Optogenetic Inhibition of Rho1-Mediated Actomyosin Contractility Coupled with Measurement of Epithelial Tension in Drosophila Embryos
Summary
Actomyosin contractility plays an important role in cell and tissue morphogenesis. However, it is challenging to manipulate actomyosin contractility in vivo acutely. This protocol describes an optogenetic system that rapidly inhibits Rho1-mediated actomyosin contractility in Drosophila embryos, revealing the immediate loss of epithelial tension after the inactivation of actomyosin in vivo.
Abstract
Contractile forces generated by actin and non-muscle myosin II (“actomyosin contractility”) are critical for morphological changes of cells and tissues at multiple length scales, such as cell division, cell migration, epithelial folding, and branching morphogenesis. An in-depth understanding of the role of actomyosin contractility in morphogenesis requires approaches that allow the rapid inactivation of actomyosin, which is difficult to achieve using conventional genetic or pharmacological approaches. The presented protocol demonstrates the use of a CRY2-CIBN based optogenetic dimerization system, Opto-Rho1DN, to inhibit actomyosin contractility in Drosophila embryos with precise temporal and spatial controls. In this system, CRY2 is fused to the dominant negative form of Rho1 (Rho1DN), whereas CIBN is anchored to the plasma membrane. Blue light-mediated dimerization of CRY2 and CIBN results in rapid translocation of Rho1DN from the cytoplasm to the plasma membrane, where it inactivates actomyosin by inhibiting endogenous Rho1. In addition, this article presents a detailed protocol for coupling Opto-Rho1DN-mediated inactivation of actomyosin with laser ablation to investigate the role of actomyosin in generating epithelial tension during Drosophila ventral furrow formation. This protocol can be applied to many other morphological processes that involve actomyosin contractility in Drosophila embryos with minimal modifications. Overall, this optogenetic tool is a powerful approach to dissect the function of actomyosin contractility in controlling tissue mechanics during dynamic tissue remodeling.
Introduction
Actomyosin contractility, the contractile force exerted by non-muscle myosin II (hereafter 'myosin') on the F-actin network, is one of the most important forces in changing cell shape and driving tissue-level morphogenesis1,2. For example, the activation of actomyosin contractility at the apical domain of the epithelial cells results in apical constriction, which facilitates a variety of morphogenetic processes, including epithelial folding, cell extrusion, delamination, and wound healing3,4,5,6,7. The activation of myosin requires phosphorylation of its regulatory light chain. This modification alleviates the inhibitory conformation of the myosin molecules, allowing them to form bipolar myosin filament bundles with multiple head domains on both ends. The bipolar myosin filaments drive the anti-parallel movement of actin filaments and result in the generation of contractile force1,8,9.
The evolutionarily conserved Rho family small GTPase RhoA (Rho1 in Drosophila) plays a central role in the activation of actomyosin contractility in various cellular contexts10,11. Rho1 functions as a bimolecular switch by binding either GTP (active form) or GDP (inactive form)12. The cycling between GTP- or GDP-bound Rho1 is regulated by its GTPase-activating proteins (GAPs) and guanine nucleotide-exchange factors (GEFs)13. GEFs function to facilitate the exchange of GDP for GTP and thus activate Rho1 activity. GAPs, on the other hand, enhance the GTPase activity of Rho1 and thus deactivate Rho1. Activated Rho1 promotes actomyosin contractility through interacting with and activating its downstream effectors, Rho-associated kinase (Rok) and Diaphanous14. Rok induces myosin activation and actomyosin contractility by phosphorylating the regulatory light chain of myosin15. In addition, Rok also inhibits the myosin regulatory light chain phosphatase, and hence further promotes myosin filament assembly16. Rok can also phosphorylate LIM kinases, which, when activated, prevent actin disassembly by phosphorylating and inhibiting the actin-depolymerization factor cofilin17,18. Diaphanous is a formin family actin nucleator that promotes actin polymerization, providing a base for myosin to interact with19,20,21.
While the cellular mechanisms that activate actomyosin contractility have been well elucidated, our understanding of its function in regulating dynamic tissue remodeling remains incomplete. Filling this knowledge gap requires approaches that can rapidly inactivate actomyosin at specific tissue regions in vivo and record the immediate impact on tissue behavior and properties. This protocol describes the use of an optogenetic approach to acutely inhibit actomyosin contractility during Drosophila mesoderm invagination, followed by measurement of epithelial tension using laser ablation. During Drosophila gastrulation, the ventrally localized mesoderm precursor cells undergo apical constriction and invaginate from the surface of the embryo by forming an anterior-posteriorly oriented furrow22,23. The formation of ventral furrows has long been used as a model for studying the mechanism of epithelial folding. Ventral furrow formation is administered by the dorsal-ventral patterning system in Drosophila24,25,26,27. The expression of two transcription factors, Twist and Snail, located at the ventral side of the embryo, controls ventral furrow formation and specifies mesodermal cell fate28. Twist and Snail activate the recruitment of the Rho1 GEF RhoGEF2 to the apex of the mesoderm precursor cells via a G-protein coupled receptor pathway and a RhoGEF2 adaptor protein, T4829,30,31,32,33. Next, RhoGEF2 activates myosin throughout the apical surface of the potential mesoderm through the Rho-Rho kinase pathway34,35,36,37,38,39. Activated myosin forms a supracellular actomyosin network throughout the apical surface of the mesoderm primordium, the contractions of which drive apical constriction and result in a rapid increase in apical tissue tension14,37,40.
