Enhancing the Engraftment of Human Induced Pluripotent Stem Cell-derived Cardiomyocytes via a Transient Inhibition of Rho Kinase Activity
Summary
In this protocol, we demonstrate and elaborate on how to use human induced pluripotent stem cells for cardiomyocyte differentiation and purification, and further, on how to improve its transplantation efficiency with Rho-associated protein kinase inhibitor pretreatment in a mouse myocardial infarction model.
Abstract
A crucial factor in improving cellular therapy effectiveness for myocardial regeneration is to safely and efficiently increase the cell engraftment rate. Y-27632 is a highly potent inhibitor of Rho-associated, coiled-coil-containing protein kinase (RhoA/ROCK) and is used to prevent dissociation-induced cell apoptosis (anoikis). We demonstrate that Y-27632 pretreatment for human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs+RI) prior to implantation results in a cell engraftment rate improvement in a mouse model of acute myocardial infarction (MI). Here, we describe a complete procedure of hiPSC-CMs differentiation, purification, and cell pretreatment with Y-27632, as well as the resulting cell contraction, calcium transient measurements, and transplantation into mouse MI models. The proposed method provides a simple, safe, effective, and low-cost method which significantly increases the cell engraftment rate. This method cannot only be used in conjunction with other methods to further enhance the cell transplantation efficiency but also provides a favorable basis for the study of the mechanisms of other cardiac diseases.
Introduction
Stem cell-based therapies have shown considerable potential as a treatment for cardiac damage caused by MI1. The use of differentiated hiPSCs provides an inexhaustible source of hiPSC-CMs2 and opens the door for the rapid development of breakthrough treatments. However, many limitations to therapeutic translation remain, including the challenge of the severely low engraftment rate of implanted cells.
Dissociating cells with trypsin initiates anoikis3, which is only accelerated once these cells are injected into harsh environments like the ischemic myocardium, where the hypoxic environment accelerates the course toward cell death. Of the remaining cells, a large proportion is washed out from the implantation site into the bloodstream and spread throughout the periphery. One of the key apoptotic pathways is the RhoA/ROCK pathway4. Based on previous research, the RhoA/ROCK pathway regulates the actin cytoskeletal organization5,6, which is responsible for cell dysfunction7,8. The ROCK inhibitor Y-27632 is widely used during somatic and stem cell dissociation and passaging, to increase cell adhesion and reduce cell apoptosis9,10,11. In this study, Y-27632 is used to treat hiPSC-CMs prior to transplantation in an attempt to increase the cell engraftment rate.
Several methods aimed at improving the cell engraftment rate, such as heat shock and basement membrane matrix coating12, have been established. Aside from these methods, genetic technology can also promote cardiomyocyte proliferation13 or reverse nonmyocardial cells into cardiomyocytes14. From the bioengineering perspective, cardiomyocytes are seeded onto a biomaterial scaffold to improve the transplantation efficiency15. Unfortunately, the majority of these methods are complicated and costly. On the contrary, the method proposed here is simple, cost-efficient, and effective, and it can be used as a basal treatment before transplantation, as well as in conjugation with other technologies.
Protocol
All animal procedures in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Alabama at Birmingham and were based on the National Institutes of Health Laboratory Animal Care and Use Guidelines (NIH Publication No 85-23).
1. Preparation of Culture Media and Culture Plates
- Medium preparation
- For hiPSC medium, mix 400 mL of human pluripotent stem cell (hPSC) basal medium (Table of Materials 1) and 100 mL of hPSC 5x supplement; store the mixture at 4 °C.
- For RPMI 1640/B27 minus insulin (RB-) medium, mix 500 mL of RPMI 1640 and 10 mL of B27 supplement minus insulin.
- For RPMI 1640/B27 (RB+) medium, mix 500 mL of RPMI 1640, 10 mL of B27 supplement, and 5 mL of penicillin-streptomycin (pen-strep) antibiotic.
- For purification medium16, mix 500 mL of no-glucose RPMI 1640, 10 mL of B27 supplement, 5 mL of pen-strep antibiotic, and 1,000 μL of sodium DL-lactate solution (at a final concentration of 4 mM).
- For neutralizing solution, mix 200 mL of RPMI 1640, 50 mL of fetal bovine serum (FBS), 2.5 mL of pen-strep antibiotic, and 50 μL of Y-27632 (at a final concentration of 10 μM).
- For freezing medium, mix 9 mL of FBS, 1 mL of dimethyl sulfoxide (DMSO), and 10 μL of Y-27632 (at a final concentration of 10 μM).
- For extracellular matrix solution (EMS), thaw concentrated extracellular matrix (Table of Materials 1) at 4 °C until it has reached an evenly consistent liquid state. Precool pipette tips and tubes prior to making matrix aliquots of 350 µL each on ice, and store the aliquots at -20 °C. Before coating the plates, dilute one thawed aliquot into 24 mL of ice-cold DMEM/F12 medium (EMS); keep the EMS in 4 °C for up to 2 weeks.
