Spiral Ganglion Neuron Explant Culture and Electrophysiology on Multi Electrode Arrays
Summary
We present a protocol to culture primary murine spiral ganglion neuron explants on multi electrode arrays to study neuronal response profiles and optimize stimulation parameters. Such studies aim to improve the neuron-electrode interface of cochlear implants to benefit hearing in patients as well as the energy consumption of the device.
Abstract
Spiral ganglion neurons (SGNs) participate in the physiological process of hearing by relaying signals from sensory hair cells to the cochlear nucleus in the brain stem. Loss of hair cells is a major cause of sensory hearing loss. Prosthetic devices such as cochlear implants function by bypassing lost hair cells and directly stimulating SGNs electrically, allowing for restoration of hearing in deaf patients. The performance of these devices depends on the functionality of SGNs, the implantation procedure and on the distance between the electrodes and the auditory neurons.
We hypothesized, that reducing the distance between the SGNs and the electrode array of the implant would allow for improved stimulation and frequency resolution, with the best results in a gapless position. Currently we lack in vitro culture systems to study, modify and optimize the interaction between auditory neurons and electrode arrays and characterize their electrophysiological response. To address these issues, we developed an in vitro bioassay using SGN cultures on a planar multi electrode array (MEA). With this method we were able to perform extracellular recording of the basal and electrically induced activity of a population of spiral ganglion neurons. We were also able to optimize stimulation protocols and analyze the response to electrical stimuli as a function of the electrode distance. This platform could also be used to optimize electrode features such as surface coatings.
Introduction
In accordance with the World Health Organization, 360 million people worldwide suffer from hearing loss with profound consequences on professional and private life. Hearing aids can restore sensory function in moderate forms of hearing loss; however, for the most severe cases, the most effective treatment option is a prosthetic device called a cochlear implant (CI). CIs contain a linear electrode array of up to 24 electrodes, which is surgically inserted into the scala tympani of the cochlea. The electrodes directly stimulate the spiral ganglion neurons, forming the auditory nerve 1.
With more than 300,000 devices implanted worldwide, CIs are very successful medical implants and rank among the most cost-effective procedure ever reported. Despite its success the cochlear implant still has limitations such as reduced frequency resolution compared to physiological hearing. This can lead to deficits in effective communication in groups or noisy environments, and the ability to decipher very complex sounds such as music. This reduced frequency resolution is likely due to the gap between the CI electrodes and the spiral ganglion neurons, leading to stimulation of large groups of neurons. This gap is in the range of hundreds of micrometers 2,3. Elimination of this gap would facilitate the stimulation of smaller groups of neurons per electrode, thereby increasing frequency resolution and overall performance of the device 4.
To study the influence of the gap between the electrode and the neuron and the effect of various optimized stimulation protocols, we have developed an in vitro bioassay based on a non-invasive electrophysiological characterization of SGNs on multi electrode arrays (MEAs) 5. Additionally, MEAs can be easily modified to vary electrode shape, size, material and surface roughness, to optimize the neuron-electrode interface. The following is a step-by-step protocol to reproducibly obtain recordings from murine spiral ganglion neuron cultures and assess the dependency on the above-mentioned parameters.
Protocol
Animal care and experimentation was performed in accordance with the guidelines of the Swiss local authorities (Amt für Landwirtschaft und Natur des Kantons Bern, Switzerland).
1. Prepare Solutions for Experiments
- Prepare the extracellular matrix (ECM) coating solution (see Material Table, point 6): Thaw ECM mix on ice. Dilute the ECM mix 1:10 in basic culture medium (without neurotrophic factors or Fetal Bovine Serum (FBS)) and store on ice.
- Prepare the culture medium (see Material Table, point 1): Prepare a stock of Neurobasal medium not containing FBS or Brain Derived Neurotrophic Factor (BDNF). Supplement neurobasal media with fresh FBS (10%) and BDNF (5 ng/ml) just prior to adding to the cell culture.
