While BTB can be compromised with certain types of stimuli, such as treatment with cadmium chloride (CdCl2)12, BTB becomes accessible to small molecules, which eventually enter the adluminal compartment as indicators.
An early in vivo BTB integrity assay involves injecting biotin or inulin-FITC into the jugular vein, which involves surgery, and is invasive, complicated, and time-consuming. Besides, as the reporter substances diffuse through the whole body via the circulation, the local concentration of biotin or inulin-FITC in the seminiferous tubules is limited. Moreover, systemic exposure may induce immune reactions. Here, we present a simple and effective in vivo BTB integrity assay enabling direct injection of a small aliquot of inulin-FITC into the interstitium of a testis. Using the fluorescent labeling method, the staining process is convenient, as secondary antibodies are not required. Here, the process of fluorescent dye entering the testis is visualized.
Protocol
All performed animal experiments have been approved by the Nanjing Medical University committee. Male C57BL/6 mice were kept under controlled photoperiod conditions and were supplied with food and water.
1. Preparations
- Microinjection capillaries
- Use microinjection capillaries with an outer diameter, inner dimeter, and length of 1.0 mm, 0.8 mm, and 10.0 cm, respectively.
- Pull glass capillaries with a capillary puller (Figure 1A). Test and adjust the settings depending on the capillary puller machine that is being used.
- Break the pipette tips with forceps to use to obtain capillaries of a 50-µm diameter at the tip.
Note: The tips may be too long to penetrate the testis. - Sharpen the tip in a 30° angle by using a micropipette beveler (Figure 1C).
- Reagents
- Prepare 1% pentobarbital sodium: dissolve pentobarbital sodium 100 mg in 10 mL of phosphate-buffered saline (PBS) (step 2.3.3).
- Prepare 10 mg/mL inulin-FITC: dissolve inulin-FITC 1 mg in 100 μL of PBS (step 2.1.6).
- Prepare 4% paraformaldehyde: dissolve paraformaldehyde (PFA) 4 g in 100 mL of PBS (step 2.3.3).
- Prepare 30% sucrose: dissolve sucrose 3 g in 10 mL of PBS (step 2.3.4).
- Prepare 0.1% cadmium chloride: dissolve cadmium chloride 10 mg in 10 mL of PBS (step 2.1.1).
2. Methods
- Anesthesia and presurgery preparation
- Weigh 8-week-old C57BL/6 male mice and calculate the required dose of CdCl2. Treat a group of mice (n = 3) with CdCl2 (5 mg/kg b.w., i.p.) for 3 d before surgery. Treat another group of 8-week-old C57BL/6 male mice (n = 3) with PBS as control.
- Perform surgery under aseptic conditions by using sterile syringe, needle, scissors, and forceps.
- Prepare anesthesia working solution freshly. The required dose of anesthesia is 70 mg of pentobarbital sodium/kg of body weight. On average, an adult C57BL/6 mouse at 8 weeks of age weighs ~25 g, which corresponds to 175 μL of 1% pentobarbital sodium (1.75 mg per mouse).
NOTE: The pentobarbital sodium is dissolved in sterile phosphate-buffered saline (PBS). The solution is filtered by 0.22 um filter unit before being used. - Clean the area for surgery with 75% ethanol and cover the area with a clean tissue towel.
- Turn on the thermostatic heater and adjust the temperature to 37 °C.
- Prepare inulin-FITC working solution (10 mg/mL) on the day of the surgery.
- Surgical procedure
- Weigh 8-week-old C57BL/6 male mice and calculate the required dose of anesthesia.
- Perform an intraperitoneal injection of pentobarbital sodium using the 1-mL sterile syringe. Keep the mouse in a clean cage.
- Observe the eye reflex response and breathing pattern of the mouse to confirm that it is under complete anesthesia.
NOTE: Usually, 10 – 15 min are needed until the mouse is deeply anesthetized, with a total lack of toe pinch response and maintenance of slow, steady breathing. - Move the mouse to the operation area and cover its eye area with a moist tissue paper to avoid dryness during anesthesia (Figure 2A).
- Shave its abdominal hair with a shaver and disinfect the surgery area with 75% ethanol.
- With sharp scissors, make a 1-cm skin incision above the preputial glands to expose the abdominal wall. Lift the abdominal wall with small forceps and make a 0.5-cm incision to expose the peritoneal cavity (Figure 2B).
