Measuring Enzymatic Stability by Isothermal Titration Calorimetry
Summary
The thermal stability of enzyme activity is readily measured by isothermal titration calorimetry (ITC). Most protein stability assays currently used measure protein unfolding, but do not provide information about enzymatic activity. ITC enables direct determination of the effect of enzyme modifications on the stability of enzyme activity.
Abstract
This work demonstrates a new method for measuring the stability of enzyme activity by isothermal titration calorimetry (ITC). The peak heat rate observed after a single injection of the substrate solution into an enzyme solution is correlated with enzyme activity. Multiple injections of the substrate into the same enzyme solution over time show the loss of enzyme activity. The assay is autonomous, requiring very little personnel time, and is applicable to most media and enzymes.
Introduction
Enzymes are proteins capable of catalyzing a wide array of organic reactions. Most enzymes function in aqueous solution at near neutral pH thus avoiding the use of harsh solvents. Because of their high selectivity, enzyme catalyzed reactions produce fewer (in some cases no byproducts) byproducts than non-selective catalysts such as acids and bases1. This is especially relevant in food manufacturing where all chemical reactions must be done so the final product is safe for human consumption. Currently, enzymes are used to produce high fructose corn syrup2, cheese3, beer4, lactose-free milk5, and other important food products. While this paper focuses on enzyme use in the food industry, there are many other uses for enzymes including in green chemistry and drug synthesis.
The utility of enzymes is limited by the stability of enzyme activity, which depends on maintaining the three-dimensional structure of the enzyme. The enzyme structure can be stabilized by modifications such as PEGylation6, immobilization on a solid support7, genetic modifications8, and formulations. Currently, enzyme stability is typically measured by differential scanning calorimetry (DSC) and endpoint enzyme activity assays9. DSC measures the temperature at which an enzyme unfolds; the higher the temperature, the more stable the structure. However, loss of activity often occurs at a lower temperature than required to unfold the enzyme or domains within the enzyme10. Therefore, DSC is not sufficient to determine whether an enzyme modification increases the stability of enzyme activity. Endpoint enzyme assays are usually time intensive, require multiple samples, and often involve a coupled colorimetric reaction that is not applicable to highly colored or opaque solutions or suspensions.
This work demonstrates a method for direct measurement of the stability of enzyme activity by isothermal titration calorimetry (ITC). ITC measures the rate of heat released or absorbed during the course of a reaction. Since nearly all reactions produce or absorb heat, ITC can be used for most enzyme-catalyzed reactions, including reactions that do not have a coupled reaction or occur in opaque media such as milk. ITC has been used for many decades to measure chemical kinetic parameters for many kinds of reactions, but the protocol presented here focuses on using ITC to measure the peak heat rate of enzyme-catalyzed reactions and demonstrates that enzyme activity is linearly correlated with the peak heat rate. ITC measurements of peak heat rates are mostly autonomous and require very little personnel time to setup and analyze.
Protocol
1. Preparing samples
- 1,000 mL of 0.1 M sodium acetate buffer at pH 4.6
- Measure 800 mL of distilled water in a 1,000 mL graduated beaker.
- Weigh 8.2 g of anhydrous sodium acetate and add it to the beaker.
- Place the beaker on a stir plate, place a stir rod into the beaker, turn on the stir plate and stir until completely dissolved.
- When the anhydrous sodium acetate is completely dissolved, measure the pH of the solution with a calibrated pH meter.
- Add 1 M HCl or NaOH accordingly to obtain the desired pH 4.6.
- Add distilled water until the total volume is 1,000 mL.
- Store at room temperature until use.
- Enzyme solution
- Prepare 10 mL of the enzyme solution within the 10-30 mg/mL range by first measuring 8 mL of the 0.1 M sodium acetate buffer pH 4.6 in a 15 mL graduated cylinder.
- Add the buffer solution into a 15 mL conical tube with enzyme and shake vigorously until the enzyme has dissolved.
- Add more buffer solution until the total volume is 10 mL.
- Store the enzyme solution at 4 °C until use.
