טכניקה אספטית במדעי הסביבה
English
소셜에 공유하기
개요
מקור: מעבדות של ד”ר איאן פפר וד”ר צ’ארלס גרבה – אוניברסיטת אריזונה
הפגנה סופרת: לואיזה איקנר
טכניקה אספטית היא מיומנות בסיסית הנהוגה בתחום המיקרוביולוגיה הסביבתית הדורשת איזון של תשומת לב ותרגול במעבדה. שימוש נכון בטכניקה זו מפחית את הסבירות לזיהום חיידקי או פטרייתי של ריאגנטים, מדיה תרבותית ודגימות סביבתיות. טכניקה אספטית חיונית גם כדי להבטיח שלמות נתונים ולשמור על טוהר ספריות התרבות שעשויות להיות מורכבות מטילוקים נדירים וקשים מאוד לתרבות. מקורות הזיהום בסביבת המעבדה כוללים מיקרואורגניזמים מוטסים (כולל אלה הנצמדים לאבק וחלקיקי מוך), חיידקים הנמצאים בסביבת העבודה של ספסל המעבדה או על כלי זכוכית או ציוד לא סטריליים, ומיקרואורגניזמים המועברים מגופו ושיערו של החוקר. השימוש בטכניקה אספטית הוא גם אמצעי בטיחות שמוריד את הפוטנציאל להעברת מיקרואורגניזמים לחוקרים, וזה חשוב במיוחד בעת עבודה עם פתוגנים.
Principles
Procedure
Results
The outcome of the procedure demonstrates proper aseptic technique and poor aseptic technique. Figure 7 illustrates the contamination that can arise from poor aseptic technique when pouring agarose plates (top plate: sterile medium; bottom plates: contaminated media).
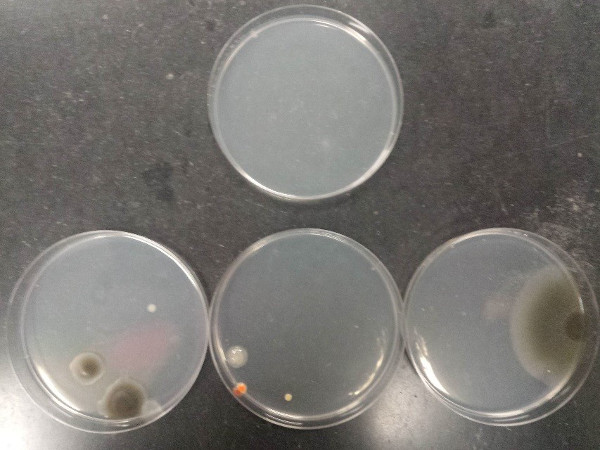
Figure 7: Contamination that can arise from poor aseptic technique when pouring agarose plates. Top plate: sterile medium; bottom plates: contaminated media.
Applications and Summary
Other than using Bunsen burners, aseptic working environments can also be maintained in specialized workstations known as laminar flow hoods, which use directed airflow and filters to maintain sterility.
Proper use of aseptic technique is vital for environmental microbiologists when sampling in the field and in the laboratory when working with media, reagents, and cultured isolates. Poor aseptic technique in the field can result in the transfer of microorganisms from the technician to critical environmental samples, as well as the cross-contamination of microbes from one sample to another. Such events are of importance, for example, in microbial ecology studies seeking to identify and compare bacterial and fungal populations that may be present in a given biome. Contamination of such samples can result in a loss of data integrity. Aseptic technique is also critical for the maintenance of laboratory culture isolates originating from field sampling or from well-established microbial and cell culture repositories. The time, labor, and financial costs that would be required of a lab in an effort to “clean-up” or replace contaminated cultures, particularly rare isolates from unique environments, could be very high and prohibitive, as some isolates may be irreplaceable.
내레이션 대본
Aseptic technique is a fundamental skill in microbiology, and has crucial applications in environmental research.
If microbiological cultures are contaminated, the time, labor, and financial costs that would be required of a lab to “clean up” or replace the cultures, particularly rare isolates from unique environments, could be very high and prohibitive, and some samples may be irreplaceable.
Proper use of aseptic techniques reduces the likelihood of bacterial and fungal contamination of reagents, culture media, and environmental samples, and also avoids cross-contamination between samples. It is also a safety measure that diminishes the potential transmission of microbes to the experimenter, which is especially important when working with pathogens.
This video will introduce the principles of asepsis; several important techniques to maintain sterile reagents and cultures; and finally, some of their uses in environmental science.
The goal of using aseptic techniques is to create and maintain a sterile working environment, equipment, and reagents, so as to minimize contamination of biological samples. Common sources of contamination include airborne microorganisms, microbes present on the laboratory bench or equipment, and those from the hair, body, and clothing of the researcher.
Two types of agents are central to removing or preventing contamination in the laboratory: disinfectant chemicals and heat. Solutions such as 70% ethanol and dilute bleach are often used to disinfect equipment, working surfaces, and experimenters’ gloves before engaging in aseptic work.
At the same time, microbes on or in tools, glassware, and liquid media can be heat-killed in an autoclave, which is a chamber that sterilizes contents via exposure to high-temperature pressurized steam. In addition, tools such as glass rods used for spread plating and metal inoculation loops can be heat-sterilized using a flame source, such as a Bunsen burner.
The use of a flame source is also one of the most common ways to establish an aseptic working environment. The heat from the flame causes air convection, generating an updraft that lifts any airborne contaminants away from the vicinity of the burner, and creating a “sterile field” in which to conduct aseptic experimental work.