- Use forceps to search for fat pads around the epididymis and testis. Carefully pull the fat pads out to expose the attached testis clearly (Figure 2C and 2D). Usually, operate on one testis at a time.
NOTE: Avoid touching the fat pads and testis with hands. - Place a paper (9 cm in diameter) underneath the fat pads and testis (Figure 2C).
- Inject inulin-FITC into a microinjection pipette and connect it to the micromanipulator unit (Figure 1D). Gently insert the microinjection pipette under the tunica albuginea and load a total of 20 μL of inulin-FITC into the interstitium of the testis (Figure 2E and 2F).
NOTE: Carefully depress the microinjection pipette to avoid moving it. - Monitor the movement of inulin-FITC in the testis (Figure 2G).
- Immediately put the testis back into the abdominal cavity after the completion of the injection.
- Repeat the procedure on the contralateral testis. This testis is injected with 20 μL of PBS as a control.
- Close the skin with a surgical suture and move the mouse to a heat pad of a thermostatic heater (Figure 1B). Administer a dose of 1 mg of analgesic (buprenorphine) per 1 kg of body weight via subcutaneous injection.
- Harvesting the testes and frozen sections preparation
- 40 min after the injection, euthanize the recipient mouse by cervical dislocation according to animal care and use guidelines.
- Use a pair of sharp scissors to collect the testes. Place the testes into 1 mL of ice-cold PBS to remove any blood contamination.
- Fix the testes in 4% paraformaldehyde (PFA) at 4 °C for 12 – 24 h. Discard the 4% PFA and wash the tissue 3x with 1.5 mL of 1% PBS at room temperature.
- Dehydrate the tissue in 30% sucrose overnight. Put the testis in the embedding frame and cover the tissue with optimal cutting temperature compound (OCT). After the OCT is frozen, fill the embedding frame with OCT so that the whole testis can be covered with OCT.
- Cut 5-μm-thick, frozen cross sections of the testes in a cryostat at -20 °C and let them adhere to microscope slides.
NOTE: Refreezing embedded tissues in dry ice may increase the rigidity for cutting.
- Image requisition
- Place the slides in a humidified box. Warm the slides at room temperature for 10 min.
- Wash the sections 3x with Tris-buffered saline (TBS) at room temperature.
- Air-dry for 5 min in the dark. Wipe off any residual TBS with dust-free paper.
- Cover the cross sections with 4’,6-diamidino-2-phenylindole (DAPI). Place the inverted coverslip on a microscope slide.
- Acquire images using a confocal microscope. A total of 20X magnification is generally sufficient for detecting the brightly fluorescent signal.
Representative Results
The experimental set-up for performing the BTB integrity assay is shown in Figure 1. Pull and sharpen microinjection capillaries with a capillary puller and micropipette beveler, respectively (Figure 1A and 1C). The thermostatic heater and equipment for microinjection are illustrated in Figure 1B and 1D.
Figure 2 displays some of the key steps for the injection of inulin-FITC. Use scissors to make a small incision after the mouse has undergone complete anesthesia (Figure 2A and 2B). The mouse testis is exposed and injected with fluorescent dye using a microinjection pipette (Figure 2C – 2G).
Figure 3 displays typical images of a study to assess the BTB integrity based on an in vivo assay. The mice in the CdCl2-treatment group are injected with an acute dose of CdCl2 (5 mg/kg b.w., i.p.) for 3 days. The diffusion of inulin-FITC from the basal compartment is blocked by BTB structure in the control group, while the BTB construction is damaged and inulin (green fluorescence) passages into the apical compartment of the seminiferous epithelium in the CdCl2-treatment group. White line segments indicate the distance travelled by the inulin from the basement membrane (Figure 3A). The extent of BTB damage is determined by the distance. For an elliptical lumen, the radius is the average of the shortest and the longest distance from the basal compartment to the center of the tubule. We use such a ratio as an index of the extent of the BTB damage:
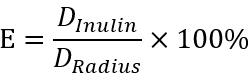
Here, DInulin is the distance traveled by inulin from basal compartment and DRadius is the radius of the same seminiferous tubule (Figure 3B). The intact BTB in Rictorfl/+ mice blocks the diffusion of inulin across the barrier to enter the apical compartment. In contrast, Rictorcko mice have a compromised BTB permitting inulin diffusion (Figure 3C).