- Substrate solution
- To prepare a substrate solution within the 300-600 mM range, calculate the amount of substrate needed in grams to make the desired concentration.
- Weigh out the substrate and place into a 100 mL glass beaker
- Measure 20 mL of the buffer solution using a 25 mL graduated cylinder and then add it to the glass beaker.
- Place the beaker on a stir plate and place a magnetic stir rod into the beaker. Turn on the heat and adjust the stirring speed accordingly.
- Allow stirring to continue until the substrate has dissolved.
- Pour the substrate solution into a 50 mL conical tube and add 0.1 M sodium acetate buffer pH 4.6 until the total volume is 45 mL. Mix by shaking.
- Store the substrate solution at room temperature until use.
2. Performing the experiment
- Preparing the ITC instrument
- Ensure that the reference cell is loaded with 350 µL of distilled water. Before loading the enzyme into the sample cell, verify that the sample cell has been cleaned.
- Cleaning protocol- Fill the loading syringe with 500 µL of 2% cleaning solution (Table of Materials), carefully insert the needle into the sample cell, fill the cell and slowly remove the liquid using the same syringe. Dispose the liquid into a beaker. Repeat this step twice with 2% cleaning solution (Table of Materials), three times with 70% ethanol and then wash ten times with distilled water.
- Fill the loading syringe with 450 µL of enzyme solution, carefully insert the needle all the way to the bottom of the sample cell and press the plunger down to the 100 µL line slowly to prevent formation of air bubbles.
- Wash the 50 µL titration syringe with distilled water three times by placing the needle tip into water then slowly taking up the water into the syringe, then dispensing the water into a waste container.
- Remove residual water by rinsing with substrate solution three times.
- Fill the titration syringe with substrate solution by drawing the solution up until the syringe is full without any air bubbles.
- With the syringe still in the substrate solution, remove the plunger and allow approximately 2 µL of air to enter the top of the syringe and reinsert the plunger.
- Remove the buret handle of the ITC, place the syringe inside the buret handle and screw until tight.
- Wipe the tip of the stirrer with a lint-free tissue, then carefully place the buret handle into the ITC instrument and lock it in place.
3. Setting up ITCrun
- On the computer, open ITCrun and click Set up.
- Click Stirring rate and set to 350 RPM. Check the syringe size (µL) and ensure it is at 50 µL.
- Set the temperature and press Update. It is recommended that this step be performed at least 1 h before preparing the ITC. This allows enough time for the instrument to heat up or cool down as needed.
- For the experiment setup, select incremental titration.
- Click Insert to setup the injections. Adjust the injection interval to 5,400 s, injection volume (µL) to 4 and number of injections to 4. Press OK to confirm settings.
- In the Equilibration box, select Auto-equilibrate and Large expected heats. (If the expected heats are small, one can select Small under expected heats; however, this will increase the equilibration time.)
- Set the initial baseline to 300 s.
- To start the run, click the Start symbol next to Stirring rate and then click Start which is located next to the wrench symbol.
- Save the file and allow the instrument to run.
4. Analyzing data
- Open the file in NanoAnalyze. Click Data and select Data columns.
- Select all data, copy and then paste the data into Microsoft Excel.
- Adjust zero baseline by adding the value required at 300 s to make it zero. Apply this correction to the entire column of heat rate values.
- Find the minimum or maximum value of the heat rate for each injection by using the equation: =MIN(cell:cell) or MAX(cell:cell). Each data point represents the peak enzymatic activity of the enzyme at each injection.
- Plot graphs of the MIN or MAX values against the time at which the value occurred during the titration.
Representative Results
The representative results in Figure 1 and Figure 5 show data from two enzymes, lactase and invertase. Lactase and invertase catalyze the hydrolysis of a disaccharide into two monosaccharides, endothermically and exothermically, respectively. Both enzymatic reactions were run at concentrations that precluded saturation of the enzyme.