NOTE: Perform all coating steps on ice to prevent basement membrane matrix solidification. - For Tyrode’s solution, take 90% of the final required volume of tissue culture grade water, add the appropriate amount of the following reagents, and stir until dissolved. Then, use 1 N NaOH to adjust the pH to 7.4. Add extra water to a final concentration of 140 mmol/L sodium chloride, 1 mmol/L magnesium chloride, 10 mmol/L HEPES, 5 mmol/L potassium chloride, 10 mmol/L glucose, and 1.8 mmol/L calcium chloride. Sterilize by filtration, using a 0.22 µm membrane.
- Cell culture plate coating
- For hiPSC culture plates, apply 1 mL of EMS to each well of a 6-well plate and incubate the plate for at least 1 h at 37 °C. Aspirate DMEM/F12 solution before seeding cells.
- For gelatin coating, dissolve 0.2 g of gelatin powder in tissue culture grade water to make a 0.2% (w/v) solution. Sterilize by autoclaving at 121 °C, 15 psi for 30 min. Coat each well of a 6-well plate with 2 mL of the solution and incubate for 1 h at 37 °C. Before passaging the cells, aspirate the solution and allow the plate to dry for at least 1 h at room temperature inside the tissue culture hood.
2. hiPSC maintenance and Cardiomyocyte Ddifferentiation
- Culture the hiPSCs in hiPSC medium (see step 1.1.1) with a daily medium change until the cells reach 80%–90% confluence per well.
NOTE: For maintenance purposes, hiPSCs are generally ready for passage every 4 days. - To passage hiPSCs, aspirate the medium and wash the well 1x with 1x phosphate-buffered saline (PBS). Add 0.5 mL of stem cell detachment solution (Table of Materials 1) to each well and incubate at 37 °C for 5–7 min.
NOTE: The incubation time depends on the density of the cells and the rate of dissociation, which can be observed under a microscope. - Neutralize the stem cell detachment solution with 1 mL of hiPSC medium supplemented with 5 μM Y-27632. Collect the mixture in a 15 mL centrifuge tube. Centrifuge the tube for 5 min at 200 x g, aspirate the supernatant without disturbing the cell pellet, and then, resuspend the cells in 1 mL of hiPSC medium containing 5 μM Y-27632.
- Seed the hiPSCs onto a new EMS-coated 6-well plate at a ratio between 1:6 to 1:18. Change the medium to hiPSC medium without Y27632 after 24 h.
- To freeze the cells, resuspend the dissociated hiPSCs in freezing medium at a concentration of 0.5–1 million/500 μL, place the suspension in cryovials, and store them in a -80 °C freezer. Transfer the frozen vials to liquid nitrogen after 24 h.
- To differentiate, replace the hiPSC medium with 2 mL of RB- medium supplemented with 10 μM GSK-3β inhibitor CHIR99021 (CHIR) at 80%–90% cell-confluence. Replace the medium with 3 mL of RB- medium after 24 h and incubate for an additional 48 h (day 3).
- On day 3, change the medium to 3 mL of RB- medium with 10 μM Wnt inhibitor IWR1 for 48 h (day 5), and then replace the medium with 3 mL of RB- medium and maintain for 48 h (day 7).
- On day 7, replace the medium with 3 mL of RB medium and change the medium every 3 days going forward. Spontaneous beating of hiPSC-CMs should be observed between days 7–10.
3. hiPSC-CMs Purification and Small Molecule Pre-treatment
NOTE: Highly purified, recombinant cell-dissociation enzymes (Table of Materials 1) were used to dissociate hiPSC-CMs.
- On day 21 after hiPSC-CM differentiation, aspirate the medium and wash the cells 1x with sterile PBS. Incubate the cells with 0.5 mL of cell-dissociation enzymes per well for 5–7 min at 37 °C. Repeatedly pipette the cell suspension using a 1 mL pipette in order to thoroughly dissociate the cells.
- After the cells are dissociated, add 1 mL of neutralizing solution to each well, collect the cell mixture into a 15 mL centrifuge tube and centrifuge at 200 x g for 3 min. Discard the supernatant and resuspend the cells in neutralizing solution.
- Replant the cells onto gelatin-coated 6-well plates at a density of 2 million cells per well.
- After 24–48 h, replace the culture medium with purification medium for 3–5 days.
NOTE: Purification is complete when more than 90% of the cells in each microscope view field are beating. To prevent further damage to the cardiomyocytes, do not extend the purification duration beyond 5 days. If the first round does not yield the necessary purity, replace the purification medium with RB+ medium for 1 day, and then complete a second round of purification. - Prior to transplantation, culture the cells in the treatment group for 12 h in RB+ medium supplemented with 10 μM Y-27632. Similarly, perform verapamil treatment on the cells in the RB+ medium with 1 μM verapamil for 12 h (hiPSC-CMs+VER).
