생물학적 시료의 SEM 이미징
English
소셜에 공유하기
개요
출처: 페이만 샤베이기-루드포스티와 시나 샤바즈모하마디, 생물의학 공학과, 코네티컷 대학교, 스토스, 코네티컷
스캐닝 전자 현미경(SEM)은 전자 빔을 사용하여 진공 상태에서 전도성 물질을 비파괴적으로 이미지화하고 특성화하는 계측기입니다. 비유로서, 전자빔은 광현미경에 빛이 있는 것처럼 SEM에 있다. 차이점은 전자 현미경이 훨씬 더 높은 해상도와 배율의 이미지를 산출한다는 것입니다. 최고의 광학 현미경은 일반적으로 200 nm까지 해상도를 가지고, SEM은 일반적으로 0.5 nm의 해상도를 주장하는 반면. 이는 광현미경이 가시광선의 약 500nm인 파장의 화절에 의해 광학 현미경이 제한되어 있기 때문이다. 반대로, SEM은 1nm의 파장으로 활력을 공급되는 전자 빔을 사용합니다. 이 특성은 나노 및 미세 구조의 연구를위한 매우 신뢰할 수있는 도구를 만든다. 전자 현미경은 또한 광학 현미경검사에 너무 작은 특징 크기를 가진 생물학 견본의 연구 연구를 가능하게 합니다.
본 데모는 스캐닝 전자 현미경을 이용한 생물학적 시료의 샘플 제제 및 초기 이미지 수집에 대한 소개를 제공한다. 이 경우 콜라겐-하이드록시아파티트(HA) 세포 비계를 연구할 것이다. SEM의 진공 환경과 비전도성 샘플(예: 유기물)에 전자 빔에 의한 유도된 충전은 준비 과정에서 해결될 과제를 생성합니다. 해상도, 초점 깊이 및 샘플 유형과 관련이 있는 다양한 이미징 방법의 장점과 단점도 논의될 것입니다. 이 데모의 목적은 참가자에게 SEM에 대한 자세한 정보를 제공하여 이 현미경 모듈이 생물학적 샘플 유형에 가장 적합한지 확인하는 것입니다.
Principles
Procedure
Results
The SEM images in Figures 3 and 4 show that the imaged structure is highly three dimensional with microscale features. Image quality is affected by the focus and the thickness of the sputter coating.
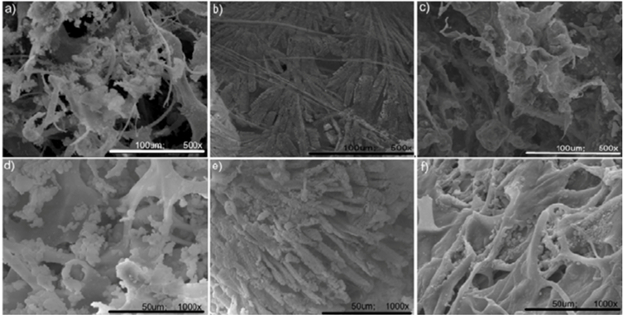
Figure 3: The following images demonstrate how the sample focus can affect image quality. In the image on the right, the whole field of view is in focus, whereas it is out of focus on the left. Playing with parameters like the focus can provide a much better image.

Figure 4: Images of collagen-hydroxyapatite sample.
Applications and Summary
Here we demonstrated the depth of focus, field of view and maximum resolution and magnification of an electron microscope and how these properties can be used to view biological samples. This demonstration was designed to help viewers decide which microscopy module is the best for a certain application. As demonstrated, SEM has a very high depth of focus, much higher resolution and greater magnifications. However, it is not appropriate for all sample types.
This demonstration introduced the principles of electron microscopy and showed several of their applications in research labs. Electron microscopes are used for inspection, characterization and quality control. For example, they are used to visualize ICs, circuit boards, crack propagation and nano-electromechanical systems. In the field of biology these instruments play a key role as well. There are even electron microscopes especially designed to accommodate wet biological samples. These biological samples range from tissues to bone, cells and microorganisms. Using additional detectors can enable even more analysis, such as precise surface analysis.
Materials List
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Biosample | |||
| Carbon or Gold coater | |||
| Cross beam-SEM | ZEISS | ||
| Collagen-Hydroxyappetite Cellular Scaffolds | Developed by Wei Laboratory at University of Connecticut |
References
- Oatley, C. W., W. C. Nixon, and R. F. W. Pease. "Scanning electron microscopy." Advances in Electronics and Electron Physics 21 (1966): 181-247.
- Goldstein, Joseph, et al. Scanning electron microscopy and X-ray microanalysis: a text for biologists, materials scientists, and geologists. Springer Science & Business Media, 2012.
- Carol Heckman, et al. Preparation of cultural cells for scanning electron microscope. Nature Protocols Network, 2007, doi:10.1038/nprot.2007.504
내레이션 대본
Scanning electron microscopy, or SEM, is often used to image biological materials on the nano scale. Optical microscopes, which use light to image a sample, are heavily used to non-destructively image biological samples, however, their resolution and depth of field is limited, thus SEM is used in order to achieve higher resolution down to one nanometer.