- Prepare the extracellular solution (see Material Table, point 2).
2. Washing and Sterilization of MEAs
NOTE: The MEAs used for the experiments contain 68 electrodes arranged in a rectangular grid (Figure 1E). Each electrode has a size of 40 x 40 µm2 with a spacing of 200 µm from center to center. The electrodes are made of platinum. The electrodes are connected to the corresponding contacts by a circuit made of indium tin oxide. This circuit is insulated by a 5 μm layer of SU-8. See Material table for details on the provider. Other MEA layouts may be suitable for these experiments.
- For new MEAs: Rinse them by immersing in 70% ethanol for 30 sec and wash with distilled water for 30 sec. Work in a laminar flow hood.
- Let the MEA dry for 30 min in a laminar flow hood.
- Put each MEA into an individual sterile Petri dish (35 mm x 10 mm) and seal with foil. The MEAs can be stored until used.
- For used MEAs: Incubate the MEAs in a Petri dish (35 mm x 10 mm) containing 1 ml of enzymatic solution (see Materials Table, point 6) O/N on orbital shaker at RT to remove biological material. Then continue as in step 2.1.
NOTE: Handle the MEAs with care using forceps with rubber-covered tips.
3. Preparation of MEAs for Culture Experiments
- Remove the sealing foil and leave the MEA in the Petri dish (35 mm x 10 mm) for the whole experiment.
- Coat the MEAs with the solution prepared in 1.1: Use a cold 200 µl pipette tip (stored at -20 °C) to pipet 50 µl of coating solution to each MEA, covering the whole electrode area.
- Allow MEAs to coat for 30 min to 1 hr at RT.
- Remove the coating solution using a pipette. Apply 100 µl of culture medium supplemented with 10% FBS and 5ng/ml BDNF and leave it at RT until plating the tissue.
- Place 2 Petri dishes containing the coated MEAs into a large petri dish (94 mm x 16 mm) and add a third small petri dish containing 1.5 ml of Phosphate Buffered Saline (PBS) for humidification.
NOTE: Adding a small petri dish (step 3.5) containing PBS is crucial to significantly minimize evaporation of culture medium.
4. Spiral Ganglion Dissection
NOTE: Gross dissection can be done outside the laminar flow hood (Step 4.1 to 4.4). For fine dissection sterile conditions (laminar flow hood) are mandatory (from step 4.5).
- Euthanize animals (5-7 day old mice) by decapitation without prior anesthesia.
- Sterilize the head by spraying with 70% ethanol.
- By holding the head, cut the connection between the skin and the skull with a sharp/sharp scissor along the sagittal line.
- Cut the skull sagittally and remove the brain using sharp/blunt scissors.
- Cut the temporal bones from the skull and place those in a petri dish containing sterile ice cold Hanks' Balanced Salt Solution (HBSS).
- Using a dissection microscope, dissect the tympanic bulla using fine forceps and isolate the inner ear.
- Remove the bone of the cochlea, using fine forceps.
- Remove the spiral ligament and stria vascularis (SV) together by holding with forceps the basal portion of the spiral ganglion (SG) and the SV and slowly unwinding the SV from base -to-apex
- Isolate the organ of Corti (OC), the SG and the modiolus (Figure 1A–C)
- Separate the OC from the SG and modiolus by holding with forceps the basal portion of the SG and the OC and slowly unwinding the OC from base-to-apex.
- Cut lateral explants (200 to 500 µm in diameter) from the spiral ganglion still attached to the modiolus (Figure 1D) using a forceps and a micro scalpel.
5. Spiral Ganglion Explant Culture on MEAs
- Place two spiral ganglion explants next to the electrodes on the MEA previously prepared with 100 µl of culture medium.
- Place the organ of Corti approximately 5 mm away from the electrode area.
- Use forceps to pin the explants and the organ of Corti onto the MEA while avoiding damaging the tissue.