- After treatment, wash the hiPSC-CMs 1x with PBS and incubate the cells with 0.5 mL of cell-dissociation enzymes per well for a maximum of 2 min. Repeatedly pipette the cell suspension, using a 1 mL pipette, to thoroughly dissociate the cells. Neutralize the cells with neutralizing solution, collect the cell mixture in a 15 mL centrifuge tube, and centrifuge at 200 x g for 3 min. Resuspend the cells in PBS at a concentration of 0.1 million cells/5 μL in preparation for injection.
4. Myocardial Infarction and Cell Transplantation
NOTE: All surgical instruments are presterilized with autoclave and are maintained in aseptic condition during multiple surgeries via a hot bead sterilizer (Table of Materials 2).
- Anesthetize NOD/scid mice (Table of Materials 2) with inhaled isoflurane (1.5%–2%). Monitor the anesthesia levels by the mice’s responses to a toe pinch. Place vet optical ointment onto the eyes to prevent them from drying during surgery.
- Supply 0.1 mg/kg buprenorphine subcutaneously for pain management prior to surgery.
- Position and secure the mouse in a supine position on a heated operating table, remove the hair from the ventral neck region and the left thorax using a depilatory cream, expose the skin, and disinfect it with 70% alcohol.
- Cut a 0.5 cm incision at the center of the neck using surgical scissors, separate the subcutaneous fat with sterile forceps, and expose the trachea. Introduce orally the intubation cannula into the trachea, connect the cannula to a small animal ventilator, and adjust the ventilation settings (set the tidal volume at 100–150 μL and the respiration rate at 100x–150x per min).
- After the mouse stabilizes, cut a 0.5 cm incision in the middle of the left chest skin. Use forceps to bluntly separate the muscle layer and make a small incision in the fifth intercostal space to expose the chest cavity. Place the retractor in the incision to open the thoracic cavity and locate the left descending coronary artery (LAD). Under a dissecting surgical microscope, ligate the LAD with an 8-0 nonabsorbable suture.
- Immediately following MI induction, inject 5 μL of hiPSC-CMs-RI, hiPSC-CMs+RI, hiPSC-CMs-VER, hiPSC-CMs+VER, or an equal volume of PBS into the mouse’s myocardium at each site (3 x 105 cells/animal, 1 x 105 cells/site), one in the infarct area and two in the areas around the infarct.
NOTE: Since 5 μL is very small volume, it is not easy to accurately control the volume by directly sucking it with a syringe. The lid of a sterile Petri dish can be turned over, and 5 μL of the cells can be first deposited on the lid with a pipette. Due to the surface tension, it will not spread but will condense into a small liquid mass. This can be easily absorbed and injected into the mouse’s myocardium. - Eliminate residual air in the thoracic cavity by filling it up with warm isotonic saline solution. Stitch the ribs, muscles, and skin in sequence with a 6-0 absorbable suture. Turn off the isoflurane anesthesia injection. Keep the surgery animals on the heating pad and closely observe them while they regain full consciousness. Then, place the animals in a clean cage. Do not return the animals to the company of other animals until they are fully recovered.
- Following surgery, inject the mice intraperitoneally with buprenorphine (0.1 mg/kg) every 12 h for 3 consecutive days and inject them with ibuprofen (5 mg/kg) every 12 h for one day. Perform subsequent analysis studies at specified time points.
NOTE: Follow your local animal care committee guidelines for analgesia.
5. Calcium Transient and Contractility Recording
- Autoclave 15 mm-diameter coverslips (Table of Materials 2) and tweezers in a small glass beaker at 121 °C, 15 psi for 15 min. Precool the coverslips and tweezers at 4 °C.
- Using the tweezers, place the coverslip into a 12-well plate and pipette 300 μL of extracellular matrix onto each coverslip.
NOTE: Do not let the matrix exceed the edge of the coverslip to prevent reducing the matrix coating amount. Incubate the 12-well plate in a 37 °C cell culture incubator for 1 h. - Aspirate the medium in the gelatin-coated 6-well plate with purified hiPSC-CMs, wash it 1x with PBS, and then digest it with 0.5 mL of cell-dissociation enzymes for 1.5 min. Add 1 mL of neutralizing solution, pipette repeatedly for no more than 5x–10x with a 1 mL pipette, and centrifuge the mixture at 200 x g for 3 min.
- Aspirate the coated extracellular matrix from the coverslips. Count the cell number and resuspend the cells in the neutralizing solution to a concentration of 40,000/300 μL, and then add 300 μL of the cell suspension onto each coverslip. Culture the plate in a 37 °C incubator.
- After 24 h, gently aspirate the neutralized solution, add 500 μL of RB+ medium to each well of the plate, and continue to culture for 2 days.
- Add Y-27632 (10 μM), Rho kinase activator (RA, 100 nM), verapamil (1 μM), or an equal volume of PBS into the culture medium of hiPSC-CMs, 12 h before calcium transient and contractility detection.