The lactase data demonstrate how ITC data can be used to estimate enzyme stability. Four sequential 4 µL injections of 600 mM lactose (Figure 1) were titrated into 20 mg/mL lactase. An interval of 5,400 s between each injection was applied, and this allows enough time to return to the initial baseline before each injection. The protocol described above was performed at 25 °C, 35 °C, 45 °C, and 55 °C. Additionally, 600 mM lactose was titrated into 100 mM sodium acetate buffer only at 55 °C as a control for the heat of mixing. For each injection of enzyme into substrate, there is an initial exothermic heat of mixing, and subsequently the lactase catalyzed endothermic hydrolysis of lactose occurs until the reaction is completed and the heat rate returns to the baseline. The injection of lactose into the lactase solution is then repeated three more times with the peak height of the endothermic reaction from each subsequent injection a little less than the preceding injection because of the decrease of enzymatic activity. The raw data can then be converted to show the peak heat relative to time (Figure 2A-D). The peak height for each injection can then be fit to a linear regression and the slope indicates enzyme stability at the chosen temperature. The more negative the slope, the less stable the enzyme. As expected, enzyme stability decreases with increasing temperature (Figure 2E).
Each injection of lactose described in Figure 1 results in dilution of lactase. To demonstrate that this dilution is not the cause of the decrease in enzymatic activity, the ITC lactase activity assay was also done at 25 °C, the lactase concentration at the first injection and at the last injection (Figure 3). This dilution results in an 8% decrease in enzyme activity, whereas the fourth injection in the assay shows a 73% decrease in activity. The dilution of the lactase during the four-injection experiment thus had a relatively small effect (i.e., 11%) on enzyme activity. The actual loss of activity was therefore 73-8 = 65%.
As mentioned in the introduction one of the advantages of using ITC is that these reactions can be done in opaque media, such as milk. To demonstrate this capability of ITC, milk was injected directly into lactase (Figure 4). Because the pH of the milk is not matched to the sodium acetate buffer, there is a large exothermic heat of mixing immediately following the injection. The exothermic heat of mixing peak is seen in the control that lacks lactase (Figure 4, gray line) and in the two injections with milk (Figure 4, black and dotted lines) subsequent to the heat of mixing peak. The endothermic reaction indicating lactase activity occurs. Milk contains a complex mixture of proteins, vitamins, minerals, and lactose. Because of the complexity of milk, it is probable that other reactions occur during the course of our reaction. To demonstrate that the endothermic peak is due to lactase activity, the milk was spiked with 146 mM lactose. In the reaction with the lactose spiked milk (Figure 4, dotted line), the endothermic peak and area under the curve are larger than in the milk alone (Figure 4, black line), indicating that the endothermic peak is indeed due to the lactase activity. The baseline offset indicates that a slow reaction continues after the lactose reaction is finished.
To demonstrate how this assay can be used to compare the stability of enzymatic activity of two different enzyme preparations, the stability of free invertase and invertase immobilized on a nylon-6 nanofiber membrane are compared in Figure 5 at 35 °C11. As in the lactase assay, four 4 µL injections of sucrose were made sequentially and the maximum heat rate determined from the peak height for each injection. As shown in Figure 6, the peak heights decrease linearly with time indicating decreasing enzyme activity.
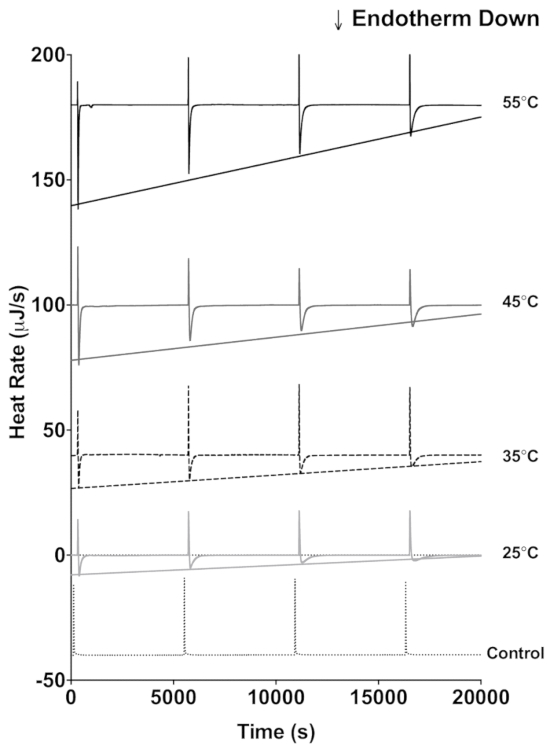
Figure 1: ITC data traces of lactase activity. Each trace shows four sequential 4 µL injections of 600 mM lactose into a lactase solution in pH 4.6 buffer. The traces were done at 55 °C (black), 45 °C (dark gray), 35 °C (dashed), 25 °C (light gray), and a no enzyme control (dotted) at 55 °C. The straight lines represent the fit to the endothermic peak minimum following each injection. Please click here to view a larger version of this figure.
