An Improved Method to Isolate Mitochondrial Contact Sites
Summary
Mitochondrial contact sites are protein complexes that interact with mitochondrial inner and outer membrane proteins. These sites are essential for the communication between the mitochondrial membranes and, thus, between the cytosol and the mitochondrial matrix. Here, we describe a method to identify candidates qualifying for this specific class of proteins.
Abstract
Mitochondria are present in virtually all eukaryotic cells and perform essential functions that go far beyond energy production, for instance, the synthesis of iron-sulfur clusters, lipids, or proteins, Ca2+ buffering, and the induction of apoptosis. Likewise, mitochondrial dysfunction results in severe human diseases such as cancer, diabetes, and neurodegeneration. In order to perform these functions, mitochondria have to communicate with the rest of the cell across their envelope, which consists of two membranes. Therefore, these two membranes have to interact constantly. Proteinaceous contact sites between the mitochondrial inner and outer membranes are essential in this respect. So far, several contact sites have been identified. In the method described here, Saccharomyces cerevisiae mitochondria are used to isolate contact sites and, thus, identify candidates that qualify for contact site proteins. We used this method to identify the mitochondrial contact site and cristae organizing system (MICOS) complex, one of the major contact site-forming complexes in the mitochondrial inner membrane, which is conserved from yeast to humans. Recently, we further improved this method to identify a novel contact site consisting of Cqd1 and the Por1-Om14 complex.
Introduction
Mitochondria perform a variety of different functions in eukaryotes, with the most well-known being the production of ATP through oxidative phosphorylation. Other functions include the production of iron-sulfur clusters, lipid synthesis, and in higher eukaryotes, Ca2+ signaling, and the induction of apoptosis1,2,3,4. These functions are inseparably linked to their complex ultrastructure.
The mitochondrial ultrastructure was first described by electron microscopy5. It was shown that mitochondria are rather complex organelles consisting of two membranes: the mitochondrial outer membrane and the mitochondrial inner membrane. Thus, two aqueous compartments are formed by these membranes: the intermembrane space and the matrix. The mitochondrial inner membrane can be even further divided into different sections. The inner boundary membrane stays in close proximity to the outer membrane, and the cristae form invaginations. So-called crista junctions connect the inner boundary membrane and the cristae (Figure 1). Furthermore, electron micrographs of osmotically shrunken mitochondria reveal that sites exist at which the mitochondrial membranes are tightly connected6,7. These so-called contact sites are formed by protein complexes spanning the two membranes (Figure 1). It is thought that these interaction sites are essential for cell viability due to their importance for the regulation of mitochondrial dynamics and inheritance, as well as the transfer of metabolites and signals between the cytosol and the matrix8.
The MICOS complex in the mitochondrial inner membrane is probably the best characterized and the most versatile contact site-forming complex. MICOS was described in yeast in 2011, and it consists of six subunits9,10,11: Mic60, Mic27, Mic26, Mic19, Mic12, and Mic10. These form a complex of approximately 1.5 MDa that localizes to the crista junctions9,10,11. The deletion of either core subunit, Mic10 or Mic60, leads to the absence of this complex9,11, meaning these two subunits are essential for the stability of MICOS. Interestingly, MICOS forms not only one but multiple contact sites with various mitochondrial outer membrane proteins and complexes: the TOM complex11,12, the TOB/SAM complex9,12,13,14,15,16, the Fzo1-Ugo1 complex9, Por110, OM4510, and Miro17. This strongly indicates that the MICOS complex is involved in various mitochondrial processes, such as protein import, phospholipid metabolism, and the generation of the mitochondrial ultrastructure18. The latter function is probably the major function of MICOS, as the absence of the MICOS complex induced through the deletion of MIC10 or MIC60 leads to an abnormal mitochondrial ultrastructure that virtually completely lacks regular cristae. Instead, internal membrane vesicles without connection to the inner boundary membrane accumulate19, 20. Importantly, MICOS is conserved in form and function from yeast to human21. The association of mutations in MICOS subunits with severe human diseases also emphasizes its importance for higher eukaryotes22,23. Although MICOS is highly versatile, additional contact sites must exist (based on our unpublished observations). Indeed, several other contact sites have been identified, for instance, the mitochondrial fusion machineries Mgm1-Ugo1/Fzo124,25,26 or Mdm31-Por1, which is involved in the biosynthesis of the mitochondrial-specific phospholipid cardiolipin27. Recently, we improved the method that led us to the identification of MICOS to identify Cqd1 as part of a novel contact site formed with the outer membrane complex Por1-Om1428. Interestingly, this contact site also seems to be involved in multiple processes such as mitochondrial membrane homeostasis, phospholipid metabolism, and the distribution of coenzyme Q28,29.
Here, we used a variation of the previously described fractionation of mitochondria9,30,31,32,33. Osmotic treatment of mitochondria leads to the disruption of the mitochondrial outer membrane and to a shrinkage of the matrix space, leaving the two membranes only in close proximity at contact sites. This allows for the generation of vesicles that consist exclusively of mitochondrial outer membrane or mitochondrial inner membrane or at contain contact sites of both membranes through mild sonication. Due to the mitochondrial inner membrane possessing a much higher protein-to-lipid ratio, mitochondrial inner membrane vesicles exhibit a higher density compared to mitochondrial outer membrane vesicles. The difference in density can be used to separate the membrane vesicles through sucrose buoyant density gradient centrifugation. Thus, the mitochondrial outer membrane vesicles accumulate at low sucrose concentrations, while the mitochondrial inner membrane vesicles are enriched at high sucrose concentrations. The vesicles containing contact sites concentrate at intermediate sucrose concentrations (Figure 2). The following protocol describes this improved method, which requires less specialized equipment, time, and energy compared to our previously established one32, in detail and provides a useful tool for the identification of possible contact site proteins.