NOTE: In addition to the detection of the cells’ calcium transient and contractility after 12 h of chemical treatment, the detection of these parameters was also tested at 12, 24, 48, and 72 h after chemical withdrawal. - Take out the plate, discard the medium, and wash the plate 1x with PBS. Change the medium to a phenol-red-free DMEM containing 0.02% (w/v) of surfactant polyol (Table of Materials 1), 5 μM calcium indicator (Table of Materials 1), and the corresponding Y-27632 (10 μM), RA (100 nM), verapamil (1 μM), or an equal volume of PBS, and incubate the plate for 30 min at 37 °C.
- Discard the supernatant, wash the cells 3x with dye-free DMEM medium, and let the medium rest for 30 min to de-esterify the mapping dye.
- Place the cell-seeded coverslip in an open bath chamber and insert the chamber into a microincubation system equilibrated to 37 °C with an automatic temperature controller (Table of Materials 2), perfuse it with Tyrode’s solution, and continuously add Rho kinase inhibitor (RI), RA, or PBS to the perfusion solution.
- Use an inverted fluorescence microscope (Table of Materials 2) and a laser scanning head (Table of Materials 2) to record spontaneous calcium transient by employing an x-t line scan, using a 488 nm argon laser for dye excitation and a 515 ± 10 nm barrier filter to collect emission light. Record the spontaneous contraction of hiPSC-CMs using the Differential Interference Contrast functionality of the the confocal microscope (Table of Materials 2).
NOTE: Calcium transient recordings were processed and analyzed using MATLAB R2016A software (Table of Materials 4). Cell contraction change assays were performed using ImageJ software (Table of Materials 4).
Representative Results
The hiPSC-CMs used in this study were derived from human origin with luciferase reporter gene; therefore, the survival rate of the transplanted cells in vivo was detected by bioluminescence imaging (BLI)17 (Figure 1A,B). For histological heart sections, human-specific cardiac troponin T (hcTnT) and human nuclear antigen (HNA) double-positive cells were classified as engrafted hiPSC-CMs (Figure 1C). Both results indicated that Y-27632 pretreatment significantly improved the cell engraftment rate. The luciferase activity of the hiPSC-CM+RI group was increased roughly sixfold on days 3, 7, and 28 after the transplantation, compared to that of the hiPSC-CM-RI group (Figure 1B). Moreover, the hcTnT/HNA expression increased close to sevenfold in the hiPSC-CM+RI group, relative to that in the hiPSC-CM-RI group (Figure 1C).
The results also indicated that Y-27632 pretreatment regulated cytoskeletal changes in transplanted cells. On days 7 and 28 of transplantation, hiPSC-CMs+RI exhibited a larger and more defined rod-shaped cytoskeletal structure compared to hiPSC-CMs-RI (Figure 1D).
Moreover, Y-27632 pretreatment has the potential to reduce transplanted hiPSC-CM apoptosis in vivo. TUNEL staining showed that the number of TUNEL-positive cells was significantly decreased in the hiPSC-CM+RI group relative to that in the hiPSC-CM-RI group on day 2 after transplantation (Figure 1E).
ROCK inhibition promoted the adhesion of transplanted cells and had the potential to further increase the retention of implanted cells at administration sites. Western blot and cardiac tissue immunostaining suggested that Y-27632 pretreatment reversibly promoted the increased expression of integrin β1 and N-cadherin and decreased the expression of phospho-myosin light chain 2 (p-MLC2) (Figure 2A,B).
The mouse cell line HL-1 was selected as the control CMs for in vitro cell attachment experiments. The results indicated that Y-27632 pretreatment significantly increased hiPSC-CM adherence relative to HL-1. To further confirm these findings, hiPSC-CMs+RI were incubated with N-cadherin or integrin β1 neutralizing antibody for 1 h, resulting in a vanishing of the improved adhesion seen previously (Figure 2C).
Compared with the hiPSC-CM-RI group, the contractile force in the hiPSC-CM+RI group was reduced by 32%, and in the hiPSC-CM+RA group (hiPSC-CMs pretreated with ROCK activator, Table of Materials 1), it was increased by 42% (Figure 3A). Meanwhile, compared with the hiPSC-CM-RI group, peak calcium transient fluorescence (Peak ΔF/F0) for the hiPSC-CM+RI group was reduced by 41%, and the calcium transient duration (CaTD50) was reduced by 11% (Figure 3B,C). In contrast, Peak ΔF/F0 for the hiPSC-CM+RA group was increased by 48%, and CaTD50 was increased by 13% (Figure 3B,C).
In addition, a pretreatment of the cardiomyocytes with Y-27632 for 12 h prior to the transplantation significantly reduced the expression of cTnI and cTnT (Figure 3D), both of which are troponin (Tn) subunits that regulate cardiomyocyte contraction.
Similar to Y-27632, a verapamil pretreatment (1 μM, 12 h) significantly increased the engraftment rate of hiPSC-CMs in induced MI mice. The hypothesis was confirmed through the observation of an increased luciferase signal in the bioluminescence assay (Figure 4A) and increased numbers of hcTnT/HNA double-positive cells on days 7 and 28 after cell transplantation (Figure 4B).