- Place the MEAs carefully into the incubator and culture at 37 °C and 5% CO2. The next day, visually inspect that the explants have attached to the MEA.
NOTE: If explants have not attached O/N, they will rarely do so over the next days. The OC is placed adjacent to the culture for trophic/neurotrophic support. - Add 100 µl of culture medium containing 10% FBS and BDNF daily for 5 consecutive days.
- On day 6 add 2 ml of culture medium containing 10% FBS and 5ng/ml BDNF and culture the tissue for an additional 13 days.
6. Electrophysiological Recordings to Investigate Spontaneous and Electrode Stimulation Dependent Activity
- Wash the MEA culture with extracellular solution prepared in step 1.3, at RT.
- Dry the contacts with a tissue and mount the MEA on the MEA setup.
NOTE: To keep the culture humid during the mounting, add a small drop of extracellular solution to the culture. - Add 300 µl of extracellular solution and wait for 10 min before recording, to allow the system to stabilize.
- Record spontaneous activity for 2 min from all electrodes by pressing on the record/acquire button of the software and identify recording electrodes.
- MEA electrode stimulation: Select the amplitude/duration/shape of the stimulus on the appropriate software and apply to several electrodes consecutively as described 13. Select the electrodes based on the ones showing spontaneous activity (as in step 6.4). Record from all of the remaining electrodes.
- To exclude stimulation artifact, stimulate from the same electrode 10 times. If the culture responds at least 8 out of 10 times, it can be assumed as a positive response upon electrode induced stimulation.
- To identify background noise, apply Tetrodotoxin (TTX) to the cultureat a concentration of 1 µM, to block voltage-gated sodium channels and record for 2 min. Use this to perform spike detection (7.1 and 7.2).
7. Data analysis
NOTE: Data analysis has been previously described in detail in 6 and 5. For specifics on software used in this study see Materials Table, point 7.
- Using the appropriate software detect spontaneous activity for each electrode employing a detector based on standard deviations and a subsequent discriminator. This procedure is described in 6. Activity appears as fast voltage transients (<5 msec).
- Choose a threshold that results in no activity when analyzing the TTX treated samples.Adapt the threshold value for each experiment to discriminate between false positive and false negative detections.
- Using appropriate software observe the detected neuronal activity of each electrode as a raster plot according to standard procedures (see Figure 2A–C).
- Determine and display the total network activity by summing up all detected events within a sliding window of 10 msec, shifted by 1 msec steps.
- Detect stimulation-induced activity by displaying the raw data using appropriate analysis software. Identify the single spikes offline manually. Single spikes appear as fast voltage transients, occurring after the stimulation artifact (arrow head in Figure 2E). An example is shown in Figure 2E.
- Depending on the experiment, analyze the number of responding electrodes, the threshold needed to achieve a response, number of action potential per electrodes and other parameters of interest 5.
8. Tissue Fixation and Immunohistochemistry
NOTE: The staining process will destroy the MEA. See Material Table (point 4) for reagents.
- Directly after recording wash the cultures with 37 °C warm PBS three times. Discard the PBS and apply 4% Paraformaldehyde (PFA) (in PBS), preheated to 37 °C for 10 min.
CAUTION: PFA is toxic. Work in a chemical hood with appropriate protection and discard solution according to guidelines. - Wash the cultures three times with PBS and block with 2% Bovine Serum Albumin (BSA) in PBS (0.01% Triton-X100) for 2 hr.
- Add the first antibody (Anti-βIII Tubulin, TUJ antibody) diluted in PBS (2% BSA and 0.01% Triton-X100) and leave O/N at 4 °C. Wash the culture three times with PBS.
NOTE: From now on keep the sample in the dark as often as possible to minimize bleaching of the secondary fluorescently-labeled antibody. - Add the secondary antibody, diluted in PBS (2% BSA and 0.01% Triton-X100) and incubate for 1 to 2 hr at RT. To stain nuclei add 1:10,000 DAPI (1 mg/ml stock) for 10 min.