The optogenetic tool described in this protocol, Opto-Rho1DN, inhibits actomyosin contractility through blue-light dependent plasma membrane recruitment of a dominant negative form of Rho1 (Rho1DN)41. A T19N mutation in Rho1DN eliminates the ability of the mutant protein to exchange GDP for GTP and thus renders the protein perpetually inactive34. A subsequent mutation in Rho1DN, C189Y, eliminates its naive membrane targeting signal42,43. When Rho1DN is infused to the plasma membrane, it binds to and impounds Rho1 GEFs, thereby blocking the activation of Rho1 as well as Rho1-mediated activation of myosin and actin34,44. The plasma membrane recruitment of Rho1DN is achieved through a light-dependent dimerization module derived from Cryptochrome 2 and its binding partner CIB1. Cryptochrome 2 is a blue-light activated Cryptochrome photoreceptor in Arabidopsis thaliana45. Cryptochrome 2 binds to CIB1, a basic helix-loop-helix protein, only in its photoexcited state45. It was later found that the conserved N-terminal, photolyase homology region (PHR), from Cryptochrome 2 (CRY2PHR, hereafter referred to as CRY2) and the N-terminal domain (aa 1-170) of CIB1 (hereafter CIBN) are important for light-induced dimerization46. Opto-Rho1DN contains two components. The first component is the CIBN protein fused with a CAAX anchor, which localizes the protein to the plasma membrane47. The second component is mCherry-tagged CRY2 fused with Rho1DN41. In the absence of blue light, CRY2-Rho1DN remains in the cytoplasm. Upon blue light stimulation, CRY2-Rho1DN is targeted to the plasma membrane through the interaction between membrane-anchored CIBN and excited CRY2. Opto-Rho1DN can be activated by ultraviolet A (UVA) light and blue light (400-500 nm, peak activation at 450-488 nm), or by an 830-980 nm pulsed laser when performing two-photon stimulation41,46,47,48. Therefore, Opto-Rho1DN is stimulated by wavelengths normally used for exciting GFP (488 nm for single photon imaging and 920 nm for two-photon imaging). In contrast, wavelengths commonly used for exciting mCherry (561 nm for single photon imaging and 1,040 nm for two-photon imaging) do not stimulate the optogenetic module and therefore can be used for pre-stimulation imaging. The protocol describes the approaches used to minimize the risk of unwanted stimulation during sample manipulation.
Laser ablation has been extensively employed to detect and measure tension in cells and tissues49. Previous studies have shown that, when laser intensity is appropriately controlled, two-photon laser ablation employing a femtosecond near-infrared laser can physically impair some subcellular structures (e.g., cortical actomyosin networks) without causing plasma membrane rapture50,51. If the tissue is under tension, laser ablation of a region of interest within the tissue results in an immediate outward recoil of the cells adjacent to the ablated region. The recoil velocity is a function of the magnitude of the tension and the viscosity of the media (cytoplasm) surrounding the structures undergoing recoil49. Because of the superior penetration depth of the near-infrared lasers and the ability to achieve well-confined focal ablation, two-photon laser ablation is particularly useful for detecting tissue tension in vivo. As demonstrated in this protocol, this method can be easily combined with Opto-Rho1DN-mediated inactivation of actomyosin contractility to investigate the direct impact of Rho1-dependent cellular contractility on tissue mechanics during dynamic tissue remodeling.
Protocol
1. Setting up the genetic cross and preparing the egg collection cup
- Select female flies (virgin) from the optogenetic line UASp-CIBNpm (I); UASp-CRY2-Rho1DN-mCherry (III) on a CO2 pad under a stereomicroscope and set up a cross with male flies from the maternal GAL4 driver line 67 Sqh-mCherry; 15 E-cadherin-GFP.
NOTE: The 67 and 15 stand for maternal-Tubulin-GAL4 inserted into the second (II) and third (III) chromosomes, respectively52. The GAL4 line used in this protocol also expresses the mCherry-tagged myosin regulatory light chain Sqh (Spaghetti squash)37 and GFP-tagged E-cadherin53. Flies from the optogenetic line may still contain FM7 and TM6 balancer chromosomes. Flies from the maternal GAL4 driver line may still contain the CyO and TM3 balancer chromosomes. - After ~10 days, select F1 female flies having the following genotype: UASp-CIBNpm/+; 67 Sqh-mCherry/+; 15 E-cadherin-GFP/UASp-CRY2-Rho1DN-mCherry. Set up an egg collection cup with the male flies.
- Ensure that the female flies with the correct genotype do not contain any balancer chromosomes (i.e., they should not contain curly wings [Cy], short bristles [Sb], bar- or kidney-shaped eyes [B], or extra humeral bristles [Hu]), which are markers for the CyO, TM3, FM7, and TM6 balancers used in generating the stocks, respectively. Cover the cup with an apple juice plate with a tinge of fresh yeast paste on the surface.
NOTE: In F1 females, the optogenetic components are expressed maternally. Therefore, the F1 females used for setting up the cup do not need to be virgins. It is recommended to use male flies from the same F1 population for the cup for convenience.
- Ensure that the female flies with the correct genotype do not contain any balancer chromosomes (i.e., they should not contain curly wings [Cy], short bristles [Sb], bar- or kidney-shaped eyes [B], or extra humeral bristles [Hu]), which are markers for the CyO, TM3, FM7, and TM6 balancers used in generating the stocks, respectively. Cover the cup with an apple juice plate with a tinge of fresh yeast paste on the surface.
- Keep the cup in a cardboard box covered with aluminum foil to avoid any possible light leakage from the environment. Change the apple juice plate every day for cups kept at room temperature (~21-23 °C), or every two days for cups kept at 18 °C.
- Immediately before plate change, knock the cup on a flat surface (e.g., bench top) to keep the flies at the bottom of the cup and prevent them from escaping. Change the apple juice plate in a dark room and use a headlamp with red light for illumination (see Table of Materials).
NOTE: To increase the expression of the constructs that are under the control of maternal-Tubulin-GAL4 and UASp, it is recommended to keep the cup at 18 °C for a minimum of 3 days before embryo collection. This incubation period also allows the flies to adapt to the cup and be well fed on the yeast paste to achieve optimal egg production.
- Immediately before plate change, knock the cup on a flat surface (e.g., bench top) to keep the flies at the bottom of the cup and prevent them from escaping. Change the apple juice plate in a dark room and use a headlamp with red light for illumination (see Table of Materials).