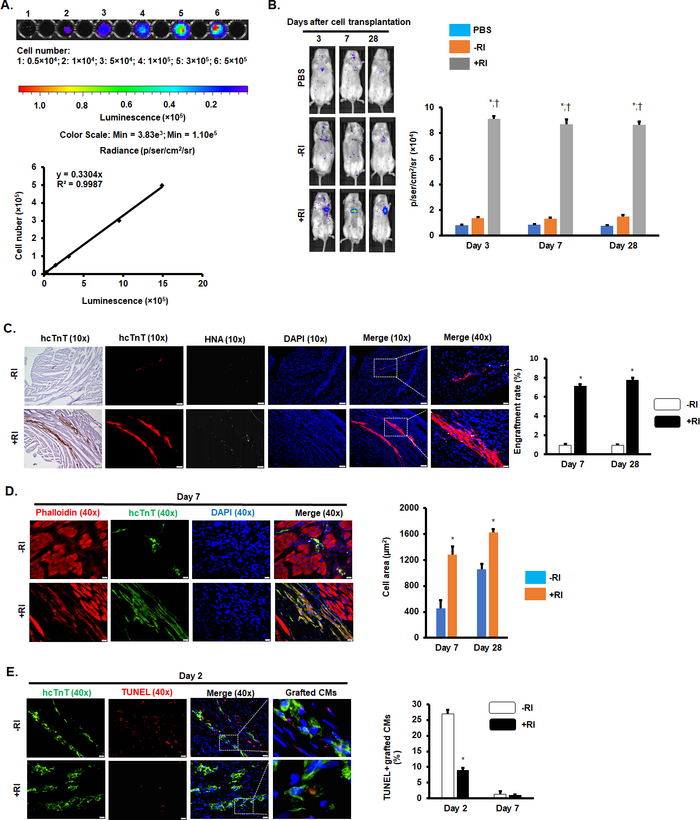
Figure 1: Y-27632 pretreatment increased the engraftment rate of hiPSC-CMs in MI mice hearts. (A) Standard curve of BLI measurements. (B) Luciferin signal in NOD/scid mice treated with PBS, hiPSC-CMs-RI, or hiPSC-CMs+RI on days 3, 7, and 28 after surgery. n = 6-9 mice per group. *P < 0.05 vs. PBS; †P < 0.05 vs. hiPSC-CMs-RI. (C) Immunostaining of heart sections for hcTnT and HNA. Scale bars = 100 µm (10x images); 20 µm (40x images). n = 5 mice per group. *P < 0.05 vs. hiPSC-CMs-RI. (D) Representative images of heart sections stained with phalloidin and hcTnT. Scale bar = 20 µm. n = 5 mice per group. *P < 0.05 vs. hiPSC-CMs-RI. (E) Representative images of heart sections for TUNEL staining. Scale bar = 20 µm. n = 4-6 mice per group. *P < 0.05 vs. hiPSC-CMs-RI. This figure has been modified from Zhao et al.18. Please click here to view a larger version of this figure.
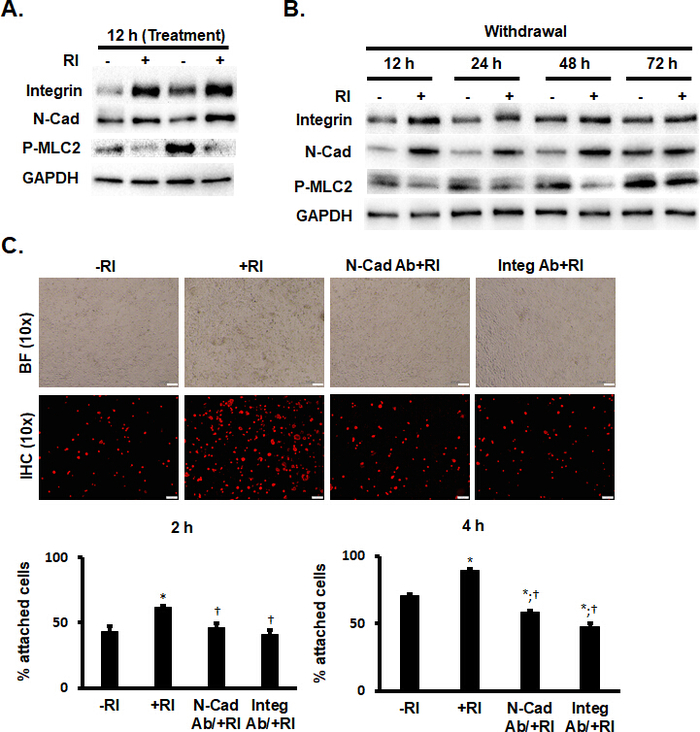
Figure 2: Y-27632 enhanced the adhesion of hiPSC-CMs by maintaining the expression of adhesion proteins. (A and B) Western blot analysis of the expression of integrin ß1, N-cadherin, and phosphorylation of MLC2 (p-MLC2) in hiPSC-CMs treated with RI and in nontreated hiPSC-CMs. n = 5 replicates per group. (C) hcTnT immunofluorescence staining analysis of -RI and +RI hiPSC-CMs with and without a pretreatment of anti-N-cadherin (N-Cad) and anti-integrin ß1 (Integ) antibodies. Scale bars = 100 µm. *P < 0.05 vs. hiPSC-CMs-RI; †P < 0.05 vs. hiPSC-CMs+RI. This figure has been modified from Zhao et al.18. Please click here to view a larger version of this figure.