- Wash three times with PBS and mount the samples backwards to thin cover slips (24 x 50 mm2) using mounting medium (see Materials Table, point 4). Image using a fluorescent microscope with 5X and 20X magnification.
Representative Results
Figure 1 summarizes the procedure for tissue isolation, preparation and culture on MEA. We show the consecutive steps of tissue dissection to isolate the spiral ganglion (SG) from the sensory epithelium of the organ of Corti (OC) and stria vascularis (SV) and annexed spiral ligament (Figure 1 A–C). Spiral ganglion explants (3-4 in number) are cut with micro-scissors from the ganglion as schematically illustrated in Figure 1D and placed on MEA (Figure 1E), over the electrode occupied area (2.2 mm2). The OC is placed in proximity, outside of the electrode surface. The growth of the culture can be monitored over time (Figure 1G). A schematic diagram of the protocol is shown in Figure 1F. Electrophysiological activity can be detected after 6 days of culture, which increase with prolonged culture time. We recommend assessing 18 day cultures which produce significantly higher numbers of recording electrodes compared to earlier time points 5.
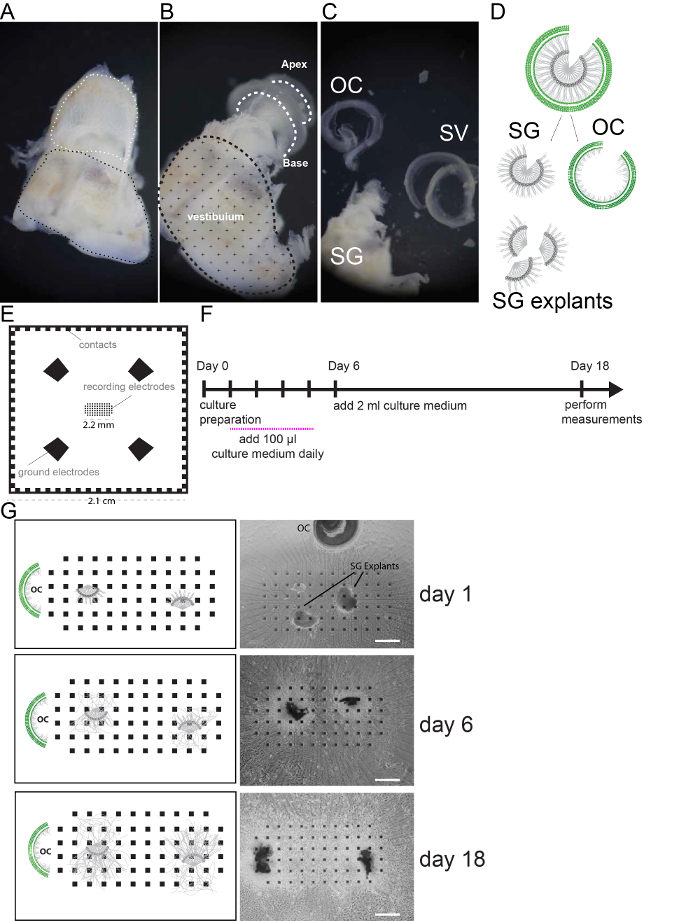
Figure 1. Cell culture preparation. (A) Freshly dissected mouse inner ear. White dashed line indicates the location of the cochlea, black dashed line indicates the vestibular region. (B) Mouse inner ear after removal of the cochlear bony wall. Cochlear turns are indicated by white dashed lines. (C) The Spiral ganglion (SG) and modiolus, the organ of Corti (OC) and the stria vascularis (SV) and spiral Ligament are shown after dissection. (D) Schematic of SG and OC dissection and SG explant preparation {figure adapted from 5 with approval from the publisher}. (E) Illustration of the multi electrode array used in this study. Recoding electrodes are organized in a rectangular grid in the center and occupy an area of 2.2 mm2. 4 Ground electrodes and side contacts are illustrated. (F) Schematic of the culture protocol. Recordings are performed at day 18. (G) Representative pictures (bright field images) and schemes of SG explants on MEA monitored in culture at day 1, 6 and 18. Scale bars = 400 µm. Please click here to view a larger version of this figure.
