Transuterine Fetal Tracheal Occlusion Model in Mice
Summary
Various animal models of congenital diaphragmatic hernia and fetal tracheal occlusion present advantages and disadvantages regarding ethical issues, cost, surgical difficulty, size, survival rates, and availability of genetic tools. This model provides a new tool to study the impact of both tracheal occlusion and increased luminal pressure on lung development.
Abstract
Fetal tracheal occlusion (TO), an established treatment modality, promotes fetal lung growth and survival in severe congenital diaphragmatic hernia (CDH). Following TO, retention of the secreted epithelial fluid increases luminal pressure and induces lung growth. Various animal models have been defined to understand the pathophysiology of CDH and TO. All have their own advantages and disadvantages such as the difficulty of the technique, the size of the animal, cost, high mortality rates, and the availability of genetic tools. Herein, a novel transuterine model of murine fetal TO is described. Pregnant mice were anesthetized, and the uterus exposed via a midline laparotomy. The trachea of selected fetuses were ligated with a single transuterine suture placed behind the trachea, one carotid artery, and one jugular vein. The dam was closed and allowed to recover. Fetuses were collected just before parturition. Lung to body weight ratio in TO fetuses was higher than that in control fetuses. This model provides researchers with a new tool to study the impact of both TO and increased luminal pressure on lung development.
Introduction
Congenital diaphragmatic hernia (CDH) occurs in 1:2500 pregnancies and results in pulmonary hypoplasia and neonatal pulmonary hypertension1,2,3,4,5,6. Fetal tracheal occlusion (TO) is an established prenatal therapy in severe CDH patients involving fetoscopy in the 26-30th gestational week in which a balloon is placed just above the carina and then removed in the 32nd gestational week. This temporary TO induces fetal lung growth and improves survival. Congenital High Airway Obstruction Syndrome is a lethal condition associated with lung hyperplasia, which inspired surgeons to perform artificial occlusion of the trachea to promote retention of the secreted epithelial fluid. This occlusion increased luminal pressure and induced lung growth7. However, the occlusion should be reversed to enable epithelial cell maturation.
Various animal models of CDH and TO – ovine, rabbit, rat, and mouse – have been developed to understand the pathophysiology of CDH and TO. All have their own advantages and disadvantages such as the difficulty of the technique, the size of the animal, cost, high mortality rates, and the availability of genetic tools. Although the surgical technique used for the ovine model is very similar to that used in humans and could be reversed, the major drawbacks of this model are the expense of the animal, the long gestational period, and the limited number of surgeries possible. The rabbit model has a shorter gestational period and is less expensive than the sheep model. However, the rabbit model is irreversible8,9. The murine model has the lowest cost, the highest number of fetuses per pregnancy, the best-characterized genome, and widely available tools for cellular and molecular analyses. However, a key drawback is the lack of reversibility of the TO, preventing full understanding of the impact of TO. Herein, a method is presented that combines all the advantages of the previously mentioned models and creates an easy, potentially reversible, and minimally invasive rodent TO model.
Protocol
All experiments have complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80023, revised 1978). The procedure was approved with IACUC protocol #2016-0068 by the Cincinnati Children's Research Foundation Institutional Animal Care and Use Committee.
1. Preparation
- To mate age-matched wild-type (WT) C57BL/6 mice, place them in the same cage at 6:00 p.m. and separate them at 9:00 a.m. the next day.
- To determine embryonic day 0 (E0), look at the vaginal plug, which has a homogeneous outer zone attached to the vaginal wall and an inner zone that is fibrous and includes some spermatozoa that form entangled masses mixed with the fibers of the plug material.
- Record the weight of the mice at the time of mating.
- Re-weigh the mice on E10 to ensure ongoing pregnancy.
- Perform the surgery on E16.5 (early canalicular stage).
- Sterilize the instruments that are going to be used during surgery: scissors, needle holder, forceps, clamps, and surgical knives and handles.
- Pre-heat the surgery platform to 24 °C and prepare warm saline (24 °C) prior to surgery.
- Create a warm environment for recovery, and leave wet food inside the cage for the early feeding.
- Stay with the operated animals until they can feed themselves.
- Keep the operated mice alone in their individual cages after the surgery.
2. Anesthesia
- Apply subcutaneous 0.1 mg/kg of buprenorphine to the pregnant dams 1 h before the procedure.
- Use inhaled 5 mL/h of isoflurane for induction and 2 mL/h continuously during the procedure for anesthesia.
- Monitor the movements of the chins of the pregnant mice.
3. Laparotomy
- Clean the abdominal surface with alcohol and povidone-iodine. Maintain sterile conditions throughout the operation.
- Perform a vertical incision for the laparotomy of pregnant dams. Cut all layers separately.