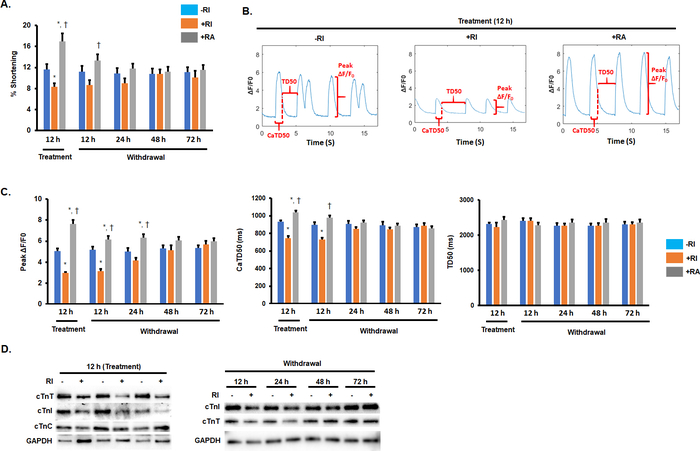
Figure 3: Pretreatment with Y-27632 reduced the contractility of hiPSC-CMs and down-regulated the expression of cardiac troponin subunits. (A) Quantification of the percentage of shortening of hiPSC-CMs exposed to RI and RA treatment. *P < 0.05 vs. hiPSC-CMs-RI; †P < 0.05 vs. hiPSC-CMs+RI. (B and C) Representative images and quantification of calcium transient measurements of hiPSC-CMs treated with RI and RA and of a nontreated group. *P < 0.05 vs. hiPSC-CMs-RI; †P < 0.05 vs. hiPSC-CMs+RI. (D) Western blot analysis of the expression of cardiac troponin subunits (cTnC, cTnT, and cTnI) in hiPSC-CMs treated with RI and RA and in a nontreated group. This figure has been modified from Zhao et al.18. Please click here to view a larger version of this figure.
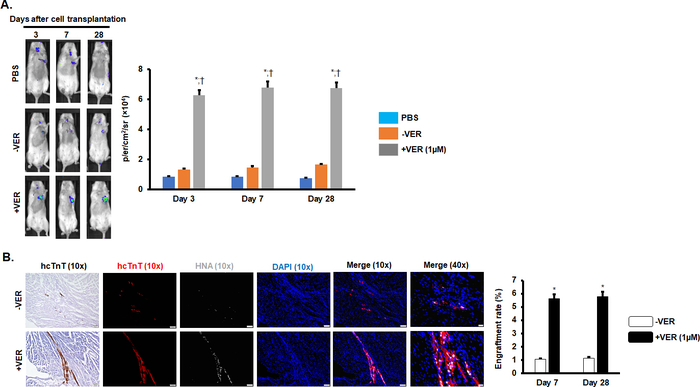
Figure 4: Verapamil pretreatment improved the engraftment rate of hiPSC-CMs in MI mice. (A) Luciferin signal in NOD/scid mice treated with PBS, hiPSC-CMs-VER, or hiPSC-CMs+VER on days 3, 7, and 28 after surgery. n = 8 mice per group. *P < 0.05 vs. PBS; †P < 0.05 vs. hiPSC-CMs-VER. (B) Immunostaining of heart sections for hcTnT and HNA. For 10x images, Scale bars = 100 µm (10x images); 20 µm (40x images). n = 5 mice per group. *P < 0.05 vs. hiPSC-CMs-VER. This figure has been modified from Zhao et al.18. Please click here to view a larger version of this figure.
Discussion
The key steps of this study include obtaining pure hiPSC-CMs, improving the activity of hiPSC-CMs through Y-27632 pretreatment, and finally, transplanting a precise amount of hiPSC-CMs into a mouse MI model.
The key issues addressed here were that, first, we optimized the glucose-free purification methods19 and established a novel efficient purification system. The system procedure included applying cell-dissociation enzymes, replanting cells in gelatin-coated plates, culturing the cells in the neutralization medium for 24 h after replating, and minimizing the digestion time of the cells before transplantation, all of which were performed to achieve the highest activity of the cells while obtaining pure cardiomyocytes.