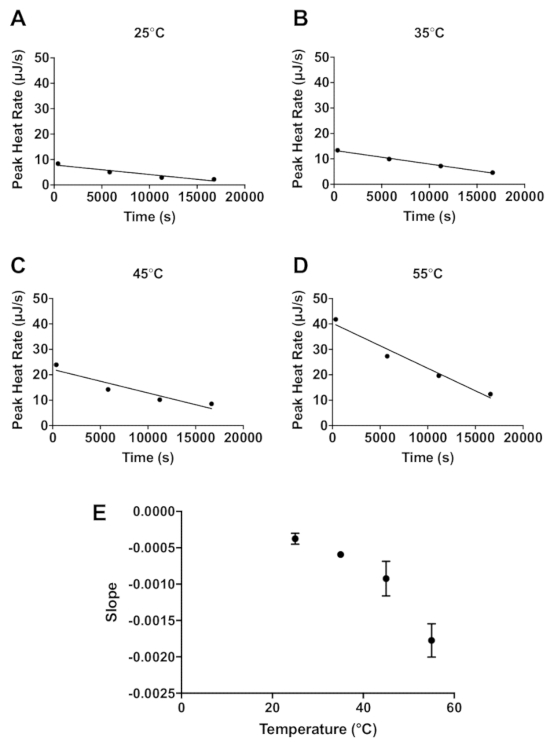
Figure 2: Stability of enzyme activity based on the rate of change in peak activity. The peak enzymatic activity for each of four injections relative to time (s) at 25 °C (A), 35 °C (B), 45 °C (C), and 55 °C (D). The linear fit of the data is represented by the solid line. The slopes of the fitted lines in parts A-D are plotted against temperature in E. Please click here to view a larger version of this figure.
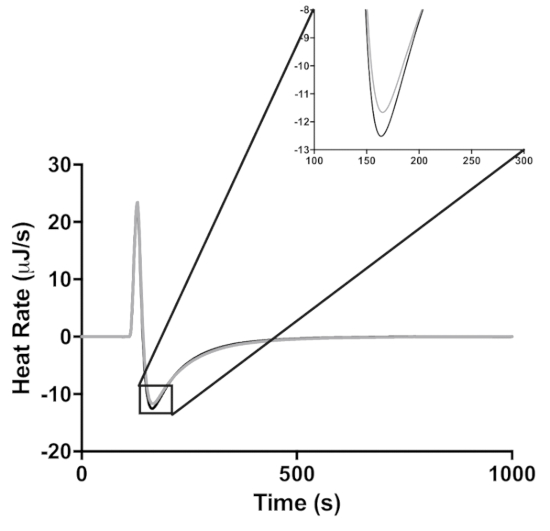
Figure 3: The effect of enzyme dilution on peak height at 25 °C. Lactase activity is measured at the enzyme concentration at the first and fourth injections of 20 mg/mL (black) and 18.62 mg/mL (gray), respectively. The inset is a zoomed in view of the peak enzyme activity. Please click here to view a larger version of this figure.
