协议测量过冷的合成砂水 - 气 - 甲烷水合物样品的热性能
Summary
We present a protocol for measuring the thermal properties of synthetic hydrate-bearing sediment samples comprising sand, water, methane, and methane hydrate.
Abstract
Methane hydrates (MHs) are present in large amounts in the ocean floor and permafrost regions. Methane and hydrogen hydrates are being studied as future energy resources and energy storage media. To develop a method for gas production from natural MH-bearing sediments and hydrate-based technologies, it is imperative to understand the thermal properties of gas hydrates.
The thermal properties’ measurements of samples comprising sand, water, methane, and MH are difficult because the melting heat of MH may affect the measurements. To solve this problem, we performed thermal properties’ measurements at supercooled conditions during MH formation. The measurement protocol, calculation method of the saturation change, and tips for thermal constants’ analysis of the sample using transient plane source techniques are described here.
The effect of the formation heat of MH on measurement is very small because the gas hydrate formation rate is very slow. This measurement method can be applied to the thermal properties of the gas hydrate-water-guest gas system, which contains hydrogen, CO2, and ozone hydrates, because the characteristic low formation rate of gas hydrate is not unique to MH. The key point of this method is the low rate of phase transition of the target material. Hence, this method may be applied to other materials having low phase-transition rates.
Introduction
天然气水合物是包含含有在轿厢1客体分子氢键的水分子的笼形结构的结晶化合物。在海底和永久冻土地区的甲烷水合物(MHS)的大量非常有趣的未来能源资源,但可能会影响到全球的气候条件2。
2013年3月,日本石油,天然气和金属国家公司进行了世界上第一个海上生产测试,使用了“减压法”3,4东部南海海槽自然承载MH沉积物中提取气体。
气体水合物可以存储气体,例如甲烷1,氢5,CO 2 1,6,和臭氧7。因此,甲烷和氢气水合物研究作为势能储存和运输介质。为了减少二氧化碳释放到大气中,CO 2 seques 2排放使用二氧化碳在深海沉积物2水合物tration进行了研究6。臭氧是目前在水净化和食品杀菌使用。臭氧保鲜技术的研究已进行了,因为它是化学上不稳定7。在水合物的臭氧浓度是在臭氧化水或冰7比要高得多。
为了开发天然含MH沉积物和基于水合物技术的天然气产量,就必须了解天然气水合物的热性能。然而,气体水合物含沉积物的热性质数据和模型研究很少8。
的“减压方法”可用于通过减少低于水合物稳定的孔隙压力以解离氢的沉积物孔隙空间。在这个过程中,沉淀物的孔隙空间的分量,从水和氢水,氢,和气体发生变化。热性能“测量后者条件是困难的,因为氢的熔化热可能会影响测量结果。为了解决这个问题,村冈等在氢形成9进行热性质“测量在过冷的条件。
与此视频协议中,我们解释过冷合成砂水煤气氢样品的测定方法。