2. Collection of embryos at the desired stage and preparing them for optogenetic stimulation
NOTE: All sample collection and preparation steps need to be performed in a dark room, using a "safe light" (e.g., red light) for illumination. The optogenetic components are immensely sensitive to ambient light. Even the slightest exposure to ambient light leads to premature stimulation of the specimen. Typically, lights in the green-red range (>532 nm) do not cause unwanted stimulation.
- For collecting embryos at early embryogenesis, place a new apple juice plate on the cup 8 to 16 h prior to embryo collection (at 18 °C).
- At the time of embryo collection, change the apple juice plate. Label the plate taken off from the cup and cover the plate's surface with a thin layer of Halocarbon oil 27 (see Table of Materials). Wait for 30-60 s for the eggshell to become transparent.
- Place an orange-red plastic shield (see Table of Materials) on the stage of an upright stereoscope. The placement of the orange-red shield prevents undesirable stimulation during sample illumination by blocking the blue-green wavelengths from the transmitted light.
NOTE: In this protocol, an upright stereoscope is used for embryo collection (see Table of Materials). - Place the apple juice plate on top of the orange-red shield. Turn on the transmitted light of the stereomicroscope to illuminate the sample. Collect 5-15 embryos at the appropriate stage from the apple juice plate using a pair of tweezers. Do not squeeze the embryos with the tweezers.
NOTE: In this protocol, the appropriate embryo should be at the early-mid cellularization stage. The embryo at this stage should have a dark opaque yolk surrounded by a clear, uniform periplasm layer that contains the forming blastoderm cells. The leading edge of the cleavage furrows ("cellularization front"), which appears as a continuous line parallel to the surface of the embryo, should not pass half of the depth of the periplasm. - Gently blot the embryos on a piece of paper towel (~1.5 cm × 1.5 cm) to remove excess oil from the embryos using the tweezers.
- Add several drops of freshly prepared 40% bleach (~3% sodium hypochlorite; see Table of Materials) to a new small square piece of paper towel (~1.5 cm × 1.5 cm) using a plastic transfer pipette so that the paper towel is covered with a thin layer of bleach. Transfer the embryo from the dry paper towel to the bleach-soaked paper towel using the tweezers and make sure the embryos are soaked in bleach. Wait 2-4 min for the embryo to become dechorionated.
- After dechorionation, use the tweezers to blot the square paper towel on a large piece of tissue paper to remove excess bleach. Ensure that the side with the embryos is facing up.
- To rinse the embryos, use the tweezers to gently soak the square paper towel in a drop of deionized water and quickly blot it on a large piece of tissue paper. Repeat this process eight times to ensure the removal of any residue bleach.
- Use an eyelash tool to transfer the embryo from the paper towel to a 35 mm glass-bottom dish (see Table of Materials). Add deionized water to the dish to cover the embryos entirely. Fine-tune the position and orientation of the embryos using the eyelash tool.
NOTE: After dechorionation, the embryo tends to stick on the surface of the glass and is immobile without perturbation, so it is not necessary to apply additional treatment (such as glue) to immobilize the embryo on the glass-bottom dish. - Place the 35 mm glass-bottom dish with the embryos inside a lightproof black box (see Table of Materials) to protect the sample from light exposure during the transfer process. Bring the box to the room with the multiphoton microscope.
3. Optogenetic stimulation, laser ablation, and imaging of the embryo
NOTE: The multiphoton system used in this experiment (see Table of Materials) is capable of simultaneous dual-wavelength imaging. It also contains a photostimulation unit with a 458 nm laser and a separate galvanometer scanner, allowing photo-activation/stimulation within a defined region of interest (ROI). Of note, the 920 nm laser, which is used to excite green-yellow fluorescent proteins, will stimulate Opto-Rho1DN, albeit more slowly compared to blue laser-mediated stimulation.
- Cover the multiphoton microscope with a lightproof black cloth (see Table of Materials) to avoid unwanted stimulation of the embryos during sample setup and imaging.
NOTE: The same approach is used during regular multiphoton imaging to protect the light-sensitive detectors equipped on the microscope. - Turn off the room light and the computer screens. Turn off the touch panel controller by clicking OFF under 'Backlight of touch panel controller' in the software. Ensure there is no other ambient light in the room.
- Open the front side of the black cloth cover on the microscope. Take the 35 mm glass-bottom dish from the black box and place it on the microscope stage.
- Illuminate embryos with a green light normally used as the excitation light for epifluorescence. To do this, turn on the fluorescence illumination unit, select Ocular under the 'Ocular' panel in the software, and change the 'Cube turret' to 4:TRITC. Use the eyepiece to identify the embryo of interest and bring it into focus.
NOTE: The green light visualization is achieved by allowing a white light generated by the fluorescence illumination unit to pass through a built-in standard TRITC filter cube, which contains a 528-553 nm excitation filter, a 565 nm beam splitter, and a 590-650 nm emission filter. Other non-blue lights should also work fine for sample illumination. It is not necessary to wear eye protection at this step, since the excitation light has a low intensity and will not be directed to the eyepiece. The embryo will appear uniformly red due to autofluorescence. - Close the black cloth cover so that the sample is fully protected from light. Turn on the computer screen to access the software that controls the microscope. Change the 'Ocular' to LSM in the software for image acquisition.
- Perform laser ablation in control, unstimulated embryos using a 25x water immersion objective.
- Click Bright Z, Sequence Manager, and LSM Stimulation from the 'Tool Window' in the software. Set the scanner type as Galvano and the scan size as 512 × 512. Turn on CH1 and CH3 under the 'PMT setting' panel to allow the use of the 1,040 nm laser, and click Live × 4 to visualize the embryo.
- Rotate the embryo using the Rotation function in the software so that the anterior-posterior axis of the embryo is vertically oriented. Set the zoom to 3. Draw a region of interest (ROI) using the shape tool under 'Scan Settings' and set the size of the ROI in the 'Reference' panel. Set the ROI as 512 pixels in width and 100 pixels in height (171 × 33 µm2).
- Set acquisition parameters for the pre-ablation Z-stack.