Protocol
1. Buffers and stock solutions
- Make a 1 M 3-morpholinopropane-1-sulfonic acid (MOPS) solution in deionized water, pH 7.4. Store at 4 °C.
- Prepare 500 mM ethylenediaminetetraacetic acid (EDTA) in deionized water, pH 8.0. Store at room temperature.
- Prepare 2.4 M sorbitol in deionized water. Store at room temperature after autoclaving.
- Prepare 2.5 M sucrose in deionized water. Store at room temperature after autoclaving.
- Prepare 200 mM phenylmethylsulfonyl fluoride (PMSF) in isopropanol. Store at −20 °C.
CAUTION: PMSF is toxic if swallowed and causes severe skin burns and eye damage. Do not breathe in the dust, and wear protective gloves and goggles. - Prepare 72% trichloroacetic acid (TCA) in deionized water. Store at 4 °C.
CAUTION: TCA may cause respiratory irritation, severe skin burns, and eye damage. Do not breathe in the dust, and wear protective gloves and goggles. - Prepare the SM buffer: 20 mM MOPS and 0.6 M sorbitol, pH 7.4.
- Prepare the swelling buffer: 20 mM MOPS, 0.5 mM EDTA, 1 mM PMSF, and protease inhibitor cocktail, pH 7.4.
- Prepare the sucrose gradient buffers: 0.8 M, 0.96 M, 1.02 M, 1.13 M, and 1.3 M sucrose in 20 mM MOPS, and 0.5 mM EDTA, pH 7.4.
NOTE: All the buffers can be stored at 4 °C; however, it is recommended to store the sucrose-containing buffers at −20 °C to avoid fungal growth. The protease inhibitors have to be added freshly prior to use.
2. Generation of submitochondrial vesicles
- Isolate crude yeast mitochondria freshly according to established protocols34,35.
NOTE: For this experiment, mitochondria were isolated from Saccharomyces cerevisiae. The generation of gradient pure mitochondria devoid of other organelles is not necessary. - Resuspend 10 mg of freshly isolated mitochondria in 1.6 mL SM buffer (4 °C) by pipetting (Figure 2, step 1).
- Transfer the mitochondrial suspension to a pre-cooled 100 mL Erlenmeyer flask.
- Slowly add 16 mL of swelling buffer using a 20 mL glass pipette. Apply constant mild stirring on ice during the addition. This osmotic treatment results in water uptake into the matrix space. The swelling of the mitochondrial inner membrane will disrupt the outer membrane. However, both membranes will stay in contact at the contact sites9(Figure 2, step 2).
- Incubate the samples under constant mild stirring for 30 min on ice.
- Slowly add 5 mL of 2.5 M sucrose solution using a 5 mL glass pipette to increase the sucrose concentration in the samples to approximately 0.55 M. This osmotic treatment results in a water efflux from the matrix36. The shrinkage of the inner membrane is intended to maximize its distance to the residual fragments of the outer membrane. This decreases the probability of generating artificial hybrid vesicles of inner and outer membranes through sonication (Figure 2, step 3).
- Incubate the samples under mild stirring for 15 min on ice.
- Subject the mitochondria to sonication to generate submitochondrial membrane vesicles (Figure 2, step 4).
- Transfer the mitochondrial suspension to a pre-cooled rosette cell.
- Sonicate the mitochondrial suspension at a 10% amplitude for 30 s while cooling the rosette cell in an ice bath.
- Rest the suspension for 30 s in an ice bath.
- Repeat step 2.8.2 and step 2.8.3 three more times.
NOTE: The sonication conditions will vary according to the sonicator used. Optimization will be necessary for individual machines. Sonication that is too harsh will lead to the artificial formation of vesicles consisting of mitochondrial inner and outer membranes.
3. Separation of submitochondrial vesicles
- Separate the generated vesicles from the remaining intact mitochondria by centrifugation at 20,000 x g for 20 min at 4 °C. The intact mitochondria will pellet while the vesicles will stay in the supernatant.
- Concentrate the vesicle mixture.
- Transfer the supernatant to fresh ultracentrifugation tubes.
- Load a cushion of 0.3 mL of 2.5 M sucrose solution at the bottom of the tube using a 1 mL syringe equipped with a 0.8 mm x 120 mm cannula.
- Centrifuge at 118,000 x g for 100 min at 4 °C.
NOTE: Here, a swinging bucket rotor with a maximum radius of 153.1 mm was used. The centrifugation conditions strongly depend on the rotor and will have to be adapted when another rotor is used.
- The vesicles will appear as a disc on the top of the sucrose cushion after the centrifugation. Discard approximately 90% of the supernatant. Now, harvest the concentrated vesicles by resuspending them in the remaining buffer including the 2.5 M sucrose by pipetting up and down. Transfer the suspension to an ice-cold Dounce homogenizer.
- Homogenize the suspension with at least 10 strokes using a polytetrafluoroethylene potter.
- Prepare the sucrose gradient.