Second, we elaborated on the pretreatment of hiPSC-CMs with Y-27632 at 12 h before cell injection. The anoikis is usually induced by the modification of cells’ adhesion proteins, such as integrins4,20 and N-cadherin21, that lead to the activation of the apoptotic pathway. We demonstrated that Y-27632 pretreatment could promote the adhesion of hiPSC-CMs to the transplantation site through maintaining the expression of integrin β1 and N-cadherin, suppressing the expression of p-MLC2, which is related to changes in the cytoskeletal architecture22,23. Seven days after hiPSC-CM transplantation, hiPSC-CMs+RI resulted in a greater cell engraftment area, finer defined rod shapes, and a more complete cytoskeletal organization compared to hiPSC-CMs-RI (Figure 1B-D).
Third, we established a novel intracellular injection system in mouse MI models, with 5 μL per injection point to accurately inject the required number of cells, while avoiding a decrease in the mouse survival rate due to excessive injection volume.
Fourth, we elaborated on how to determine the changes in cell contraction and calcium transient in hiPSC-CMs after pretreatment with RI or RA. We found that Y-27632 can reversibly reduce the cell contractility and calcium transient. The hypothesis here is that due to the lower energy requirements of the transplanted cells, the engraftment rate increased. Similar effects can be achieved using the calcium channel inhibitor verapamil. The proposed mechanism resulting in the contractility inhibition is that Y-27632 reduces the expression of troponin subunits cTnT and cTnI in hiPSC-CMs.
The main limitation is that Y-27632 only played a transient role during the transplantation. How to inhibit Rho kinase for a longer period, thus further improving the transplantation efficiency of hiPSC-CMs, is a problem to be solved in the future. As for more applications of this method, ROCK inhibitor can not only improve the activity of cardiomyocytes during transplantation but also enhance the activity of other cells; therefore, it can also be used during the pretreatment in the transplantation processes of other cells. Moreover, the approach presented here lays a good experimental foundation for more research on heart disease.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Dr. Joseph C. Wu (Stanford University) for kindly providing the Fluc-GFP construct and Dr. Yanwen Liu for excellent technical assistance. This study is supported by the National Institutes of Health RO1 grants HL95077, HL114120, HL131017, HL138023, UO1 HL134764 (to J.Z.), and HL121206A1 (to L.Z.), and a R56 grant HL142627 (to W.Z.), an American Heart Association Scientist Development Grant 16SDG30410018, and the University of Alabama at Birmingham Faculty Development Grant (to W.Z.).
Materials
| Reagent | |||
| Accutase (stem cell detachment solution) | STEMCELL Technologies | #07920 | |
| B27 minus insulin | Fisher Scientific | A1895601 | |
| B27 Supplement | Fisher Scientific | 17-504-044 | |
| CHIR99021 | Stem Cell Technologies | 72054 | |
| DMEM (1x), high glucose, HEPES, no phenol red | Thermofisher | 20163029 | |
| Fetal bovine serum | Atlanta Biologicals | S11150 | |
| Fluo-4 AM (calcium indicator) | Invitrogen/Thermofisher | F14201 | |
| Glucose-free RPMI 1640 | Fisher Scientific | 11879020 | |
| IWR1 | Stem Cell Technologies | 72562 | |
| Matrigel (extracellular matrix ) | Fisher Scientific | CB-40230C | |
| mTeSR (human pluripotent stem cells medium) | STEMCELL Technologies | 85850 | |
| Pen-strep antibiotic | Fisher Scientific | 15-140-122 | |
| Pluronic F-127 (surfactant polyol) | Sigma-Aldrich | P2443 | |
| Rho activator II | Cytoskeleton | CN03 | |
| RPMI1640 | Fisher Scientific | 11875119 | |
| Sodium DL-lactate | Sigma-Aldrich | L4263 | |
| TrypLE (cell-dissociation enzymes) | Fisher Scientific | 12-605-010 | |
| Verapamil | Sigma-Aldrich | V4629 | |
| Y-27632 | STEMCELL Technologies | 72304 | |
| Name | Company | Catalog Number | Comments |
| Equipment and Supplies | |||
| IVIS Lumina III Bioluminescence Instruments | PerkinElmer | CLS136334 | |
| 15 mm Coverslips | Warner | CS-15R15 | |
| Centrifuge | Eppendorf | 5415R | |
| Confocal Microscope | Olympus | IX81 | |
| Cryostat | Thermo Scientific | NX50 | |
| Dual Automatic Temperature Controller | Warner Instruments | TC-344B | |
| Electrophoresis Power Supply | BIO-RAD | 1645050 | |
| Fluoresence Microscope | Olympus | IX83 | |
| High Speed Camera | pco | 1200 s | |
| Laser Scan Head | Olympus | FV-1000 | |
| Low Profile Open Bath Chamber (mounts into above microincubation system) | Warner Instruments | RC-42LP | |
| Microincubation System | Warner Instruments | DH-40iL | |
| Minivent Mouse Ventilator | Harvard Apparatus | 845 | |
| NOD/SCID mice | Jackson Laboratory | 001303 | |
| Precast Protein Gels | BIO-RAD | 4561033 | |
| PVDF Transfer Packs | BIO-RAD | 1704156 | |
| Trans-Blot System | BIO-RAD | Trans-Blot Turbo | |
| Hot bead sterilizer | Fine Science Tools | 18000-45 | |
| Name | Company | Catalog Number | Comments |
| Antibody | |||
| Anti-human Nucleolin (Alexa Fluor 647) | Abcam | ab198580 | |
| Cardiac Troponin T | R&D Systems | MAB1874 | |
| Cardiac Troponin C | Abcam | ab137130 | |
| Cardiac Troponin I | Abcam | ab47003 | |
| Cy5-donkey anti-mouse | Jackson ImmunoResearch Laboratory | 715-175-150 | |
| Cy3-donkey anti-rabbit | Jackson ImmunoResearch Laboratory | 711-165-152 | |
| Fitc-donkey anti-mouse | Jackson ImmunoResearch Laboratory | 715-095-150 | |
| GAPDH | Abcam | ab22555 | |
| Human Cardiac Troponin T | Abcam | ab91605 | |
| Integrin β1 | Abcam | ab24693 | |
| Ki67 | EMD Millipore | ab9260 | |
| N-cadherin | Abcam | ab18203 | |
| Phospho-Myosin Light Chain 2 | Cell Signaling Technology | 3671s | |
| Name | Company | Catalog Number | Comments |
| Software | |||
| Matlab | MathWorks | R2016A | |
| Image J | NIH | 1.52g |
References
- Menasche, P., et al. Towards a clinical use of human embryonic stem cell-derived cardiac progenitors: a translational experience. European Heart Journal. 36 (12), 743-750 (2015).