- Register the surface of the embryo as 0 under the 'Z Section'. Set the start as 0 and the end as 100 µm. Set the step size as 2 µm. Activate the Z acquisition mode by checking Z under the 'Series' tab.
- Set 1,040 nm laser intensity to increase linearly from 3% to 7% using the Bright Z function.
- Save the current imaging setting as the first task of the pipeline by clicking LSM in 'Sequence Manager'.
NOTE: The pre-ablation Z-stack is obtained to confirm the stage of the embryo. In this experiment, CRY2-Rho1DN-mCherry and Sqh-mCherry will be excited by the 1,040 nm laser. During apical constriction, the Sqh-mCherry signal is enhanced at the medioapical region of the ventral mesodermal cells, whereas CRY2-Rho1DN-mCherry is cytosolic before stimulation. The laser intensity employed for pre- and post-stimulation imaging is decided empirically based on the balance between the optimal signal-to-noise ratio and the avoidance of photobleaching.
- Set acquisition parameters for the pre-ablation movie.
- Delete any existing ROI to allow imaging of the entire 512 × 512 pixel region (171 × 171 µm2, 3x zoom) near the ventral surface of the embryo, as described in step 3.5.2. Set the 1,040 nm laser intensity to 3%.
- Check Time and uncheck Z under the 'Series' panel. Keep the 'Interval' as FreeRun under the 'Time Lapse' panel. Set cycle as 10.
- Save the current settings as the next task of the pipeline by clicking LSM in 'Sequence Manager'.
NOTE: The purpose of this task is to obtain a 10-frame single Z-plane pre-ablation movie with a 1,040 nm laser for image acquisition. The image acquisition speed is approximately 1 s per frame.
- Set the parameters for laser ablation.
- Define a 3D region from immediately below the vitelline membrane to ~20 µm deep for laser ablation (Figure 1A). Set the start of the Z-stack as the plane that is immediately below the vitelline membrane and the end as 20 µm deeper than the start plane. Set the step size as 1.5 µm.
NOTE: The ROI of the ablated region is ~30 pixels in width and ~10 pixela in height (~10 µm along the medial-lateral axis and ~3.3 µm along the A-P axis). The purpose of ablating the sample at multiple Z-planes is to ensure that the apical surface of the ventral cells is ablated. This is specifically important for the stimulated embryos, as the ventral cells experience rapid apical relaxation after Rho1 inhibition. - Turn on CH2 and CH4 under the 'PMT setting' panel to allow the use of the 920 nm laser. Set the intensity of the 920 nm laser to 30%. Set image acquisition with the 920 nm laser for a single Z-stack within the 3D region defined in step 3.5.5.1 for laser ablation.
NOTE: The laser intensity chosen in this step is sufficient to ablate the tissue (as indicated by tissue recoil immediately after the laser treatment) but meanwhile does not obviously damage the plasma membrane (as indicated by the lack of burn marks on the cell membrane) (Figure 1B,C). - Save the current setting as the next task of the pipeline by clicking LSM in 'Sequence Manager'.
- Define a 3D region from immediately below the vitelline membrane to ~20 µm deep for laser ablation (Figure 1A). Set the start of the Z-stack as the plane that is immediately below the vitelline membrane and the end as 20 µm deeper than the start plane. Set the step size as 1.5 µm.
- Set acquisition parameters for the post-ablation movie.
- Set image acquisition for a 100-frame single Z-plane post-ablation movie using both the 1,040 nm and 920 nm lasers. Set the intensity of the 1,040 nm laser and the 920 nm laser to 3% and 0.3%, respectively. The region specified in step 3.5.4 will be imaged with identical image acquisition speed.
- Save the current setting as the next task of the pipeline by clicking LSM in 'Sequence Manager'.
NOTE: The purpose of storing the parameters described in steps 3.5.3-3.5.6 as sequential tasks in 'Sequence Manager' before any actual acquisition is to ensure the capture of the immediate tissue response after laser ablation.
- Select Sequence under 'Acquire'. Change the data-saving path and file name as needed. Click Ready and wait for the software to initialize the pipeline. Then click Start to execute the pipeline.
- Perform laser ablation in stimulated embryos.
- Set acquisition parameters for the pre-ablation Z-stack, as described in steps 3.5.1-3.5.3. Save the current setting as the first task of the pipeline by clicking LSM in 'Sequence Manager'.
- Set parameters for optogenetic stimulation within a defined ROI.
- Change the zoom to 1 and select an ROI that covers the ventral surface of the embryo (~512 × 300 µm2). Turn off the CH1-CH4 detectors.
- Click LSM Stimulation. Uncheck Continuous within Duration and type 12 s. Check the 458 nm with 0.3% laser intensity.
- Save the current setting as the next task of the pipeline by clicking Stimulation in 'Sequence Manager'.
NOTE: A more rapid stimulation may be achieved by increasing the 458 nm laser intensity. However, when using higher laser intensities, the scattered laser light may stimulate the region adjacent to the ROI, which is not ideal if a spatially confined stimulation is required.
- Set a 3 min wait time after stimulation to ensure total inactivation of myosin and disassembly of apical F-actin, and to achieve a static tissue morphology before laser ablation. This is achieved by clicking on Wait/Pause under 'Sequence Manager' and setting the desired time for 'Wait'.
NOTE: Recruitment of CRY2-Rho1DN-mCherry to the membrane caused by a single round of stimulation (0.3% 458 nm laser for 12 s) is clearly detectable 10-15 min after the stimulation. This is in alignment with the published dissociation half-time of ~9 min47. - Set acquisition parameters for the single Z-plane pre-ablation movie, as described in step 3.5.4, except that both the 1,040 nm and 920 nm lasers are used for image acquisition. Turn on the CH1-CH4 detectors. Set the intensity of the 1,040 nm laser and the 920 nm laser to 3% and 0.3%, respectively.Save the current setting as the next task of the pipeline by clicking LSM in 'Sequence Manager'.
- Set parameters for laser ablation, as described in step 3.5.5. Save the current setting as the next task of the pipeline by clicking LSM in 'Sequence Manager'.