- For an 11 mL step gradient with five steps (1.3 M, 1.13 M, 1.02 M, 0.96 M, and 0.8 M sucrose in 20 mM MOPS and 0.5 mM EDTA, pH 7.4), each layer has 2.2 mL of sucrose solution.
NOTE: A refractometer is recommended to measure and adjust the sucrose concentrations; however, it is not essential. Apply 10 µL of the buffer to the refractometer, and detect the respective refractive indices. The calculation of sucrose concentration is as follows:
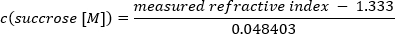
1.3333 represents the refractive index of water. 0.048403 is a sucrose specific conversion index obtained from the linear scaling of molar refractivity to sucrose concentration in aqueous solutions 37. - Add the highest sucrose concentration to the centrifugation tube, and put the tube at −20 °C. Wait until the layer is completely frozen before applying the next one. Proceed accordingly until the last layer is added, and store the gradients at −20 °C.
NOTE: Remember to transfer the frozen gradients to 4 °C when starting the experiment to allow the gradients to thaw. This will take approximately 3 h. For the centrifugation here, a swinging bucket rotor with a capacity of 13.2 mL was used. When a different rotor is used, the gradient step volumes must be adapted accordingly.
- For an 11 mL step gradient with five steps (1.3 M, 1.13 M, 1.02 M, 0.96 M, and 0.8 M sucrose in 20 mM MOPS and 0.5 mM EDTA, pH 7.4), each layer has 2.2 mL of sucrose solution.
- Measure the sucrose concentration of the samples using a refractometer. If there is not access to a refractometer, one could alternatively assume that the sucrose concentration is 2 M. This supposed sucrose concentration will then be the basis for the next step.
- Adjust the sucrose concentration to 0.6 M by the addition of an appropriate amount of 20 mM MOPS, 0.5 mM EDTA, 1 mM PMSF, and protease inhibitor cocktail, pH 7.4. If the sucrose concentration is estimated as 2 M instead of being measured, it is recommended to test whether the concentration is low enough after adjustment. Apply a small aliquot of the sample (ca. 50 µL) on top of 200 µL of 0.8 M sucrose in a test tube. The sample must stay on top of the 0.8 M sucrose.
- Load the samples (approximately 1 mL) on top of the sucrose gradient (keep 10% for later reference). Careful pipetting is important to avoid the disturbance of the gradient (Figure 2, step 5).
- Separate the vesicles by centrifugation at 200,000 x g and 4 °C for 12 h. If possible, set the centrifuge to slow acceleration and deceleration to avoid the disturbance of the gradient (Figure 2, step 6).
NOTE: Here, a swinging bucket rotor with a maximum radius of 153.1 mm was used. The centrifugation conditions strongly depend on the rotor and will have to be adapted when another rotor is used. - Harvest the gradient from top to bottom in 700 µL fractions using a 1 mL pipette. This will result in 17 fractions, which allows for a sufficient resolution.
4. Analysis of submitochondrial vesicles
- Concentrate the proteins by subjecting each fraction to two sequentially performed TCA precipitations38 to prepare the SDS-PAGE samples.
- Add 200 µL of 72% TCA to the individual fractions, and mix until the solution is homogeneous. Incubate the fractions for 30 min on ice, and pellet the precipitated proteins by centrifugation at 20,000 x g and 4 °C for 20 min. Discard the supernatant, add 500 µL of 28 % TCA solution, mix well, and repeat the centrifugation step.
- Wash the pellets with 1 mL of acetone (−20°C), and centrifuge for 10 min at 20,000 x g and 4 °C. Discard the supernatant, and let the pellets air dry. Resuspend the pellets in 60 µL of SDS sample buffer, and incubate the samples for 5 min at 95 °C.
NOTE: A large amount of sucrose will remain, particularly in the high-density fractions, after the first TCA precipitation. This will be removed through the additional TCA precipitation.
- Analyze 20 µL of each fraction by SDS-PAGE39 and immunoblotting40,41,42,43.
Representative Results
It is relatively easy to separate mitochondrial inner and outer membranes. However, the generation and separation of contact site-containing vesicles are much more difficult. In our opinion, two steps are critical and essential: the sonication conditions and the gradient used.
Usually, linear gradients are thought to have a better resolution compared to step gradients. However, their reproducible production is tedious and requires special equipment. Therefore, we established a method to generate a step gradient with relatively small differences between the individual steps (see step 3.5). We speculated that the step gradient would be balanced upon centrifugation, resulting in an almost linear gradient. Strikingly, the determination of the sucrose concentrations of the individual fractions after centrifugation using a refractometer revealed that the step gradient indeed turned virtually linear after 12 h of centrifugation at 200,000 x g (Figure 3).
The importance of mild sonication is evident in these representative results. In mild sonication conditions (Figure 4A), the mitochondrial outer membrane marker Tom40 was enriched in the early low-density fractions (Fraction No. 4) and was virtually absent in the later ones. The mitochondrial inner membrane marker Tim17 was concentrated in high-density fractions (Fraction No. 17). In contrast, the contact site protein Mic60 accumulated in fractions of intermediate sucrose concentration (Fraction No. 12-14), indicating the successful generation and subsequent separation of the various membrane vesicles. In contrast, with harsher sonication conditions, the mitochondrial outer membrane marker Tom40 could be detected in lower fractions of the gradient (Fraction No. 14 and Fraction No. 17, Figure 4B). Additionally, there was a significant amount of Tim17 in fractions of intermediate density (Fraction No. 13 and Fraction No. 14, Figure 4B). The unspecific accumulation of outer and inner membrane markers in fractions of intermediate density indicates the artificial formation of mixed membranes, which inhibits the identification of candidate proteins.