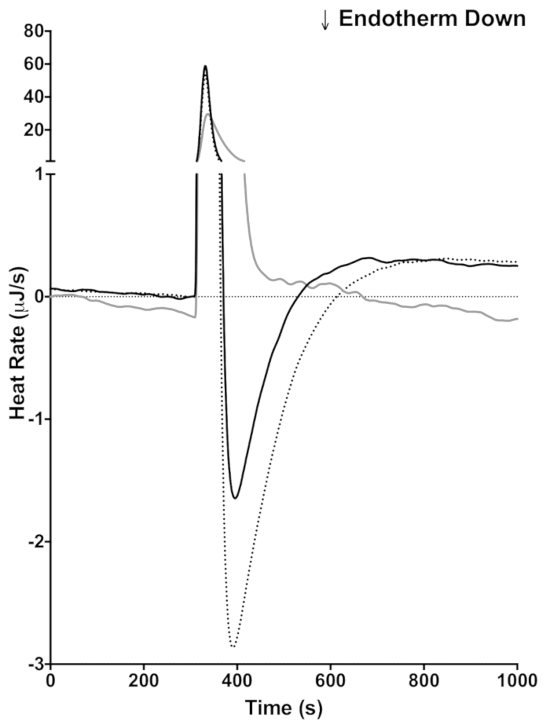
Figure 4: Measuring lactase activity in milk. ITC trace of an injection of BYU creamery fat free milk into buffer (gray), milk into 20 mg/mL lactase (black), and milk spiked with 5% additional lactose into 20 mg/mL (dotted). The y axis is discontinuous to show the endothermic enzyme reaction and the exothermic heat of mixing. Please click here to view a larger version of this figure.
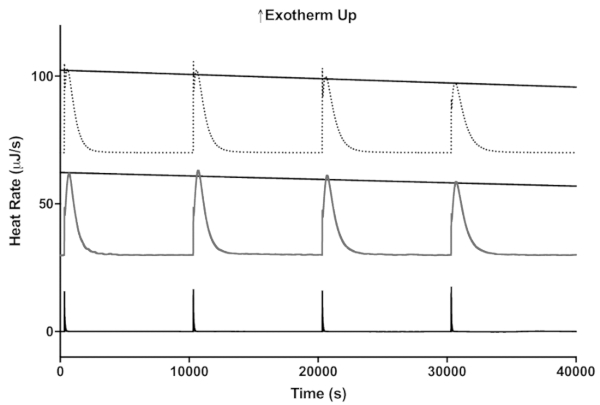
Figure 5: A representative example of how the ITC enzyme stability assay at 35 °C could be used to compare two different preparations of invertase. The activities of invertase immobilized on a nanofiber membrane and free enzyme are shown by the dotted and dark gray lines, respectively. The black line is the control with no invertase showing just the heat of mixing on appropriate scales. Please click here to view a larger version of this figure.
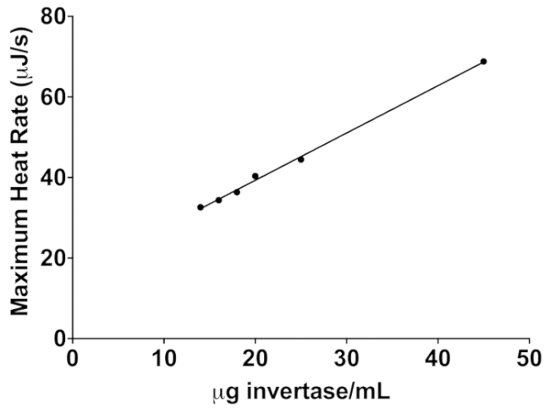
Figure 6: The linear relationship between peak exothermic heat rate and invertase concentration following a 4 µL injection of 600 mM sucrose. This standard curve can be used to determine the concentration of invertase in an unknown sample. Please click here to view a larger version of this figure.
Discussion
A major advantage of the ITC enzyme stability assay described here is automation. Once all the appropriate buffers and solutions are made, the set-up time for each assay is approximately 15 min for the person doing the assay. In contrast, the conventional assays for invertase and lactase activity require about 2 h with continual involvement of the person doing the assay and many enzymatic activity assays take considerably more person-hours. In a previous publication, we have demonstrated how data from the ITC method compares to a more traditional spectrophotrometric method for invertase activity11.