- Identify uterine horns on each side.
- Determine the candidate fetuses for the surgery.
NOTE: Do not operate on the fetuses that are the nearest to the vagina. - Operate on two fetuses in each uterine horn if there are an even number of fetuses on each side (4 most of the time), and on 1 fetus in each uterine horn if there are an odd number of the uterus(3 most of the time).
4. Tracheal occlusion
- Use 2.5x magnification glasses for visualization.
- Position the uterine horn in a transverse fashion.
- Take the pups, facing upward, between two fingers using the eyes of the pups and the tail as a guide to position the fetus.
- Apply gentle pressure to the pup's head to allow extension of the head and therefore, visualization of the neck.
- Use a 6.0 polypropylene suture with an atraumatic needle to perform TO (Figure 1). Keep the placenta on the side and far from the entrance and exit points of the needle.
- Insert the needle transversely through the side of the uterus away from the placenta through the 1/3rd anterior part of the neck.
- Move the needle gently until the midline of the neck and direct it to the anterior part, then exit the neck between the trachea and opposite the carotid sheath and uterus.
- Knot the suture, taking care to maintain the integrity of the membranes and uterine wall, and keep the umbilical cord safe during knotting.
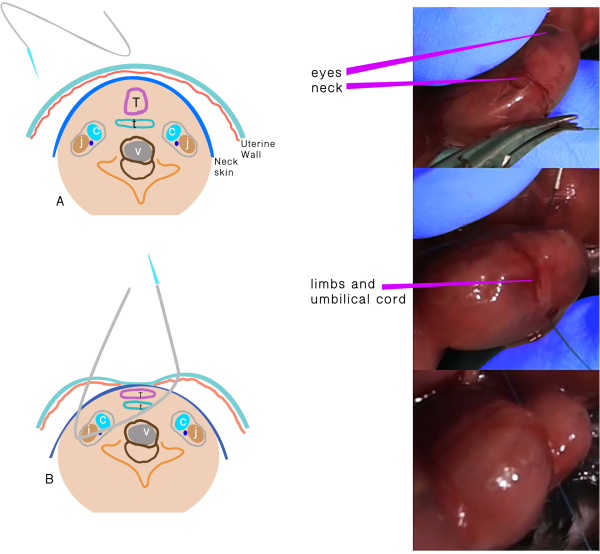
Figure 1: Tracheal occlusion. (A) The transuterine suture passing through the neck. (B) Schematic representation of the structures after the suture passes through and before the knot. Abbreviations: C = Carotid artery; J = Jugular vein; T =Trachea; E = Esophagus; V = Vertebra. Please click here to view a larger version of this figure.
5. Abdominal wall closure
- Replace the uterine horn in the abdomen.
- Inject 2 mL of warm sterile saline into the peritoneal cavity before closure.
- Put a running 5/0 polyglactin suture to close the abdominal wall, and close the skin with a non-running silk suture.
- Apply 0.1 mg/kg of buprenorphine intraperitoneally for analgesia, and allow the recovery of the dam in a warm incubator.
6. Harvest
- Apply anesthesia to the pregnant dam, and harvest all fetuses at E18.5 by cesarean section.
- Check the viability of the fetuses by watching the movements of the fetuses.
- Use at least two different techniques for the euthanasia: carbon dioxide insufflation and cervical dislocation.
- Remove the bodies per the regulation of veterinary laboratory.
- Weigh all fetuses.
- Perform a vertical incision on the thorax for thoracotomy to remove the lungs.
- Dissect the lungs of embryos, and weigh them to calculate total lung to body weight ratio (LBWR = (left lung weight + right lung weight)/body weight x100).
7. Histology
- Snap-freeze the tissues in liquid nitrogen, optimal cutting temperature compound, and dry ice.
- Cut the samples in 10 µm sections using a cryostat, and mount them on poly-lysine-coated slides.
- Bake the slides at 60 °C overnight, and stain the baked slides with hematoxylin and eosin before mounting them for image acquisition at 10-20x magnification using a widefield microscope.
8. Tissue processing for protein and DNA analyses
- Snap-freeze the dissected fetal lungs, and homogenize them in 300 µL of radioimmunoprecipitation assay buffer. Centrifuge at 4 °C for 5 min at 18,000 × g.
- Extract and quantify protein, DNA, and RNA10,12.
Representative Results
This study examined 37 fetuses: 20 (54.1%) as TO vs. 17 (45.9%) as control. As the trachea could not be occluded in 4 fetuses in the TO group, they were excluded from the study. There was no significant difference in mortality in both groups: 4 fetuses (25%) in the TO group and 2 fetuses (12%) in the control group (p=0.334, odds ratio (OR) 2.5, 95% confidence interval (CI) 0.39-16.05). The mean body weight, lung weight, and lung to body weight ratio (LBWR) were higher in the TO group than in the control group (Table 1). There was a significant difference in LBWR (p=0.006) between the TO and control groups.