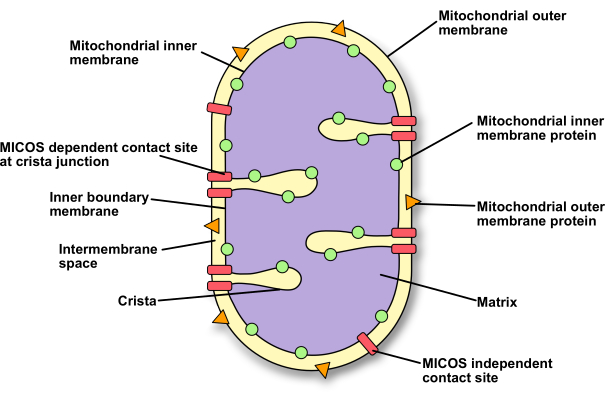
Figure 1: Schematic representation of the mitochondrial ultrastructure. A schematic representation of a mitochondrion to show the different compartments, membranes, and protein locations. Please click here to view a larger version of this figure.
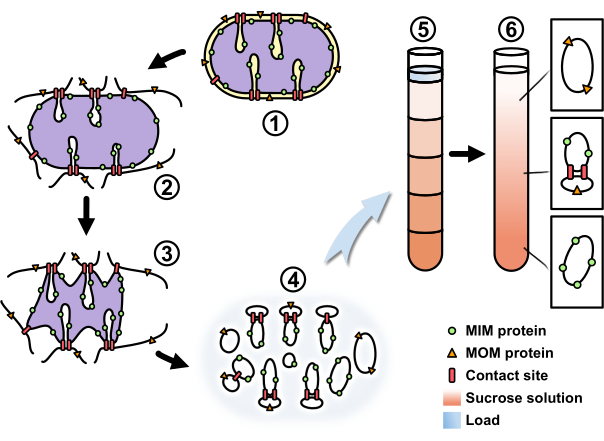
Figure 2: Workflow of mitochondrial fractionation to isolate contact site proteins. A schematic overview of the process described in the protocol. The (1) freshly isolated mitochondria were (2) osmotically swollen (3) and then shrunken. (4) The shrunken mitochondria were objected to mild sonication to generate submitochondrial vesicles. (5) The vesicle mixture was concentrated and loaded onto a sucrose step gradient. (6) Through centrifugation the mitochondrial outer membrane vesicles were enriched at a low sucrose concentration, the mitochondrial inner membrane vesicles at a high sucrose concentration, and the contact site-containing vesicles at an intermediate sucrose concentration. Abbreviations: MIM, mitochondrial inner membrane; MOM, mitochondrial outer membrane. Please click here to view a larger version of this figure.
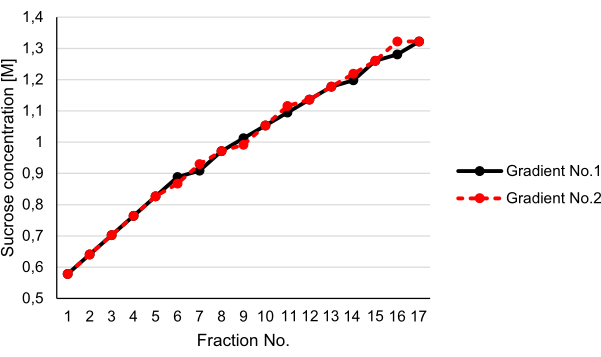
Figure 3: Generation of a linear gradient by centrifugation. Two step gradients (1.3 M, 1.13 M, then 1.02 M, 0.96 M, and 0.8 M in 20 mM MOPS and 0.5 mM EDTA, pH 7.4) were prepared in parallel, and 1 mL of 0.6 M sucrose in 20 mM MOPS and 0.5 mM EDTA, pH 7.4, was loaded onto both gradients. The gradients were subjected to centrifugation at 200,000 x g and 4°C for 12 h and harvested in 17 fractions each. The reproducibility of the method was tested by determination of the sucrose concentration of the single fractions of the individual gradients using a refractometer. Please click here to view a larger version of this figure.
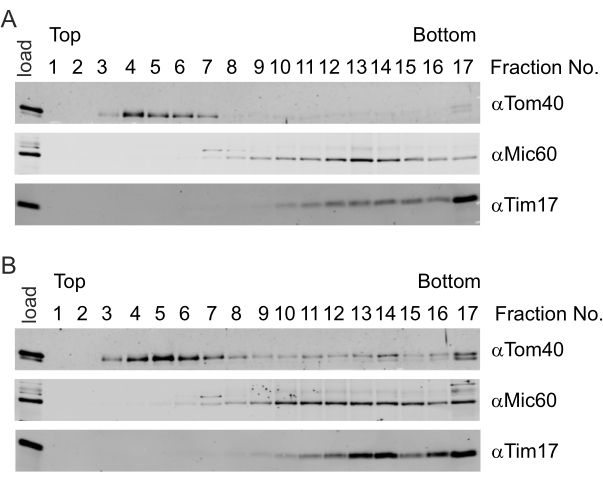
Figure 4: Isolation of contact site proteins with a well-defined ultrasonic pulse. (A,B) Freshly isolated yeast mitochondria were subjected to osmotic treatment and exposed to sonication. The generated vesicles were separated using sucrose buoyant density gradient centrifugation. The gradient was fractionated, and the proteins in the various fractions were subjected to TCA precipitation. The distribution of the marker proteins for the mitochondrial outer membrane (Tom40), the mitochondrial inner membrane (Tim17), and contact sites (Mic60) were analyzed by immunoblotting using the indicated antibodies. (A) Mild sonication (4 x 30 s, 10% amplitude) resulted in a clear separation of the outer membrane proteins (low-density fractions) and inner membrane proteins (high-density fractions). Additionally, the contact site protein Mic60 was successfully enriched in fractions of intermediate density. (B) Harsher sonication (4 x 30 s, 20% amplitude) resulted in the generation of hybrid vesicles and, thus, did not allow for a clear separation of contact sites. Please click here to view a larger version of this figure.