- Set acquisition parameters for the single Z-plane post-ablation movie, as described in step 3.5.6. Save the current setting as the next task of the pipeline by clicking LSM in 'Sequence Manager'.
- Select Sequence under 'Acquire'. Change the data-saving path and file name as needed. Click Ready and wait for the software to initialize the pipeline. Then, click Start to execute the pipeline.
4. Quantifying the rate of tissue recoil after laser ablation
- Open the post-ablation movie in ImageJ.
- Draw a small ROI along the ventral midline that covers the ablated region using the Rectangle Selection tool. The width of the ROI is nine pixels. The height of the ROI is set such that the ROI is large enough to cover the full range of tissue recoil after laser ablation.
- Click Duplicate under the 'Image' tab. In the pop-up window, check Duplicate stack and then click OK to duplicate the stack within the selected ROI.
- Generate a montage from the duplicated stack through Image > Stacks > Make Montage. In the pop-up window, set the row number to 1 and the column number to the total number of frames in the stack (100 in this case).
- Measure the A-P width of the ablated region over time from the generated montage.
- Use the Multi-point tool in ImageJ to mark out the A-P boundaries of the ablated region for each time point on the montage.
- Save the coordinates of the marked dots using File > Save as > XY Coordinates.
- Import the measured XY coordinates into MATLAB. Calculate the A-P width of the ablated region for each time point by subtracting the Y coordinate of the upper boundary from the Y coordinate of the lower boundary.
- Determine the rate of tissue recoil by fitting the first 20 s of the "width over time" curve into a line using the 'polyfit' function in MATLAB. Report the slope of the fitted line as the rate of tissue recoil.
- Perform statistical tests to compare the rate of tissue recoil between the stimulated and non-stimulated samples.
NOTE: It is recommended to obtain data from at least five embryos per condition. Both the two-sided Wilcoxon rank-sum test and the two-sided student t-test can be used for statistical comparison.
Representative Results
In the unstimulated embryos undergoing apical constriction, Sqh-mCherry became enriched at the medioapical region of the ventral mesodermal cells, whereas CRY2-Rho1DN-mCherry was cytosolic (Figure 1A). Laser ablation within the constriction domain led to a rapid tissue recoil along the A-P axis (Figure 1B,C). In the stimulated embryos, the CRY2-Rho1DN-mCherry signal became plasma membrane localized, whereas the medioapical signal of Sqh-mCherry completely disappeared (Figure 1A). Laser ablation in the stimulated embryos did not result in obvious tissue recoil, as exemplified in Figure 1B,C and quantified in Figure 1D. These results indicate that the generation of tissue tension requires active apical actomyosin contractility; when actomyosin becomes inactive upon Rho1 inhibition, the apical tissue tension also diminishes41. These observations are consistent with the previous findings that activation of apical myosin contractility in ventral mesodermal cells results in an increase in tissue tension at the ventral surface of the embryo40.
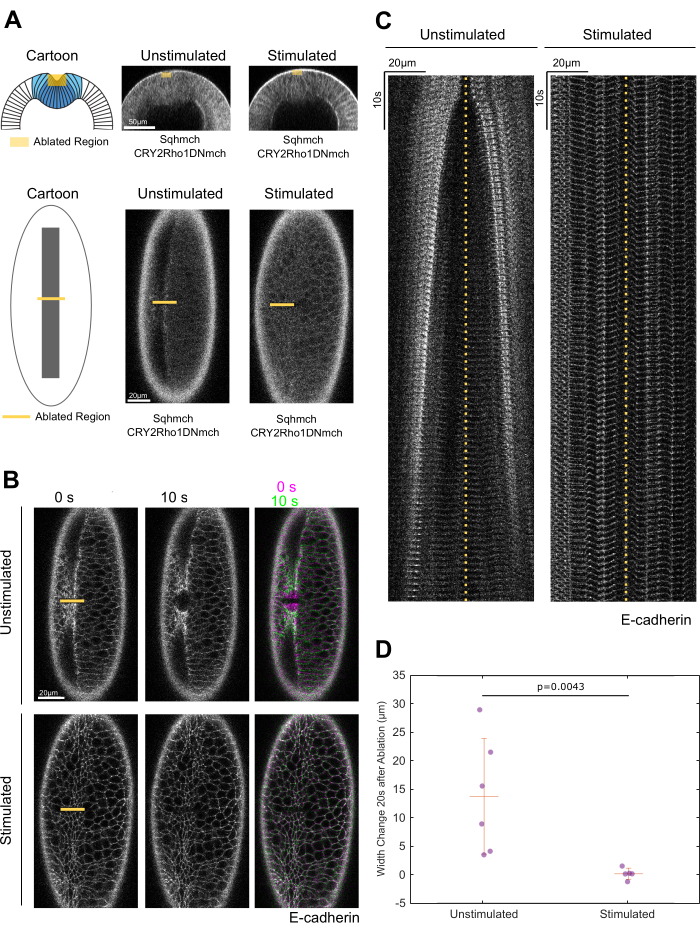
Figure 1: Opto-Rho1DN stimulation during apical constriction results in an immediate loss of cortical tension at the ventral surface of the embryo. (A) Cartoon depicting the experimental setup for laser ablation to detect cortical tension. Yellow-shaded regions indicate the ablated regions. For stimulated embryos, light-activation of Opto-Rho1DN was performed 3 min before the laser ablation. Due to apical relaxation after stimulation, multiple z-planes were ablated (yellow-shaded region) in order to ensure the ablation of the very apical surface of the ventral cells. (B,C) Comparison between unstimulated and stimulated embryos. No obvious tissue recoil was observed in the stimulated embryos (N = 6 for unstimulated embryos and N = 5 for stimulated embryos). (B) En face view of the laser-ablated embryos. Yellow-shaded boxes mark the ablated region. (C) Kymographs representing the width change of the ablated region. Yellow dotted lines indicate the ablation site. (D) Width changes of the ablated region along the A-P axis during the first 20 s after laser ablation. A clear tissue recoil was observed after laser cutting in the unstimulated control embryos. In contrast, little to no tissue recoil was observed in the stimulated embryos, indicating a lack of apical tension after Rho1 inhibition. Error bar is standard deviation. The p-value was calculated using a two-sided Wilcoxon rank-sum test. This figure is reused from Guo et al.41. Please click here to view a larger version of this figure.