DNA, RNA, and protein were quantified to determine the reason for the difference in LBWR (Figure 2). Lung DNA amounts and the DNA/protein ratio were higher in the TO group, no difference was observed in lung RNA, and protein amounts were lower in the TO group than in the control group,as previously observed in the rabbit TO model in which epithelial hyperplasia was noted12. The diameters of the airways in the TO group also demonstrated an increase.
Histological analyses of the E18.5 lungs showed the late canalicular/early saccular stage of lung development with developing airspaces and thickened interstitium between epithelial surfaces in the control samples while the lungs in the TO group had dilated central and distal airspaces with subjectively higher numbers of nuclei (Figure 2). This increased cellularity is consistent with the noted increase in the amount of lung DNA.
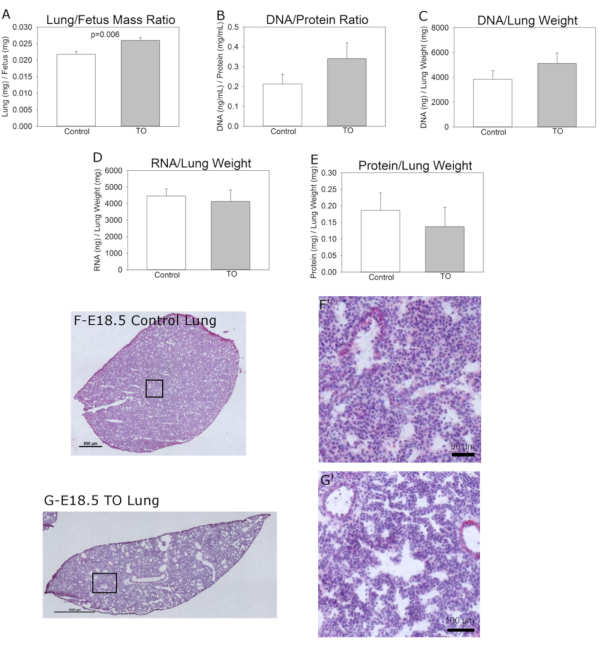
Figure 2: Features of the groups. (A) Normalized lung to fetus weight ratio, (B) Lung DNA to protein ratio, (C) Lung DNA content normalized to lung weight, (D) Lung RNA content normalized to lung weight, and (E) Lung protein content normalized to lung weight. (F) Representative hematoxylin and eosin images of C57BL/6 E18.5 lungs without (scale bar = 50 µm) and (G) with fetal transuterine tracheal occlusion showing hyperplasia of conducting airways and increased size of distal airspaces; scale bar = 100 µm. Comparison of control (n=9) and tracheal occlusion (TO) (n=6) was performed using Student's t-test. Please click here to view a larger version of this figure.
| Table 1: Morphometrical results of groups | |||
| TO | Control | p | |
| Fetus weight (mg) | 1100.52 ± 229.38 | 1087.15 ± 172.32 | 0.896 |
| Lung weight (mg) | 28.41 ± 5.87 | 23.38 ± 3.09 | 0.043 |
| LBWR | 0.0259 ± 0.0021 | 0.0217 ± 0.0028 | 0.006* |
| Values expressed as means ± standard deviations. Abbreviations: LBWR = Lung to Fetal Body Weight Ratio; TO = tracheal occlusion. | |||
| *95% Confidence Interval 0.0222–0.0249. Groups compared by Student's t-test. | |||
Table 1: Morphometrical results of groups
Discussion
This method describes a surgical procedure of fetal tracheal occlusion in mice and its impact on lung development. There are some critical steps in the protocol that should be carefully performed for successful TO. The warmth of the platform on which the surgery takes place and the saline introduced into the peritoneal cavity is crucial for the progression of the pregnancy. In addition, a slight pressure has to be applied to the head of the pups to ensure exposure of the neck.
A 6.0 polypropylene suture is the only suture that can be used for this technique. The needles of sutures larger than 6.0 are thicker and destroy the structures around the trachea in the neck, resulting in the loss of the fetus. The needles of the thinner sutures are very short and cannot pass through the neck of an E16.5 pup (early canalicular stage). Moreover, a cutting needle is not appropriate as it might destroy the adjacent structures.
This model has some limitations. First, there is a difference in the correlation of the lung developmental stages and gestational period between mice and humans. Second, it is difficult to develop CDH in mice and finally, hemodynamic studies are difficult to conduct in mouse models. However, the short learning curve in this study resulted in a dramatic decrease in the fetal mortality rate. As stated earlier, the cost of the animals as well as their maintenance, the number of fetuses per pregnancy, the length of the pregnancy period, and limited availability of genetic tools are the main limiting factors in rabbit and sheep models.