Discussion
Mitochondrial subfractionation is a complicated experiment with several highly complex steps. Therefore, we aimed to further improve and, to a certain degree, simplify our established method32. Here, the challenges were the requirement for complicated and highly specialized equipment, which are often individual constructions, and the enormous time and energy consumption. To this end, we tried to remove the pumps and individual constructions used for casting and harvesting the linear gradient and changed to a step gradient. Importantly, we realized that, at least when prepared as described here, the gradient becomes linear during centrifugation, making this a valid replacement. This method (i) eliminates the need for a gradient mixer, hence decreasing the required equipment; (ii) saves time on the day of the experiment, since the gradient can be prepared in advance; and (iii) is highly reproducible (Figure 3) when prepared according to the described method, which involves freezing the individual steps before layering the next on top. These gradients can also be stored indefinitely at −20°C. Additionally, the loading of the here suggested sedimentation gradient is also easier than having to layer the vesicle mixture under the gradient, as described in similar methods9,32. Loading a floatation gradient requires a syringe with a long cannula to pass the already cast gradient. This step was always problematic because of the risk of destroying the gradient. Another advantage of this method is the lower centrifugation time of 12 h compared to 24 h32, which greatly reduces the time and energy required for this method. The feasibility and reproducibility of the improved procedure described here are highlighted by the recent identification of the MICOS-independent contact site formed by Cqd1 and Por1-Om1428.
However, this experiment is still highly complex. In order to obtain optimal results, there are several essential criteria that have to be taken into consideration when applying this protocol.
One of the most important aspects is the quality of the mitochondria. These have to be freshly isolated. Therefore, the cultivation of yeast as well as the isolation of mitochondria have to be taken into account while planning the procedure. Gentle treatment of the mitochondria during the isolation will prevent the disruption of the membranes, while it is also critical to inhibit proteases with an appropriate concentration of PMSF or, alternatively, commercially available protease inhibitor cocktails34.
Another important step, and one of the most critical aspects of the procedure, is the sonication. Importantly, it is possible to estimate the correct intensity of the ultrasonic pulse by the sound generated through the swirling of the suspension (see video). However, the optimal conditions have to be experimentally determined for each experimental setup, not only based on different brands and models but also different machines of the same model. Notably, the disassembly and cleaning of the tip also affect the intensity of the sonication, thus making a readjustment of the conditions necessary. The recommendation in the protocol is also to use a rosette cell for higher efficiency, but other vessels might work as well. Sonication that is too harsh will lead to the formation of artificially mixed vesicles. This will result in a strong accumulation of inner and outer membrane proteins in fractions of intermediate density, which can be even stronger, as in the shown example (Figure 4B). If this happens, one should reduce the amplitude or time of the sonication. However, issues can also arise when the sonication is not intense enough. In this case, the yield of the generated vesicles might be too low to detect proteins by immunoblotting, and, thus, one would have to increase the amplitude, time, or cycles of sonication.
Finally, the gradient is important for the separation of the various vesicle species generated. However, the optimal maximum and minimum of the sucrose gradient, as well as the number of steps, can also vary depending on the individual setup. Here, a five-step gradient with a maximum of 1.3 M sucrose and a minimum of 0.8 M sucrose sufficed for a visible and reproducible separation of the contact site-containing vesicles from the mitochondrial inner and outer membrane vesicles. In the beginning, it is essential to choose conditions that leave empty fractions above the mitochondrial outer membrane vesicles and below the mitochondrial inner membrane vesicles to ensure correct separation. Compressing either the top or the bottom fractions might lead to artifacts. At later steps, when the precise molarity of sucrose at which the inner and outer membrane vesicles will accumulate is known, the gradient can be optimized by increasing or decreasing the maximum and minimum sucrose concentrations to enhance the resolution among the inner membrane, outer membrane, and contact sites. Of note, the growth conditions of yeast can affect the behavior of the generated vesicles upon density centrifugation. Here, the yeast was grown on rich YP medium (1% [w/v] yeast extract, 2% [w/v] peptone, pH 5.5) containing glycerol (3 % [v/v]) as a carbon source at 30 °C34,44. Other growth conditions might alter the protein and lipid composition of the mitochondria and, thus, the density of the generated vesicles.