Another advantage of the ITC assay is its applicability to almost any enzyme12. Thus, testing the stability of different enzymes under a particular set of conditions does not require setting up several different assays. Additionally, the ITC assay is useful for determining the stability of the activity of a novel enzyme or of an enzyme that does not have a suitable colorimetric assay. The ITC assay can also be done in opaque media which is a major advantage in food science. Since most previous assays of enzyme activity use spectrophotometry, fluorescence, or luminescence to measure enzyme activity, they are not compatible with opaque or highly colored media. Although this work demonstrates only the effect of temperature on the stability of enzyme activity, the ITC method is applicable to most other conditions that affect enzyme stability (e.g., pH, salts, non-aqueous solvents, and denaturing agents).
As with all ITC experiments, the buffer used for the enzyme and substrate solution should match as closely as possible. Mismatch of the concentration or pH can cause large heat effects that exceed the dynamic range of the calorimeter. Use of the same stock buffer to prepare both the enzyme and substrate solutions is usually sufficient. But if not, the solutions can be matched by dialyzing both solutions against the same buffer solution.
A further condition is that the enzyme should not be substrate saturated, in which case, the peak height becomes independent of substrate concentration. Also, the time for the reaction to go to completion or to equilibrium between injections can become excessive and/or the signal can exceed the dynamic range of the calorimeter. However, the substrate concentration must be large enough to provide a strong heat signal. If the enzyme is substrate saturated, the substrate concentration can be decreased, or the enzyme concentration increased. When loading the calorimeter cell and syringe, one must ensure that no bubbles are present. If a bubble is injected or released from the cell during the course of an experiment, it will cause an anomalous event in the heat rate signal.
The ITC method is dependent on the enthalpy change of the catalyzed reaction and on the rate and amount of reaction. If the enthalpy change for the reaction is too small, the substrate is too insoluble, or the enzyme does not have sufficient activity, the heat rate may be too small to obtain sufficient signal. Additionally, if the enzyme is inhibited by low concentrations of the products, the decrease in enzymatic activity observed during the course of the experiment will be caused by product inhibition as well as by loss of activity over time. For example, in the lactase titration, the final concentration of 54 mM glucose and galactose causes a 27% decrease in the peak height of the enzyme activity at 55°C (data not shown). This is significantly less than the 78% decrease in peak height from injection 1 to injection 4 in Figure 1. However, this can be corrected by measuring the enzyme activity in the presence of the product concentration reached during the course of the assay. Further, titrating the enzyme with less substrate can reduce the amount of product inhibition.
Other limitations can occur because of the enzyme. For example, with each injection the enzyme will be the diluted. Because this ITC uses an overflow reaction vessel, during the course of the experiment a volume of solution equivalent to the injection size is removed with each injection. Thus, a small amount of enzyme is removed, and the products of the reaction increase with each injection. The effects of dilution and the amount of enzyme removed can be kept small if the injection size is kept small, and therefore a large concentration of substrate is often necessary. Because the ratio of injection volume to reaction vessel volume is smaller in ITCs with larger reaction vessels the dilution effect is smaller than in the low volume ITC used in this work. Additionally, each reaction for many enzymes will require microgram to low milligram quantities, so if you have an enzyme that is expensive or difficult to purify, this could prohibit the use of this assay.
Most of the issues that can arise during this assay are related to proper maintenance and cleaning of the ITC reaction vessel. If the ITC does not reach a stable baseline, this is often due to the reaction vessel not being sufficiently clean. We recommend using a strong detergent after each experiment when using proteins in the ITC because proteins can stick to the reaction vessel and interfere with the heat rate measurement. For a deeper clean, a 50% formic acid solution incubated at 55 °C for up to 12 h followed by cleaning with cleaning solution (Table of Materials), ethanol, and water will remove most contaminants.