Discussion
This protocol described the combined use of optogenetics and laser ablation to probe changes in tissue tension immediately after the inactivation of actomyosin contractility. The optogenetic tool described here takes advantage of the dominant negative form of Rho1 (Rho1DN) to acutely inhibit endogenous Rho1 and Rho1-dependent actomyosin contractility. Previous characterization of Opto-Rho1DN in the context of Drosophila ventral furrow formation demonstrated that the tool is highly effective in mediating the rapid inactivation of apical actomyosin contractility through simultaneous myosin inactivation and actin disassembly41. In particular, the stimulation of embryos at the time of apical constriction resulted in reduction of the apical myosin signal within 60 s in cells undergoing apical constriction41. This rapid removal of cortical myosin upon Rho1 inhibition is likely due to the fast cycling of Rho1 and myosin through active and inactive states, caused by the activities of GTPase activating proteins (GAPs) for Rho1 and myosin light chain phosphatases, respectively19,54. Consistent with the impact on actomyosin, coupling optogenetics with laser ablation demonstrated that Opto-Rho1DN stimulation during apical constriction resulted in an immediate loss of epithelial tension at the ventral region of the embryo41 (Figure 1). This combined approach allowed us to investigate the function of Rho1-mediated cellular contractility in regulating tissue mechanics with unprecedented spatial and temporal precision, making it possible to dissect immediate impacts from long-term effects that are difficult to achieve using conventional genetic approaches.
An important technical consideration when using Opto-Rho1DN is that the tool is highly sensitive to ambient light. A commonly encountered problem in the experiment is the recruitment of CRY2-mCherry-Rho1DN to the plasma membrane before the stimulation step, which is typically caused by premature stimulation of the sample during one of the following steps: sample preparation, sample transfer to the microscope room, sample positioning on the microscope stage, and pre-stimulation image acquisition. In our protocol, multiple procedures are employed to prevent undesired stimulation, including handling the fly cups and embryos in a dark room under red light, filtering out blue wavelengths from the illumination light when selecting and mounting embryos under the stereomicroscope, and avoiding exposure of embryos to a 400-500 nm laser (single photon excitation) or an 830-980 nm pulsed laser (multiphoton excitation) prior to stimulation. It is critical to practice extra attention at multiple steps of the experiment to prevent unwanted stimulation of the sample. In addition, when using Opto-Rho1DN to inhibit Rho1 within a specific region of interest (ROI) in the embryo41, it is recommended to use the lowest laser intensity that can achieve a robust translocation of CRY2-Rho1DN to the plasma membrane. Because Opto-Rho1DN is extremely sensitive to blue wavelengths, a high-intensity blue laser can result in unwanted stimulation in cells outside of the ROI or even neighboring embryos due to scattered light.
In its current version, the Opto-Rho1DN tool has several limitations. First, for the experiments described in this protocol, a plasma membrane localized CIBN anchor was used to recruit activated CRY2-Rho1DN from the cytosol to the plasma membrane41,47. By this design, it is difficult to perform confined Rho1 inhibition within a specific plasma membrane domain due to the diffusion of activated CRY2-Rho1DN proteins in the cytosol. Further improving the spatial precision at the subcellular scale awaits the development of new CIBN anchors that have more specific subcellular localization patterns. Second, Opto-Rho1DN is designed to inhibit Rho1 during early embryogenesis. The expression of CIBNpm and CRY2-Rho1DN-mCherry is controlled by UASp, which is standardized for expression in female germlines55. The expression of these modules in somatic tissues beyond early embryogenesis may require the replacement of UASp with a promoter that is more effective for driving somatic expression (e.g., UASt56). Finally, the effectiveness of Opto-Rho1DN is contingent upon the abundance of the CIBN anchor and CRY2-Rho1DN proteins. In the current version of the tool, it is determined by the GAL4 driver line used to drive the expression of the optogenetic modules. When using the maternal GAL4 driver described in this protocol, it is critical to use the line that provides two copies of the GAL4 gene (e.g., both 67 and 15) in order to achieve the rapid and potent inhibition of actomyosin contractility. Reducing the copy number of maternal GAL4 in the F1 females from two to one significantly reduced the inhibitory effect.
Compared to conventional genetic approaches, the optogenetic approach described in this protocol is advantageous in dissecting the stage and tissue-specific function of Rho1 in early Drosophila embryos. The function of Rho1 in early Drosophila embryogenesis is largely fulfilled by the maternally loaded gene product11. Depleting Rho1 maternally blocks oogenesis57, preventing the study of its function during early embryogenesis. In the past few years, several optogenetic tools have been developed to regulate endogenous Rho1 activity in Drosophila embryos. Both Izquierdo et al. and Rich et al. developed optogenetic tools to activate Rho1 activity by regulating the localization of the catalytic domain of Rho GEFs in Drosophila embryos48,58. In addition, Herrera-Perez et al. developed two optogenetic tools using either full-length RhoGEF2 (optoGEF) or full-length C-GAP (optoGAP) to activate or inhibit endogenous Rho1 activity, respectively59. Since optoGAP functions by recruiting a Rho1 GAP to the plasma membrane, its application may be sensitive to the presence of endogenous Rho1 GEFs, which can offset or even override the effect of ectopic recruitment of the GAP. In contrast, by directly sequestering Rho1 GEFs, Opto-Rho1DN may provide a more robust way to inhibit endogenous Rho1 and Rho1-dependent actomyosin contractility.