The described method provides a reliable and reproducible way to isolate contact sites. However, the identification of the protein composition of the respective contact site (inner and outer membrane components) needs additional assays such as immunoprecipitation, possibly in combination with mass spectrometry. For this, specific antibodies and model organisms expressing tagged versions of the protein candidates are necessary. When using yeast as a model organism, protein candidates can be readily obtained due to the variety of genetic modification methods available and the existence of tagged libraries45,46,47. Therefore, we decided to establish and optimize this protocol for yeast mitochondria. Although it should be possible to adapt the method for mitochondria isolated from other organisms, yeast has the advantage of allowing high amounts of mitochondria to be obtained in a cheap and fast way. Moreover, using yeast as a model organism for gathering the initial results is a well-proven way to identify protein complexes and processes that are conserved in higher eukaryotes up to humans. As stated before, after the identification and characterization of MICOS in yeast9,10,11, it was shown that MICOS is conserved in form and function in higher eukaryotes21. Cqd1 and Por1, components of the recently discovered MICOS-independent contact site28, are also conserved, indicating that this contact site is also present in higher eukaryotes.
The importance of continued research on these contact sites is underlined by the links of MICOS to various neurodegenerative diseases18,48,49,50,51,52,53,54. The correct phosphorylation of Mic60 by PINK1 has been linked to the maintenance of crista junctions in Drosophilia, and, thus, this pathway, which is conserved to humans, may be connected to Parkinson's disease48. Moreover, a point mutation in the human MICOS subunit Mic14 results in destabilization of MICOS and the subsequent alteration of the mitochondrial ultrastructure, which have been found in patients suffering from frontotemporal dementia and amyotrophic lateral sclerosis50,51,52,53,54. Since Mic14 is conserved from yeast to humans, further studies on this protein could be conducted using yeast as a model organism. This basic research might lead to a better understanding of the underlying mechanisms of these diseases, which could speed up the development of efficient treatment and therapies for them. Moreover, the recent discoveries of additional MICOS-independent contact sites27,28 indicate that this field is still growing. Therefore, the here presented protocol might help to progress this promising and fascinating research.
Disclosures
The authors have nothing to disclose.
Acknowledgements
M.E.H. acknowledges the Deutsche Forschungsgemeinschaft (DFG), project number 413985647, for financial support. The authors thank Dr. Michael Kiebler, Ludwig-Maximilians University, Munich, for his generous and extensive support. We are grateful to Walter Neupert for his scientific input, helpful discussions, and ongoing inspiration. J.F. thanks the Graduate School Life Science Munich (LSM) for support.
Materials
| 13.2 mL, Open-Top Thinwall Ultra-Clear Tube, 14 x 89mm | Beckman Instruments, Germany | 344059 | |
| 50 mL, Open-Top Thickwall Polycarbonate Open-Top Tube, 29 x 104mm | Beckman Instruments, Germany | 363647 | |
| A-25.50 Fixed-Angle Rotor- Aluminum, 8 x 50 mL, 25,000 rpm, 75,600 x g | Beckman Instruments, Germany | 363055 | |
| Abbe refractometer | Zeiss, Germany | discontinued, any pipet controller will suffice |
|
| accu-jet pro Pipet Controller | Brandtech, USA | BR26320 | discontinued, any pipet controller will suffice |
| Beaker 1000 mL | DWK Life Science, Germany | C118.1 | |
| Branson Digital Sonifier W-250 D | Branson Ultrasonics, USA | FIS15-338-125 | |
| Branson Ultrasonic 3mm TAPERED MICROTIP | Branson Ultrasonics, USA | 101-148-062 | |
| Branson Ultrasonics 200- and 400-Watt Sonifiers: Rosette Cooling Cell | Branson Ultrasonics, USA | 15-338-70 | |
| Centrifuge Avanti JXN-26 | Beckman Instruments, Germany | B37912 | |
| Centrifuge Optima XPN-100 ultra | Beckman Instruments, Germany | 8043-30-0031 | |
| cOmplete Proteaseinhibtor-Cocktail | Roche, Switzerland | 11697498001 | |
| D-Sorbit | Roth, Germany | 6213 | |
| EDTA (Ethylendiamin-tetraacetic acid disodium salt dihydrate) | Roth, Germany | 8043 | |
| Erlenmeyer flask, 100 mL | Roth, Germany | X747.1 | |
| graduated pipette, Kl. B, 25:0, 0.1 | Hirschmann, Germany | 1180170 | |
| graduated pipette, Kl. B, 5:0, 0.05 | Hirschmann, Germany | 1180153 | |
| ice bath | neoLab, Germany | S12651 | |
| Magnetic stirrer RCT basic | IKA-Werke GmbH, Germany | Z645060GB-1EA | |
| MOPS (3-(N-Morpholino)propanesulphonic acid) | Gerbu, Germany | 1081 | |
| MyPipetman Select P1000 | Gilson, USA | FP10006S | |
| MyPipetman Select P20 | Gilson, USA | FP10003S | |
| MyPipetman Select P200 | Gilson, USA | FP10005S | |
| Omnifix 1 mL | Braun, Germany | 4022495251879 | |
| Phenylmethylsulfonyl fluoride (PMSF) | Serva, Germany | 32395.03 | |
| STERICAN cannula 21 Gx4 4/5 0.8×120 mm | Braun, Germany | 4022495052414 | |
| stirring bar, 15 mm | VWR, USA | 442-0366 | |
| Sucrose | Merck, Germany | S8501 | |
| SW 41 Ti Swinging-Bucket Rotor | Beckman Instruments, Germany | 331362 | |
| Test tubes | Eppendorf, Germany | 3810X | |
| Tissue grinders, Potter-Elvehjem type, 2 mL glass vessel | VWR, USA | 432-0200 | |
| Tissue grinders, Potter-Elvehjem type, 2 mL plunger with serrated tip | VWR, USA | 432-0212 | |
| Trichloroacetic acid (TCA) | Sigma Aldrich, Germany | 33731 | discontinued, any TCA will suffice (CAS: 73-03-9) |
| TRIS | Roth, Germany | 4855 |
References
- Braymer, J. J., Freibert, S. A., Rakwalska-bange, M., Lill, R. BBA – Molecular Cell Research Mechanistic concepts of iron-sulfur protein biogenesis in Biology * General concepts of FeS protein biogenesis. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research. 1868 (1), 118863 (2021).