Figure 1: Equipment for mouse testicular interstitial microinjection. (A) Pull glass capillaries with a vertical capillary puller. (B) This panel shows the thermostatic heater. (C) The tips are sharpened using the micropipette beveler. (D) The unit for microinjection includes a microinjection pump and a stereo microscope. Please click here to view a larger version of this figure.
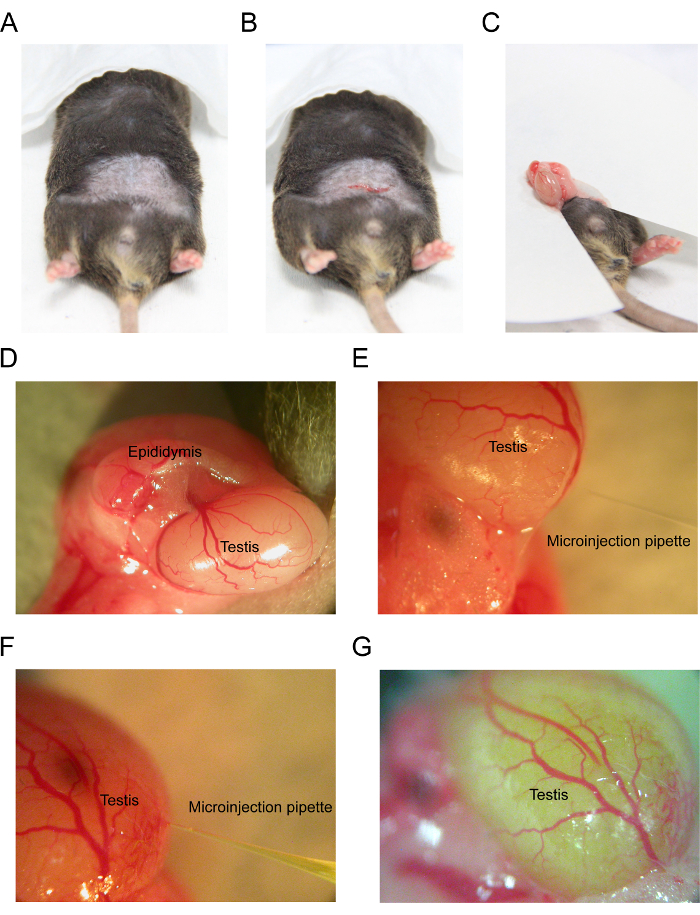
Figure 2: Representative images of an in vivo testicular interstitial microinjection proceeding in a mouse. (A) This panel shows the removal of abdominal hair by a shaver. (B) This panel shows the 0.5-cm abdominal wall incision to expose the peritoneal cavity. (C) Pull the fat pads out to expose the attached testis clearly. (D) This panel shows the position of the testis and epididymis. (E) This panel shows the position of the microinjection pipette. (F) Insert the microinjection pipette into the interstitium of the testis. (G) This panel shows a testis with a successful injection into the interstitium of the testis. Please click here to view a larger version of this figure.
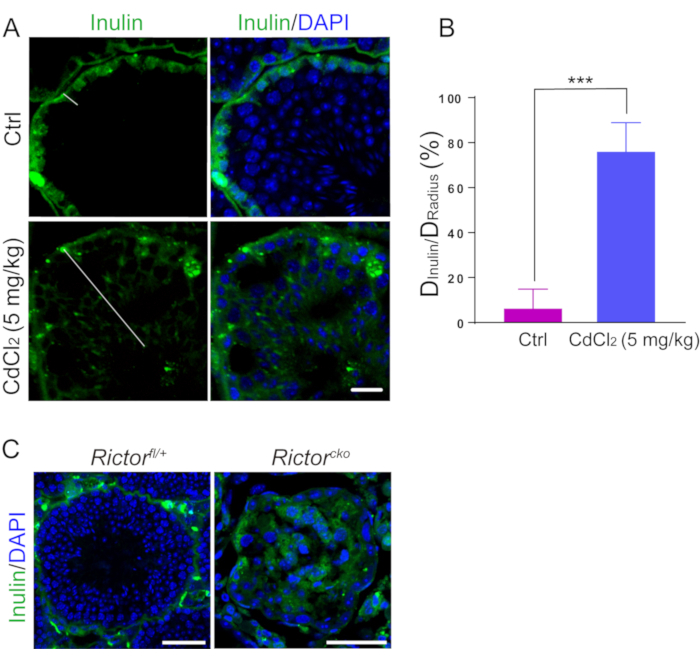
Figure 3: A study to assess the BTB integrity based on an in vivo functional assay. Adult male mice (n = 3 in both the treatment group and the control group) are treated with CdCl2 (5 mg/kg b.w., i.p.) for 3 days (treatment group) or treated with PBS for 3 days (control group). Inulin-FITC (green fluorescence) is located near the base of the seminiferous tubule in the control group, while inulin-FITC initiates a passage across the BTB in the CdCl2-treatment group. (A) In this panel, white line segments indicate the distance inulin-FITC invades. The scale bars are 20 μm. (B) Data from the BTB integrity assays are shown in this histogram, which show the distance traveled by inulin (DInulin) vs. the radius of the same tubule (DRadius). Eighty tubules are randomly selected. *** P < 0.001 (Student's t-test). (C) The BTB integrity is compromised in testes of Rictorcko mice. In Rictorcko mouse tubules, the inulin penetrates deep inside the seminiferous epithelium, reaching the tubule lumen. The scale bars are 50 μm. Please click here to view a larger version of this figure.