Now that you understand the principles behind aseptic techniques and why they are important, let’s go through a protocol for creating an aseptic working environment, making up sterile growth media, and aseptically transferring bacteria between different culturing conditions.
Before beginning aseptic work, it is important for the experimenter to don proper personal protective equipment, or PPE. The purpose of this is both to prevent the experimenter from contaminating the samples and lab cultures, and also to prevent the transfer of potentially pathogenic microbes to the researcher. PPE items include a lab coat, gloves, and safety goggles.
The next step is to properly sterilize and store the growth media to be used for culturing the microbial samples. First, weigh out the proper amount of solid medium components, and add them to the proper volume of liquid solvent specified by the manufacturer, such as deionized water, in an autoclavable container Add a magnetic stir bar, place the container on a hot plate stirrer, and dissolve the solid medium components with low heat and stirring.
Close the medium containers. If using a glass vessel with screw-on cap, be sure to not tighten the cap completely, as the air inside the vessels will expand due to heating during autoclaving and needs to escape. Without escape, the gas could cause the vessel to rupture. Put a piece of autoclave tape on the vessels, and autoclave the media according to the manufacturer’s instructions, such as 20 min at 121 °C. After autoclaving, verify that the stripes on the autoclave tapes turned black, indicating the proper temperature was reached.
For liquid growth media, let them cool to room temperature, and store them at room temperature or with refrigeration as appropriate. For agar-based solid growth media, let them cool to approximately 50 °C, then pour into sterile Petri dishes. Allow the agar to cool and solidify before storing at the appropriate temperature.
For media that cannot be autoclaved due to the presence of heat-sensitive components, filter sterilize using a 0.22-μm filter.
A core technique in microbiological work is to aseptically transfer bacterial cultures between different growth media, both solid and liquid. Prior to beginning, clean the lab bench surface with a disinfectant. This lowers the risk of contaminating cultures or sterile media.
Transferring bacterial cultures commonly makes use of a tool called an inoculating loop, which needs to be sterilized prior to use by heating in a flame.
Turn on a flame source. Slowly pass the inoculating loop through the tip of the flame. The loop will turn red hot. To transfer a bacterial colony from a solid agar plate, open the Petri plate slightly, and gently tap the hot inoculating loop onto an empty part of the agar surface to avoid heat-killing the bacteria. Scrape a single colony with the inoculating loop, and close the plate.
To transfer bacteria from a liquid growth medium, remove the cap from the culture container. To help prevent contamination, avoiding setting the cap down onto the bench. Pass the mouth of the container 2-3 times through the hottest portion of the flame. Then, carefully touch the hot, sterilized inoculation loop onto the inside of the container and let it cool before inserting it into the broth culture. Remove one loopful of the culture, and immediately close the cap.
For transferring the obtained bacteria to a sterile growth medium, remove the cap from a container with the sterile broth and pass the container’s opening through the flame 2-3 times. Then, carefully lower the inoculation loop into the medium, and agitate gently to release the bacteria. Immediately close the cap. Sterilize the inoculation loop after use.
If transferring bacteria onto a sterile agar plate, open the lid of a fresh Petri plate with uninoculated agar. Streak the inoculation loop with the bacterial culture back-and-forth across one sector of the agar. Sterilize the loop and cool it by touching an empty part of the agar, then make another streak on the agar at an obtuse angle to the first streak, making sure to cross the first streak on the first 1-2 strokes but avoid touching the first streak on subsequent strokes. Repeat the sterilization and streaking 2 more times. Close the Petri plate, and sterilize the inoculation loop.
Once inoculated, the broth or agar plate should then be incubated at the ideal growth temperature for the given microorganism to obtain viable culture. On solid medium, a lawn or continuous strand of bacteria would be visible on agar covered by the first two streaks, but individual colonies should be obtained on the final streak. Poor aseptic techniques would result in the growth of mold and other contaminants on the plate.
Aseptic techniques are important in many experiments involving microbial samples from the environment. In this study, researchers isolated bacteriophages, which are bacteria-infecting viruses, from the common soil bacterium Arthrobacter. Arthrobacter cultures were first grown under aseptic conditions. Soil samples were then washed and filtered in phage buffer, and the phage solution was mixed with the bacterial culture and plated onto agar plates. A bacterial lawn would form on the plate, but there would be clearings, or “plaques”, at spots where the virus had infected and killed the bacteria. Phage could then be purified from these plaques for further study.
Other than using Bunsen burners, aseptic working environments can also be maintained in specialized workstations known as laminar flow hoods, which use directed airflow and filters to maintain sterility. Here, scientists worked in a flow hood to isolate potential pathogenic bacteria and viruses from water samples. These isolates were then cultured together with amoebae. Because amoebae normally eat or “phagocytose” bacteria, any bacteria that were able to resist amoebal digestion and remain in these organisms can also potentially remain viable in human cells and cause diseases.
Finally, sterile conditions permit detailed study of ecological mechanisms such as the formation of root nodules in legume plants – bacteria-filled organs that “fix” atmospheric nitrogen into ammonia, which is used by the plant for growth. Researchers here created “microcosms” for studying the nodulation process using notched Petri plates with plant growth medium, placed seedlings into them and inoculated the seedlings with nodule-forming rhizobial bacteria. The aseptic environment of the flow hood prevents contamination of the cultures with other bacteria or fungi.
You’ve just watched JoVE’s video on aseptic techniques in environmental science. You should now understand why aseptic working conditions are important; how to aseptically perform microbiological experiments; and some applications of aseptic techniques to environmental research. As always, thanks for watching!