- Osman, C., Merkwirth, C., Langer, T. Prohibitins and the functional compartmentalization of mitochondrial membranes. Journal of Cell Science. 122 (21), 3823-3830 (2009).
- Smaili, S. S., Hsu, Y., Youle, R. J., Russell, J. T. Mitochondria in Ca 2 %. Signaling and Apoptosis. Journal of Bioenergetics and Biomembranes. 32 (1), (2000).
- Rolland, S. G., Conradt, B. New role of the BCL2 family of proteins in the regulation of mitochondrial dynamics. Current Opinion in Cell Biology. 22 (6), 852-858 (2011).
- Palade, G. E. An electron microscope study of the mitochondrial structure. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1 (4), 188-211 (1953).
- Hackenbrock, C. R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. The Journal of cell biology. 30 (2), 269-297 (1966).
- Hackenbrock, C. R. Chemical and physical fixation of isolated mitochondria in low-energy and high-energy states. Proceedings of the National Academy of Sciences of the United States of America. 61 (2), 598-605 (1968).
- Reichert, A. S., Neupert, W. Contact sites between the outer and inner membrane of mitochondria – Role in protein transport. Biochimica et Biophysica Acta – Molecular Cell Research. 1592 (1), 41-49 (2002).
- Harner, M., et al. The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO Journal. 30 (21), 4356-4370 (2011).
- Hoppins, S., et al. A mitochondrial-focused genetic interaction map reveals a scaffold-like complex required for inner membrane organization in mitochondria. Journal of Cell Biology. 195 (2), 323-340 (2011).
- vonder Malsburg, K., et al. Dual Role of Mitofilin in Mitochondrial Membrane Organization and Protein Biogenesis. Developmental Cell. 21 (4), 694-707 (2011).
- Bohnert, M., et al. Role of mitochondrial inner membrane organizing system in protein biogenesis of the mitochondrial outer membrane. Molecular Biology of the Cell. 23 (20), 3948-3956 (2012).
- Darshi, M., et al. ChChd3, an inner mitochondrial membrane protein, is essential for maintaining Crista integrity and mitochondrial function. Journal of Biological Chemistry. 286 (4), 2918-2932 (2011).
- Körner, C., et al. The C-terminal domain of Fcj1 is required for formation of crista junctions and interacts with the TOB/SAM complex in mitochondria. Molecular Biology of the Cell. 23 (11), 2143-2155 (2012).
- Xie, J., Marusich, M. F., Souda, P., Whitelegge, J., Capaldi, R. A. The mitochondrial inner membrane protein Mitofilin exists as a complex with SAM50, metaxins 1 and 2, coiled-coil-helix coiled-coil-helix domain-containing protein 3 and 6 and DnaJC11. FEBS Letters. 581 (18), 3545-3549 (2007).
- Zerbes, R. M., et al. Role of MINOS in mitochondrial membrane architecture: Cristae morphology and outer membrane interactions differentially depend on mitofilin domains. Journal of Molecular Biology. 422 (2), 183-191 (2012).
- Modi, S., et al. Miro clusters regulate ER-mitochondria contact sites and link cristae organization to the mitochondrial transport machinery. Nature Communications. 10 (1), 4399 (2019).
- Khosravi, S., Harner, M. E. The MICOS complex, a structural element of mitochondria with versatile functions. Biological Chemistry. 401 (6-7), 765-778 (2020).
- John, G. B., et al. The mitochondrial inner membrane protein mitofilin controls cristae morphology. Molecular Biology of the Cell. 16 (3), 1543-1554 (2005).
- Rabl, R., et al. Formation of cristae and crista junctions in mitochondria depends on antagonism between Fcj1 and Su e/g. Journal of Cell Biology. 185 (6), 1047-1063 (2009).
- Alkhaja, A. K., et al. MINOS1 is a conserved component of mitofilin complexes and required for mitochondrial function and cristae organization. Molecular Biology of the Cell. 23 (2), 247-257 (2012).
- Eramo, M. J., Lisnyak, V., Formosa, L. E., Ryan, M. T. The "mitochondrial contact site and cristae organising system" (MICOS) in health and human disease. Journal of Biochemistry. 167 (3), 243-255 (2020).
- Ikeda, A., Imai, Y., Hattori, N. Neurodegeneration-associated mitochondrial proteins, CHCHD2 and CHCHD10-what distinguishes the two. Frontiers in Cell and Developmental Biology. 10, 1-12 (2022).