In SEM, a beam of electrons is focused through a series of condenser lenses, which then hits the sample. When the beam hits the sample, electrons on the surface are scattered and measured by the detector.
In this video, we will discuss how SEM works, demonstrate how to image a biological sample in the laboratory, and finally, introduce some techniques used to image sensitive samples.
A scanning electron microscope uses a high energy electron beam, which is generated by an electron gun fitted with a filament cathode. The generated electrons are propelled toward the anode and then focused using condenser lenses before entering the objective lens. The objective lens is calibrated to focus the beam on the sample, where it is raster scanned across the surface. The interactions of the electrons with the atoms in the sample are used to study the topography, elemental composition, and crystallinity of the sample. When the incident electron beam hits the surface, it emits secondary and backscattered electrons. Secondary electrons are low energy electrons that are emitted from the sample close to the surface and provide topographical information.
Backscattered electrons, on the other hand, are reflected in the opposite direction of the incident beam. The interaction intensity increases with increasing atomic weight, enabling the user to distinguish compositional differences. Special consideration is needed to image biological samples with SEM, since SEM utilizes a high vacuum, thus biological samples, which typically have a high water content, must be dried first. This can cause collapse of the structure of sensitive samples, especially cells. Thus, cells are treated with a fixative, rinsed, and then dehydrated slowly by washing with increasing amounts of ethanol.
For rigid biological materials, such as the collagen-hydroxyapatite tissue scaffold used in this demonstration, the sample is dried over a period of several days under high vacuum.
Finally, since typical SEM imaging requires a conductive surface, biological samples are often sputter coated with a thin layer of metal prior to imaging. Now that we’ve discussed how SEM works and how to prepare a biological sample for imaging, let’s take a look at how to prepare and image a collagen-hydroxyapatite tissue scaffold.
First, mount the biomaterial sample onto an SEM stub using conductive carbon tape and ensure that the sample is dry and has no contamination on the surface.
Then, place the mounted sample in the chamber of a sputter coater, pump down the chamber, and sputter coat the sample for around 40 seconds to achieve a thin, four- to six-nanometer thick coating of metal, in this case gold, with adequate coverage. Once coated, remove the sample and use conductive tape to connect the stub to the top of the sample, which is now coated with conductive metal.
Finally, mount the stub on the SEM stage and tighten the screw on the side. Now the sample is ready to image with SEM. First, load the stage into the SEM chamber and seal the door, then hit the transfer button to open the passage from the loading chamber to the vacuum. Once the internal door is open, screw the metal rod into the stage and push the sample into the vacuum chamber, then unscrew the metal rod and fully retract it into the load chamber, then press store to close the vacuum chamber.
Now let’s image the sample using SEM.
First, move the stage using the controller and navigate the sample into the field of view, then move the sample vertically until the working distance is five to 10 millimeters. Turn the electron beam on and select the detector for secondary electrons, set the beam to five kiloelectron volts initially, then increase up to 20 to 30 kiloelectron volts as needed. If the image is not clear, turn the focus, brightness, and contrast knobs until a clear image appears.
Use the stage navigation and the X and Y directions to locate a new spot on the sample, then increase the magnification until the desired features are visible. Adjust the focus, contrast, and brightness as needed to improve the image quality. You may need to decrease the scan speed and turn on line averaging to acquire a better image, then save the image.
The SEM images reveal a highly three-dimensional and porous structure with fibrous features smaller than 25 microns. These features would be difficult to visualize using optical microscopy, as optical microscopy has a much lower depth of field.
There are many challenges associated with the imaging of biological structures with SEM, including structure collapse or damage from the high energy electron beam. Let’s now take a look at how the general SEM technique is applied to these types of sensitive samples. Delicate biological structures, such as these young plant tissues, or those with a high water content, must be treated through a fixation process prior to imaging.
These floral meristems were immediately treated with a freshly prepared formalin/acetic acid fixative solution. The fixed tissue was dissected in ethanol, placed in a mesh container, and dehydrated through an ethanol series of 70%, 80%, 90%, and 100% ethanol. Finally, the plant tissues were dried using a critical point dryer, mounted, and sputter coated with a thin metal coating.
After SEM imaging, it is clear that the untreated structures were heavily damaged by the drying process and showed considerable collapse in structure, while those that were fixed maintained their native structure. Alternatively, cells and other high water content specimens can be imaged using environmental SEM, or ESEM. ESEM utilizes a high energy electron beam that is raster scanned over the sample, as with conventional SEM, however, it enables the imaging of wet or uncoated samples by maintaining a gaseous environment within the chamber.
This is done by separating the high vacuum chamber containing the electron gun from the specimen chamber using two apertures. The electron beam does incur significant losses due to scattering by gas molecules, but is typically a high enough energy for imaging. Here, cells were grown on a silicon chip, functionalized with quantum dots, and fixed using a glutaraldehyde fixation protocol. The cells were imaged in water and show the uncollapsed structure of the cell with individual quantum dots visible on the cell surface.
You’ve just watched JoVE’s introduction to visualizing biomaterials using SEM. You should now understand how SEM works, how biological samples are prepared and imaged, as well as some applications of the technique for sensitive structures.
Thanks for watching!