The first advantage of this mouse model is that it eliminates the need for hysterotomy for TO and has the potential to be reversed in utero11, which accounts for low mortality rates observed in this study. Second, the reduction in the cost of the animals and their maintenance and a shorter pregnancy period facilitates a greater range of experiments. Third, non-technical causes of complications, such as hypothermia and anesthesia, are prevented by the short duration of surgery. Finally, the wide variety of genetic tools available in mice will lead to more studies to understand the pathophysiology of CDH. The removal of the transuterine suture with the live birth of the fetus in the nitrofen and knockout models of CDH will be the future applications of this technique.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. All authors have made substantial contributions to the conception and design of the study, acquisition, analysis, and interpretation of data, drafting the article, and revising it for important intellectual content and final approval of the version to be submitted. The authors thank Can Sabuncuoğlu for his kind efforts on the production of the artwork of the surgical technique.
Materials
| Buprenorphine | Par Pharmaceutical | NDC 42023-179-05 | For regional anesthesia |
| Isoflurane | Halocarbon Life Sciences | NDC 66794-017-25 | For general anesthesia |
| Magnification glasses | USA Medical-Surgical | SLR-250LBLK | At least 2.5x |
| Nikon 90i microscope | Nikon | 3417 | Motorized Fluorescence |
| Nucleospin Tissue Kit | Macherey-Nagel, Düren, Germany | 740952.5 | DNA isolation |
| Pierce BCA Protein Assay Kit | Thermo Fisher, IL, USA | 23225 | Protein quantification |
| Polyglactin suture | Ethicon | VCP451H | 4-0, 24 mm, cutting |
| Polylysine slides | VWR | 48382-117 | Microscope adhesion slides |
| Polypropylene suture | Ethicon | Y432H | 6-0, 13 mm 1/2c Taperpoint |
| RIPA buffer | Sigma-Aldrich, Missouri, USA | R0278-50ml | Protein isolation |
| Silk suture | Ethicon | VCP682G | 4-0, 24 mm, cutting |
| Trizol | Invitrogen | 15596026 | RNA isolation |
References
- Wright, N. J. Global PaedSurg Research Collaboration. Management and outcomes of gastrointestinal congenital anomalies in low, middle and high income countries: protocol for a multicentre, international, prospective cohort study. BMJ Open. 9, 030452 (2019).
- Aydin, E. Current approach for prenatally diagnosed congenital anomalies that requires surgery. Turkish Clinics Journal of Gynecology and Obstetrics. 27, 193-199 (2016).
- Nolan, H., et al. Hemorrhage after on-ECMO repair of CDH is equivalent for muscle flap and prosthetic patch. Journal of Pediatric Surgery. 54 (10), 2044-2047 (2019).
- Aydin, E., et al. Congenital diaphragmatic hernia: the good, the bad, and the tough. Pediatric Surgery International. 35 (3), 303-313 (2019).
- Aydın, E., Özler, O., Burns, P., Lim, F. Y., Peiró, J. L. Left congenital diaphragmatic hernia-associated musculoskeletal deformities. Pediatric Surgery International. 35 (11), 1265-1270 (2019).
- Aydın, E., et al. When primary repair is not enough: a comparison of synthetic patch and muscle flap closure in congenital diaphragmatic hernia. Pediatric Surgery International. 36 (4), 485-491 (2020).
- Wilson, M., Difiore, J. W., Peters, C. A. Experimental fetal tracheal ligation prevents the pulmonary hypoplasia associated with fetal nephrectomy: Possible application for congenital diaphragmatic hernia. Journal of Pediatric Surgery. 28 (11), 1433-1440 (1993).
- Mudri, M., et al. The effects of tracheal occlusion on Wnt signaling in a rabbit model of congenital diaphragmatic hernia. Journal of Pediatric Surgery. 54 (5), 937-944 (2019).
- Khan, P. A., Cloutier, M., Piedboeuf, B. Tracheal occlusion: a review of obstructing fetal lungs to make them grow and mature. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 145 (2), 125-138 (2007).
- Chomczynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 15 (3), 532-537 (1993).
- Beurskens, N., Klaassens, M., Rottier, R., De Klein, A., Tibboel, D. Linking animal models to human congenital diaphragmatic hernia. Birth Defects Research Part A: Clinical and Molecular Teratology. 79 (8), 565-572 (2007).
- Varisco, B. M., et al. Excessive reversal of epidermal growth factor receptor and ephrin signaling following tracheal occlusion in rabbit model of congenital diaphragmatic hernia. Molecular Medicine. 22, 398-411 (2016).