- Sesaki, H., Southard, S. M., Yaffe, M. P., Jensen, R. E. Mgm1p, a dynamin-related GTPase, is essential for fusion of the mitochondrial outer membrane. Molecular Biology of the Cell. 14 (6), 2342-2356 (2003).
- Fritz, S., Rapaport, D., Klanner, E., Neupert, W., Westermann, B. Connection of the mitochondrial outer and inner membranes by Fzo1 is critical for organellar fusion. Journal of Cell Biology. 152 (4), 683-692 (2001).
- Wong, E. D., et al. The intramitochondrial dynamin-related GTPase, Mgm1p, is a component of a protein complex that mediates mitochondrial fusion. Journal of Cell Biology. 160 (3), 303-311 (2003).
- Miyata, N., Fujii, S., Kuge, O. Porin proteins have critical functions in mitochondrial phospholipid metabolism in yeast. Journal of Biological Chemistry. 293 (45), 17593-17605 (2018).
- Khosravi, S., et al. The UbiB family member Cqd1 forms a novel membrane contact site in mitochondria. J Cell Sci. , (2023).
- Kemmerer, Z. A., et al. UbiB proteins regulate cellular CoQ distribution in Saccharomyces cerevisiae. Nature Communications. 12 (1), 4769 (2021).
- Pon, L., Moll, T., Vestweber, D., Marshallsay, B., Schatz, G. Protein import into mitochondria: ATP-dependent protein translocation activity in a submitochondrial fraction enriched in membrane contact sites and specific proteins. Journal of Cell Biology. 109, 2603-2616 (1989).
- Lithgow, T., Timms, M., Hj, P. B., Hoogenraad, N. J. Identification of a GTP-binding protein in the contact sites between inner and outer mitochondrial membranes. Biochemical and Biophysical Research Communications. 180 (3), 1453-1459 (1991).
- Harner, M. Isolation of contact sites between inner and outer mitochondrial membranes. Methods in Molecular Biology. 1567, 43-51 (2017).
- Adams, V., Bosch, W., Schlegel, J., Wallimann, T., Brdiczka, D. Further characterization of contact sites from mitochondria of different tissues: topology of peripheral kinases. BBA – Biomembranes. 981 (2), 213-225 (1989).
- Izawa, T., Unger, A. K. Isolation of mitochondria from Saccharomyces cerevisiae. Methods in Molecular Biology. 1567, 33-42 (2017).
- Gregg, C., Kyryakov, P., Titorenko, V. I. Purification of mitochondria from yeast cells. Journal of Visualized Experiments. (30), 17-19 (2009).
- Beavis, A. D., Brannan, R. D., Garlid, K. D. Swelling and Contraction of the Mitochondrial Matrix I. A structural interpretation of the relationship between light scattering and matrix volume. Journal of Biological Chemistry. 260 (25), 13424-13433 (1985).
- Born, M., Wolf, E. . Principles of optics electromagnetic theory of propagation, interference and diffraction of light. , (1999).
- Koontz, L. TCA precipitation. Methods in Enzymology. 541, 3-10 (2014).
- Laemmli, U. K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature. 227, 680-685 (1970).
- Renart, J., Reiser, J., Stark, G. R. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: A method for studying antibody specificity and antigen structure. Proceedings of the National Academy of Sciences of the United States of America. 76 (7), 3116-3120 (1979).
- Al-Tubuly, A. A. SDS-PAGE and Western Blotting. Methods Mol Med. 40, 391-405 (2000).
- Kurien, B. T., Hal Scofield, R. Western blotting: Methods and protocols. Western Blotting: Methods and Protocols. (3), 1 (2015).
- Gallagher, S., Chakavarti, D. Immunoblot analysis. Journal of Visualized Experiments. (16), (2008).
- Sherman, F. Getting Started with Yeast. Methods in Enzymology. 194, 3-21 (1991).
- Howson, R., et al. Construction, verification and experimental use of two epitope-tagged collections of budding yeast strains. Comparative and Functional Genomics. 6 (1-2), 2-16 (2005).
- Knop, M., et al. Epitope tagging of yeast genes using a PCR-based strategy: More tags and improved practical routines. Yeast. 15, 963-972 (1999).
- Longtine, M. S., et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 14 (10), 953-961 (1998).
- Tsai, P. I., et al. PINK1 Phosphorylates MIC60/Mitofilin to Control Structural Plasticity of Mitochondrial Crista Junctions. Molecular Cell. 69 (5), 744-756 (2018).
- Xiao, T., et al. Identification of CHCHD10 Mutation in Chinese Patients with Alzheimer Disease. Molecular Neurobiology. 54 (7), 5243-5247 (2017).
- Bannwarth, S., et al. A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain. 137 (8), 2329-2345 (2014).
- Chaussenot, A., et al. Screening of CHCHD10 in a French cohort confirms the involvement of this gene in frontotemporal dementia with amyotrophic lateral sclerosis patients. Neurobiology of Aging. 35 (12), 1-4 (2014).
- Chiò, A., et al. CHCH10 mutations in an Italian cohort of familial and sporadic amyotrophic lateral sclerosis patients. Neurobiology of Aging. 36 (4), 3-6 (2015).
- Genin, E. C., et al. CHCHD 10 mutations promote loss of mitochondrial cristae junctions with impaired mitochondrial genome maintenance and inhibition of apoptosis. EMBO Molecular Medicine. 8 (1), 58-72 (2016).
- Bannwarth, S., et al. A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain. 137 (8), 2329-2345 (2014).