图1示出用于测量人工甲烷水合物轴承沉积物的热性能的实验装置。安装是如图参考9相同。该系统主要包括一高压容器中,压力和温度的控制,以及测量系统的热性能。高压容器用140毫米的内径为140毫米的高度由圆柱形不锈钢的;其与死体积除去内部容积是2110厘米3,而其压力限制为15兆帕。该transie nt的平面源(TPS)技术被用于测量热性能10。九与2.001毫米个人半径TPS探头放入容器内。九个探针9的布局示于图2中的参考9。在TPS探针被连接到热性能'分析器用电缆和在实验过程中手动切换。在TPS传感器,连接图,和设置在容器的细节示于图S1中 ,图2和的支持信息3中参考9。
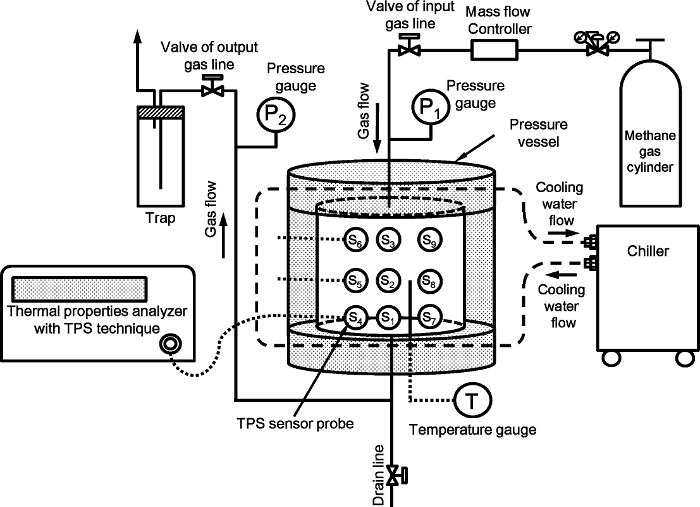
图1:该实验装置用于测量所述人工甲烷水合物轴承沉积物的热性能的数字是从参考9修改。3956fig1large.jpg“目标=”_空白“>点击此处查看该图的放大版本。
使用了TPS方法测量每个样品的热性能。该方法的原则在参照图10进行说明。在该方法中,依赖于时间的温度上升,ΔTAVE,是
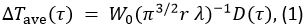
哪里
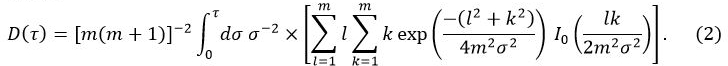
在等式1中,W 0是来自传感器的输出功率,r是传感器探头的半径,λ是样品的热导率,α为热扩散率,t是从电源供给开始的时间到传感器探头。D(τ)是一个无量纲时间相关的功能。τ </青霉>由(αT/ R)1/2给出。在等式2中,m是TPS探针的同心环的数量和I 0是一个修正贝塞尔函数。样品的热导率,热扩散率和比热同时由施加到作为电源被供给到传感器探头的温度上升反演分析确定。
Protocol
Representative Results
Discussion
估计MH的测量形成热的影响。 MH的形成热量从中文变化率的产品估计, 如图3b和形成H = 52.9千焦摩尔-1 MH 14的焓。因此,最高温度变化是0.00081℃,秒-1。这是比5秒的时间间隔期间1℃,1.5℃之间的TPS传感器的温度上升ΔTÇ低得多。详细的估计和讨论二段描述。 4参考9。
以下是至关重要的协议的步?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
这项研究是财政上MH21研究联盟甲烷水合物资源日本经济,贸易和工业部国家甲烷水合物开发计划的支持。笔者想感谢T.前川和S.转到他们与实验的援助。
经许可后转载自数字(村冈,M.,Susuki,N.,山口,H.,辻,T.,山本,Y.,能源燃料,29(3),2015年,1345年至1351年,2015年,DOI: 10.1021 / ef502350n)。版权所有(2015)美国化学学会。
Materials
| TPS thermal probe, Hot disk sensor | Hot Disk AB Co., Sweden | #7577 | Kapton sensor type, sensor radius 2.001 mm |
| Hot disk thermal properties analyzer | Hot Disk AB Co., Sweden | TPS 2500 | |
| Toyoura standard silica sand | Toyoura Keiseki Kogyo Co., Ltd., Japan | N/A | |
| Methane gas ,99.9999% | Tokyo Gas Chemicals Co., Ltd., Japan | N/A | Grade 6N, Volume 47L, Charging pressure 14.7MPa |
| Water Purification System,Elix Advantage 3 | Merck Millipore., U.S. | N/A | 5 MΩ cm (at 25°C) resistivity |
| Vibrating table, Vivratory packer | Sinfonia Technology Co. Ltd., Japan | VGP-60 | |
| Chiller, Thermostatic Bath Circulator | THOMAS KAGAKU Co., Ltd., Japan | TRL-40SP | |
| Coorant, Aurora brine | Tokyo Fine Chemical Co.,Ltd., Japan | N/A | ethylene glycol 71wt% |
| Temparature gage | Nitto Kouatsu., Japan | N/A | Pt 100, sheath-type platinum resistance temperature detector |
| Pressure gage | Kyowa Electronic Instruments., Japan | PG-200 KU | |
| Data logger | KEYENCE., Japan | NR-500 | |
| Mass flow controller | OVAL Co., Japan | F-221S-A-11-11A | Maximum flow 2000 Nml/M, maximum design pressure 19.6 MPa |
References
- Sloan, E. D., Koh, C. A. . Clathrate Hydrates of Natural Gases, 3rd ed. , (2007).