Discussion
Spermatogenesis takes place in the seminiferous epithelium and is a highly ordered and dynamic process that is governed by germ cells and somatic cells (e.g., Sertoli cells)13. The BTB structure, which is constructed by Sertoli cells, divides the seminiferous epithelium into a basal and an apical compartment. The development of meiotic and haploid germ cells occurs in the apical compartment which forms an immunological barrier14.
The BTB function can be compromised by toxicants or due to defects in genes involved in the formation of cell junctions, leading to male infertility. In order to examine BTB integrity, an in vitro Sertoli cell culture system has been established that is capable of forming functional epithelium that closely mimics the BTB in vivo15. This in vitro system provides a simple model to study the structure and function of Sertoli cell junctions. However, Sertoli cells isolated from testis and cultured in vitro are limited with respect to animal age and cell density15,16. In addition, the purity of Sertoli cells and the presence of ultrastructures mimicking BTB features must be monitored17. More important, BTB structure and integrity also require interactions between germ cells and Sertoli cells, as evident from studies of germ-cell-specific null mutant mice with BTB defects10,18,19. Thus, creating a co-culture of germ cells with Sertoli cells in vitro for the purpose of recapitulating all crucial in vivo function of BTB remains challenging11,20.
This protocol describes a method of assessing BTB integrity in vivo by injecting inulin-FITC, which was modified from a procedure by Chen et al.21. The protocol describes the anesthesia of male mice, the exposure of the peritoneal cavity, the microinjection of dye into the interstitium of the testis, harvesting the testes and cutting them into frozen sections, and the acquisition of images. For a successful completion, several steps should be noted. Firstly, it is important to use an appropriate dose of anesthesia, because severely deep anesthesia may cause death. Also, the length of the tip of the injection capillary should not be too long; otherwise, the pipette cannot penetrate the testis.
The procedure presented here can be utilized for analyzing the role of viruses, chemical toxicants, or candidate proteins involved in the regulation of BTB. This assay is sensitive, reliable, and accessible to monitor BTB integrity in vivo.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Key R&D Program of China (2016YFA0500902), the National Natural Science Foundation of China (31471228, 31771653), the Jiangsu Science Foundation for Distinguished Young Scholars (BK20150047), the Natural Science Foundation of Jiangsu Province (BK20140897, 14KJA180005) and the Innovative and Entrepreneurial Program of Jiangsu Province to K.Z.
Materials
| Capillary puller | SUTTER INSTRUMENT (USA) | P-97 | |
| 10x PBS | Hyclone (USA) | SH30258.01 | dilution to 1× in ddH2O |
| 4’,6-diamidino-2-phenylindole (DAPI) | Sigma (USA) | F6057 | |
| Adhesion microscope slides | CITOGLAS (China) | 80312-3161 | |
| Cadmium chloride | Sigma (USA) | 655198-5G | |
| Confocal microscope | Zeiss (Germany) | LSM700 | |
| Dust-free paper | Kimberly-Clark (USA) | 34120 | |
| Inulin-FITC | Sigma (USA) | F3272 | |
| Microinjection capillaries | Zhengtianyi (China) | BJ-40 | 1.0 mm × 0.8 mm × 100 mm |
| Micropipette beveler | NARISHIGE (JAPAN) | EG-400 | |
| OCT | SAKURA (JAPAN) | 4583 | |
| Paraformaldehyde | Sigma (USA) | P6148 | |
| Pentobarbital sodium | Merck (Germany) | P11011 | |
| Shaver | Yashen (China) | ||
| Stereo microscope | Nikon (JAPAN) | SMZ1000 | |
| Sucrose | Sangon Biotech (China) | A610498 | |
| Surgical instruments | Stronger (China) | scissors, forceps, needle holder | |
| Syringe | KDL (China) | 20163150518 | 0.45 mm × 0.16 mm RW LB |
| thermostatic heater | KELL (Nanjing, China) | KEL-2010 | |
| 10x TBS, pH 7.6 | |||
| 0.2 M Tris | Sangon Biotech (China) | A600194 | |
| 1.37 M Nacl | Sangon Biotech (China) | A610476 |
References
- Mruk, D. D., Cheng, C. Y. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocrine Reviews. 25 (5), 747-806 (2004).