Given the wide range of functions of Rho1 in embryogenesis and post-embryonic development, the presented protocol can be easily adapted to study the function of Rho1 and Rho1-dependent cellular reorganizations in a wide range of morphogenetic processes. In addition, a similar strategy can, in principle, be used to severely restrict other small GTPases, such as the Rho family GTPases Cdc42 and Rac, since their dominant negative forms have been widely used to inhibit the endogenous function of these proteins11. Finally, as exemplified in this protocol, combining optogenetics and laser ablation approaches can provide an effective way to investigate the immediate impact of the inactivation of a specific protein on tissue dynamics and tissue mechanics, which will bring us new insights into tissue morphogenesis that are difficult to uncover using conventional genetic approaches.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Ann Lavanway for imaging support. The authors thank the Wieschaus lab and the De Renzis lab for sharing reagents and the Bloomington Drosophila Stock Center for fly stocks. This study is supported by NIGMS ESI-MIRA R35GM128745 and American Cancer Society Institutional Research Grant #IRG-82-003-33 to BH.
Materials
| 35 mm glass-bottom dish | MatTek | P35G-1.5-10-C | Used for sample preparation |
| 60 mm × 15 mm Petri dish with lid | Falcon | 351007 | Used for sample preparation |
| Black cloth for covering the microscope | Online | NA | Used to avoid unwanted light stimulation |
| Clorox Ultra Germicadal Bleach (8.25% sodium hypochlorite) | VWR | 10028-048 | Used for embryo dechorination |
| CO2 pad | Genesee Scientific | 59-114 | Used for cross set-up |
| ddH2O | NA | NA | Used for sample preparation |
| Dumont Style 5 tweezers | VWR | 102091-654 | Used for sample preparation |
| Eyelash tool (made from pure red sable round brush #2) | VWR | 22940-834 | Used for sample preparation |
| FluoView (Software) | Olympus | NA | Used for image acquisition and optogenetic stimulation |
| Halocarbon oil 27 | Sigma Aldrich | H8773-100ML | Used for embryo stage visualization |
| ImageJ/FIJI | NIH | NA | Used for image analysis |
| MATLAB | MathWorks | NA | Used for image analysis |
| Nikon SMZ-745 stereoscope | Nikon | NA | Used for sample preparation |
| Olympus FVMPE-RS multiphoton microscope with InSight DS Dual-line Ultrafast Lasers for simultaneous dual-wavelength multiphoton imaging, , a 25x/NA1.05 water immersion objective (XLPLN25XWMP2), and an IR/VIS stimulation unit for photo-activation/stimulation. This system is also equipped with a TRITC filter (39005-BX3; AT-TRICT-REDSHFT 540/25x, 565BS, 620/60M), and a fluorescence illumination unit that emits white light. | Olympus | NA | Used for image acquisition and optogenetic stimulation |
| SP Bel-Art 100-place polypropylene freezer storage box (Black, light-proof box for sample transfer) | VWR | 30621-392 | Used to avoid unwanted light stimulation |
| UV Filter Shield for FM1403 Fluores (Orange-red plastic shield) | Bolioptics | FM14036151 | Used to avoid unwanted light stimulation |
| VITCHELO V800 Headlamp with White and Red LED Lights | Amazon | NA | Used to avoid unwanted light stimulation |
References
- Vicente-Manzanares, M., Ma, X., Adelstein, R. S., Horwitz, A. R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nature Reviews. Molecular Cell Biology. 10 (11), 778-790 (2009).
- Munjal, A., Lecuit, T. Actomyosin networks and tissue morphogenesis. Development. 141 (9), 1789-1793 (2014).
- Sawyer, J. M., et al. Apical constriction: a cell shape change that can drive morphogenesis. 발생학. 341 (1), 5-19 (2010).
- Nishimura, T., Honda, H., Takeichi, M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 149 (5), 1084-1097 (2012).
- Marinari, E., et al. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature. 484 (7395), 542-545 (2012).
- Slattum, G. M., Rosenblatt, J. Tumour cell invasion: an emerging role for basal epithelial cell extrusion. Nature Reviews. Cancer. 14 (7), 495-501 (2014).
- Antunes, M., Pereira, T., Cordeiro, J. V., Almeida, L., Jacinto, A. Coordinated waves of actomyosin flow and apical cell constriction immediately after wounding. The Journal of Cell Biology. 202 (2), 365-379 (2013).
- Yang, S., et al. The central role of the tail in switching off 10S myosin II activity. The Journal of General Physiology. 151 (9), 1081-1093 (2019).
- Yang, S., et al. Cryo-EM structure of the inhibited (10S) form of myosin II. Nature. 588 (7838), 521-525 (2020).
- Narumiya, S., Thumkeo, D. Rho signaling research: history, current status and future directions. FEBS Letters. 592 (11), 1763-1776 (2018).
- Johndrow, J. E., Magie, C. R., Parkhurst, S. M. Rho GTPase function in flies: insights from a developmental and organismal perspective. Biochemistry and Cell Biology. 82 (6), 643-657 (2004).
- Etienne-Manneville, S., Hall, A. Rho GTPases in cell biology. Nature. 420 (6916), 629-635 (2002).
- Hodge, R. G., Ridley, A. J. Regulating Rho GTPases and their regulators. Nature Reviews. Molecular Cell Biology. 17 (8), 496-510 (2016).
- Martin, A. C., Goldstein, B. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development. 141 (10), 1987-1998 (2014).
- Amano, M., Nakayama, M., Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 67 (9), 545-554 (2010).
- Kimura, K., et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 273 (5272), 245-248 (1996).
- Maekawa, M., et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 285 (5429), 895-898 (1999).
- Ohashi, K., et al. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. The Journal of Biological Chemistry. 275 (5), 3577-3582 (2000).
- Coravos, J. S., Martin, A. C. Apical sarcomere-like actomyosin contracts nonmuscle drosophila epithelial cells. Developmental Cell. 39 (3), 346-358 (2016).
- Homem, C. C. F., Peifer, M. Diaphanous regulates myosin and adherens junctions to control cell contractility and protrusive behavior during morphogenesis. Development. 135 (6), 1005-1018 (2008).
- Goode, B. L., Eck, M. J. Mechanism and function of formins in the control of actin assembly. Annual Review of Biochemistry. 76, 593-627 (2007).