Finally, because the ITC takes about 45 min to equilibrate after loading solutions, the activity of relatively unstable enzymes or under marginal conditions may not be possible. If the enzyme is inactivated during this time no signal, will be available or the decay may be too rapid after the first injection to obtain stability data.
Because the ITC assay directly measures enzyme activity, it can be extended to a number of other uses. For example, this assay could be done with increasing concentrations of an inhibitor to determine the inhibition constant, Ki, based on the decrease in enzyme activity with increasing inhibitor concentration. As shown in Figure 5, this protocol can be adapted for immobilized enzymes on a solid support. A nanofiber membrane was used in this work, but other solid supports could also be used if a method to insert and remove it through the small access tube can be developed. The ITC method could also be extended to enzymes that are altered chemically or genetically to improve thermal stability or to determine the amount of active enzyme in an unknown sample (i.e., Figure 6 shows the linear relationship between heat rate peak height and enzyme concentration).
Disclosures
The authors have nothing to disclose.
Acknowledgements
None
Materials
| a-Lactose | Fisher Scientific | unknown (too old) | 500g |
| Sodium Acetate, Anhydrous 99% min | Alfa Aesar | A13184-30 | 250g |
| Lactase | MP Bio | 100780 | 5g |
| Hydrocholric Acid Solution, 1N | Fisher Scientific | SA48-500 | 500mL |
| Benchtop Meter- pH | VWR | 89231-622 | |
| Ethanol 70% | Fisher Scientific | BP8231GAL | 1gallon |
| Micro-90 | Fisher Scientific | NC024628 | 1L (cleaning solution) |
References
- Anastas, P., Eghbali, N. Green chemistry: principles and practice. Chemical Society Reviews. 39 (1), 301-312 (2010).
- Jin, L. Q., et al. Immobilization of Recombinant Glucose Isomerase for Efficient Production of High Fructose Corn Syrup. Applied Biochemistry Biotechnoiogy. 183 (1), 293-306 (2017).
- Budak, &. #. 3. 5. 0. ;. &. #. 2. 1. 4. ;., Koçak, C., Bron, P. A., de Vries, R. P. . Microbial Cultures and Enzymes Dairy Technology. , 182-203 (2018).
- van Donkelaar, L. H. G., Mostert, J., Zisopoulos, F. K., Boom, R. M., van der Goot, A. J. The use of enzymes for beer brewing: Thermodynamic comparison on resource use. Energy. 115, 519-527 (2016).
- Rodriguez-Colinas, B., Fernandez-Arrojo, L., Ballesteros, A. O., Plou, F. J. Galactooligosaccharides formation during enzymatic hydrolysis of lactose: Towards a prebiotic-enriched milk. Food Chemistry. 145, 388-394 (2014).
- Lawrence, P. B., Price, J. L. How PEGylation influences protein conformational stability. Current Opinions in Chemical Biology. 34, 88-94 (2016).
- Bernal, C., Rodriguez, K., Martinez, R. Integrating enzyme immobilization and protein engineering: An alternative path for the development of novel and improved industrial biocatalysts. Biotechnology Advances. 36 (5), 1470-1480 (2018).
- Rigoldi, F., Donini, S., Redaelli, A., Parisini, E., Gautieri, A. Review: Engineering of thermostable enzymes for industrial applications. Applied Bioengeneering. 2 (1), 011501 (2018).
- Johnson, C. M. Differential scanning calorimetry as a tool for protein folding and stability. Archives of Biochemistry and Biophysics. 531 (1), 100-109 (2013).
- Chen, N. G., Gregory, K., Sun, Y., Golovlev, V. Transient model of thermal deactivation of enzymes. Biochimica et biophysica acta. 1814 (10), 1318-1324 (2011).
- Mason, M., et al. Calorimetric Methods for Measuring Stability and Reusability of Membrane Immobilized Enzymes. Jounal of Food Science. , (2017).
- Leksmono, C. S., et al. Measuring Lactase Enzymatic Activity in the Teaching Lab. Journal of visualized experiments : Journal of Visual Experiments. (138), e54377 (2018).