- Wen, Q., et al. Transport of germ cells across the seminiferous epithelium during spermatogenesis-the involvement of both actin- and microtubule-based cytoskeletons. Tissue Barriers. 4 (4), e1265042 (2016).
- Wang, C. Q., Cheng, C. Y. A seamless trespass: germ cell migration across the seminiferous epithelium during spermatogenesis. Journal of Cell Biology. 178 (4), 549-556 (2007).
- Fijak, M., Meinhardt, A. The testis in immune privilege. Immunological Reviews. 213, 66-81 (2006).
- O’Bryan, M. K., Hedger, M. P. Inflammatory Networks in the Control of Spermatogenesis Chronic Inflammation in an Immunologically Privileged Tissue?. Molecular Mechanisms In Spermatogenesis. 636, 92-114 (2008).
- Li, N., Wang, T., Han, D. Structural cellular and molecular aspects of immune privilege in the testis. Frontiers in Immunology. 3, 152 (2012).
- Mruk, D. D., Cheng, C. Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocrine Review. 36 (5), 564-591 (2015).
- Govero, J., et al. Zika virus infection damages the testes in mice. Nature. 540 (7633), 438-442 (2016).
- Jenabian, M. A., et al. Immune tolerance properties of the testicular tissue as a viral sanctuary site in ART-treated HIV-infected adults. AIDS. 30 (18), 2777-2786 (2016).
- Holembowski, L., et al. TAp73 is essential for germ cell adhesion and maturation in testis. Journal Of Cell Biology. 204 (7), 1173-1190 (2014).
- Legendre, A., et al. An engineered 3D blood-testis barrier model for the assessment of reproductive toxicity potential. Biomaterials. 31 (16), 4492-4505 (2010).
- Setchell, B. P., Waites, G. M. Changes in the permeability of the testicular capillaries and of the ‘blood-testis barrier’ after injection of cadmium chloride in the rat. Journal of Endocrinology. 47 (1), 81-86 (1970).
- Griswold, M. D. The central role of Sertoli cells in spermatogenesis. Seminars in Cell & Developmental Biology. 9 (4), 411-416 (1998).
- Cheng, C. Y., Mruk, D. D. The blood-testis barrier and its implications for male contraception. Pharmacological Reviews. 64 (1), 16-64 (2012).
- Mruk, D. D., Cheng, C. Y. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Molecular Biology. 763, 237-252 (2011).
- Orth, J. M. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anatomical Record. 203 (4), 485-492 (1982).
- Lee, N. P. Y., Mruk, D., Lee, W. M., Cheng, C. Y. Is the cadherin/catenin complex a functional unit of cell-cell actin-based adherens junctions in the rat testis?. Biology of Reproduction. 68 (2), 489-508 (2003).
- Bai, S., et al. A Germline-Specific Role for the mTORC2 Component Rictor in Maintaining Spermatogonial Differentiation and Intercellular Adhesion in Mouse Testis. Molecular Human Reproduction. 24 (5), 244-259 (2018).
- Korhonen, H. M., et al. DICER Regulates the Formation and Maintenance of Cell-Cell Junctions in the Mouse Seminiferous Epithelium. Biology of Reproduction. 93 (6), 139 (2015).
- Loir, M. Trout Sertoli cells and germ cells in primary culture: I. Morphological and ultrastructural study. Gamete Research. 24 (2), 151-169 (1989).
- Chen, H., et al. Monitoring the Integrity of the Blood-Testis Barrier (BTB): An In Vivo Assay. Methods in Molecular Biology. 1748, 245-252 (2018).