- Burridge, P. W., Keller, G., Gold, J. D., Wu, J. C. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 10 (1), 16-28 (2012).
- Frisch, S. M., Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. Journal of Cell Biology. 124 (4), 619-626 (1994).
- Haun, F., et al. Identification of a novel anoikis signalling pathway using the fungal virulence factor gliotoxin. Nature Communications. 9 (1), 3524 (2018).
- Ohashi, K., et al. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. Journal of Biological Chemistry. 275 (5), 3577-3582 (2000).
- Katoh, K., Kano, Y., Noda, Y. Rho-associated kinase-dependent contraction of stress fibres and the organization of focal adhesions. Journal of The Royal Society Interface. 8 (56), 305-311 (2011).
- Paoli, P., Giannoni, E., Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochimica et Biophysica Acta. 1833 (12), 3481-3498 (2013).
- Legate, K. R., Fassler, R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. Journal of Cell Science. 122 (Pt 2), 187-198 (2009).
- Watanabe, K., et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature Biotechnology. 25 (6), 681-686 (2007).
- Emre, N., et al. The ROCK inhibitor Y-27632 improves recovery of human embryonic stem cells after fluorescence-activated cell sorting with multiple cell surface markers. PLoS One. 5 (8), e12148 (2010).
- Ni, Y., Qin, Y., Fang, Z., Zhang, Z. ROCK Inhibitor Y-27632 Promotes Human Retinal Pigment Epithelium Survival by Altering Cellular Biomechanical Properties. Current Molecular Medicine. 17 (9), 637-646 (2017).
- Laflamme, M. A., et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotechnology. 25 (9), 1015-1024 (2007).
- Zhu, W., Zhao, M., Mattapally, S., Chen, S., Zhang, J. CCND2 Overexpression Enhances the Regenerative Potency of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Remuscularization of Injured Ventricle. Circulation Research. 122 (1), 88-96 (2018).
- Song, K., et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 485 (7400), 599-604 (2012).
- Ye, L., et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 15 (6), 750-761 (2014).
- Tohyama, S., et al. Glutamine Oxidation Is Indispensable for Survival of Human Pluripotent Stem Cells. Cell Metabolism. 23 (4), 663-674 (2016).
- Ong, S. G., et al. Microfluidic Single-Cell Analysis of Transplanted Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes After Acute Myocardial Infarction. Circulation. 132 (8), 762-771 (2015).
- Zhao, M., et al. Y-27632 Preconditioning Enhances Transplantation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Myocardial Infarction Mice. Cardiovascular Research. , (2018).
- Tohyama, S., et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 12 (1), 127-137 (2013).
- Silginer, M., Weller, M., Ziegler, U., Roth, P. Integrin inhibition promotes atypical anoikis in glioma cells. Cell Death & Disease. 5, e1012 (2014).
- Lelievre, E. C., et al. N-cadherin mediates neuronal cell survival through Bim down-regulation. PLoS One. 7 (3), e33206 (2012).
- Murata, K., et al. Increase in cell motility by carbon ion irradiation via the Rho signaling pathway and its inhibition by the ROCK inhibitor Y-27632 in lung adenocarcinoma A549 cells. Journal of Radiation Research. 55 (4), 658-664 (2014).
- Srivastava, K., Shao, B., Bayraktutan, U. PKC-beta exacerbates in vitro brain barrier damage in hyperglycemic settings via regulation of RhoA/Rho-kinase/MLC2 pathway. Journal of Cerebral Blood Flow & Metabolism. 33 (12), 1928-1936 (2013).