- Sweeton, D., Parks, S., Costa, M., Wieschaus, E. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development. 112 (3), 775-789 (1991).
- Leptin, M., Grunewald, B. Cell shape changes during gastrulation in Drosophila. Development. 110 (1), 73-84 (1990).
- Leptin, M. Gastrulation in Drosophila: the logic and the cellular mechanisms. The EMBO Journal. 18 (12), 3187-3192 (1999).
- Martin, A. C. The physical mechanisms of Drosophila gastrulation: mesoderm and endoderm invagination. 유전학. 214 (3), 543-560 (2020).
- Gilmour, D., Rembold, M., Leptin, M. From morphogen to morphogenesis and back. Nature. 541 (7637), 311-320 (2017).
- Gheisari, E., Aakhte, M., Müller, H. -. A. J. Gastrulation in Drosophila melanogaster: Genetic control, cellular basis and biomechanics. Mechanisms of Development. 163, 103629 (2020).
- Leptin, M. Twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes & Development. 5 (9), 1568-1576 (1991).
- Costa, M., Wilson, E. T., Wieschaus, E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell. 76 (6), 1075-1089 (1994).
- Kerridge, S., et al. Modular activation of Rho1 by GPCR signalling imparts polarized myosin II activation during morphogenesis. Nature Cell Biology. 18 (3), 261-270 (2016).
- Kölsch, V., Seher, T., Fernandez-Ballester, G. J., Serrano, L., Leptin, M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science. 315 (5810), 384-386 (2007).
- Manning, A. J., Peters, K. A., Peifer, M., Rogers, S. L. Regulation of epithelial morphogenesis by the G protein-coupled receptor mist and its ligand fog. Science Signaling. 6 (301), (2013).
- Parks, S., Wieschaus, E. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell. 64 (2), 447-458 (1991).
- Barrett, K., Leptin, M., Settleman, J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell. 91 (7), 905-915 (1997).
- Dawes-Hoang, R. E., et al. Folded gastrulation, cell shape change and the control of myosin localization. Development. 132 (18), 4165-4178 (2005).
- Häcker, U., Perrimon, N. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes & Development. 12 (2), 274-284 (1998).
- Martin, A. C., Kaschube, M., Wieschaus, E. F. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 457 (7228), 495-499 (2009).
- Mason, F. M., Tworoger, M., Martin, A. C. Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nature Cell Biology. 15 (8), 926-936 (2013).
- Nikolaidou, K. K., Barrett, K. A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation. Current Biology. 14 (20), 1822-1826 (2004).
- Martin, A. C., Gelbart, M., Fernandez-Gonzalez, R., Kaschube, M., Wieschaus, E. F. Integration of contractile forces during tissue invagination. The Journal of Cell Biology. 188 (5), 735-749 (2010).
- Guo, H., Swan, M., He, B. Optogenetic inhibition of actomyosin reveals mechanical bistability of the mesoderm epithelium during Drosophila mesoderm invagination. eLife. 11, e69082 (2022).
- Sebti, S. M., Der, C. J. Searching for the elusive targets of farnesyltransferase inhibitors. Nature Reviews. Cancer. 3 (12), 945-951 (2003).
- Roberts, P. J., et al. Rho family GTPase modification and dependence on CAAX motif-signaled posttranslational modification. The Journal of Biological Chemistry. 283 (37), 25150-25163 (2008).
- Feig, L. A., Cooper, G. M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Molecular and Cellular Biology. 8 (8), 3235-3243 (1988).
- Liu, H., et al. Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science. 322 (5907), 1535-1539 (2008).
- Kennedy, M. J., et al. Rapid blue-light-mediated induction of protein interactions in living cells. Nature Methods. 7 (12), 973-975 (2010).
- Guglielmi, G., Barry, J. D., Huber, W., De Renzis, S. An optogenetic method to modulate cell contractility during tissue morphogenesis. Developmental Cell. 35 (5), 646-660 (2015).
- Izquierdo, E., Quinkler, T., De Renzis, S. Guided morphogenesis through optogenetic activation of Rho signalling during early Drosophila embryogenesis. Nature Communications. 9 (1), 2366 (2018).
- Hutson, M. S., et al. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 300 (5616), 145-149 (2003).
- Rauzi, M., Lenne, P. -. F. Probing cell mechanics with subcellular laser dissection of actomyosin networks in the early developing Drosophila embryo. Methods in Molecular Biology. 1189, 209-218 (2015).
- Rauzi, M., Verant, P., Lecuit, T., Lenne, P. -. F. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nature Cell Biology. 10 (12), 1401-1410 (2008).
- Hunter, C., Wieschaus, E. Regulated expression of nullo is required for the formation of distinct apical and basal adherens junctions in the Drosophila blastoderm. The Journal of Cell Biology. 150 (2), 391-401 (2000).
- Oda, H., Tsukita, S. Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. Journal of Cell Science. 114, 493-501 (2001).
- Munjal, A., Philippe, J. -. M., Munro, E., Lecuit, T. A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature. 524 (7565), 351-355 (2015).
- Rørth, P. Gal4 in the Drosophila female germline. Mechanisms of Development. 78 (1-2), 113-118 (1998).
- Brand, A. H., Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118 (2), 401-415 (1993).
- Magie, C. R., Meyer, M. R., Gorsuch, M. S., Parkhurst, S. M. Mutations in the Rho1 small GTPase disrupt morphogenesis and segmentation during early Drosophila development. Development. 126 (23), 5353-5364 (1999).
- Rich, A., Fehon, R. G., Glotzer, M. Rho1 activation recapitulates early gastrulation events in the ventral, but not dorsal, epithelium of Drosophila embryos. eLife. 9, e56893 (2020).
- Herrera-Perez, R. M., Cupo, C., Allan, C., Lin, A., Kasza, K. E. Using optogenetics to link myosin patterns to contractile cell behaviors during convergent extension. Biophysical Journal. 120 (19), 4214-4229 (2021).