- Hatzikiriakos, S. G., Englezos, P. The relationship between global warming and methane gas hydrates in the earth. Chem. Eng. Sci. 48 (23), 3963-3969 (1993).
- Yamamoto, K. Overview and introduction: pressure core-sampling and analyses in the 2012-2013 MH21 offshore test of gas production from methane hydrates in the eastern Nankai Trough. Mar. Petrol. Geol. 66 (Pt 2), 296 (2015).
- Fujii, T., et al. Geological setting and characterization of a methane hydrate reservoir distributed at the first offshore production test site on the Daini-Atsumi Knoll in the eastern Nankai Trough, Japan. Mar. Petrol. Geol. 66 (Pt 2), 310 (2015).
- Mao, W. L., et al. Hydrogen clusters in clathrate hydrate. Science. 297 (5590), 2247-2249 (2002).
- Lee, S., Liang, L., Riestenberg, D., West, O. R., Tsouris, C., Adams, E. CO2 hydrate composite for ocean carbon sequestration. Environ. Sci. Technol. 37 (16), 3701-3708 (2003).
- Muromachi, S., Ohmura, R., Takeya, S., Mori, H. Y. Clathrate Hydrates for Ozone Preservation. J. Phys. Chem. B. 114, 11430-11435 (2010).
- Waite, W. F., et al. Physical properties of hydrate-bearing sediments. Rev. Geophys. 47 (4), (2009).
- Muraoka, M., Susuki, N., Yamaguchi, H., Tsuji, T., Yamamoto, Y. Thermal properties of a supercooled synthetic sand-water-gas-methane hydrate sample. Energy Fuels. 29 (3), 1345-1351 (2015).
- Gustafsson, S. E. Transient plane source techniques for thermal conductivity and thermal diffusivity measurements of solid materials. Rev. Sci. Instrum. 62 (3), 797-804 (1991).
- Sakamoto, Y., Haneda, H., Kawamura, T., Aoki, K., Komai, T., Yamaguchi, T. Experimental Study on a New Enhanced Gas Recovery Method by Nitrogen Injection from a Methane Hydrate Reservoir. J. MMIJ. 123 (8), 386-393 (2007).
- Lee, B. I., Kesler, M. G. A generalized thermodynamic correlation based on three-parameter corresponding states. AIChE J. 21 (3), 510-527 (1975).
- Reid, R. C., Prausnitz, J. M., Poling, B. E. Chapter 3, Unit 3, 7. The properties of gases and liquids. , 47-49 (1987).
- Anderson, G. K. Enthalpy of dissociation and hydration number of methane hydrate from the Clapeyron equation. J. Chem. Thermodyn. 36 (12), 1119-1127 (2004).
- Waite, W. F., deMartin, B. J., Kirby, S. H., Pinkston, J., Ruppel, C. D. Thermal conductivity measurements in porous mixtures of methane hydrate and quartz sand. Geophys. Res. Lett. 29 (24), 82-1-82-4 (2002).
- Kumar, P., Turner, D., Sloan, E. D. Thermal diffusivity measurements of porous methane hydrate and hydrate-sediment mixtures. J. Geophys. Res. 109 (B1), (2004).
- Huang, D., Fan, S. Measuring and modeling thermal conductivity of gas hydrate-bearing sand. J. Geophys. Res. 110 (B1), (2005).