Spontaneous activity can be detected on MEA and shown as a raster plot where each line of the plot represents a detected spike. A representative example showing spontaneous activity detected from several electrodes (different colors in the graphs) is shown (Figure 2A, 2B, and 2C).
Additionally, MEA electrodes can be used to stimulate the culture. A representative example showing a biphasic pulse used for stimulation (Figure 2D), 5 responding electrodes (red traces) and non-responding electrodes (blue traces) is shown in Figure 2E. For these experiments one electrode was used for stimulation and all the other electrodes for recording. Single action potentials appeared 1 msec after the stimulation artifact (black arrow head). The MEA can be immunostained at the end of the procedure in order to assess the coverage of the neuronal processes over the electrode area. The electrode indicated in green in Figure 2F was used for stimulation, and the electrodes indicated in red were used to record responses (Figure 2E).
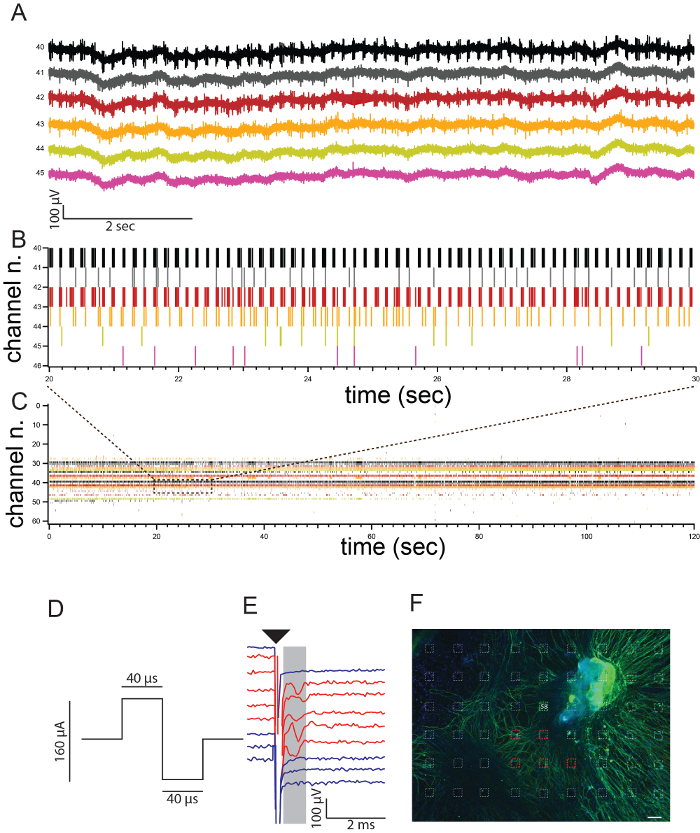
Figure 2. Data recordings on MEA. (A) Traces of original recordings of six out of 63 electrodes showing spontaneous activity. (B) Raster plot of the six electrodes of Figure 2A after spike detection. Each bar represents one action potential. (C) Raster plot including all electrodes (as in A and B) Activity is recorded from 63 electrodes (channels number 0-63) for 2 min. (D) A biphasic stimulus with total duration of 80 µsec and amplitude of 80 µA was used for stimulation of the culture from one electrode (E58 in Figure 2F). (E) Representative example of raw data traces obtained after stimulation from electrode 58 showing action potentials (red traces) or without responses (blue traces) after stimulation (black arrow-head). (F) Spiral ganglion culture on MEA immunostained for the neuronal marker TUJ (green) at the end of the experiment to visualize neuronal coverage of the electrode area {figure adapted from 5 with approval from the publisher}. Electrode 58 used for stimulation is indicated in green, responding electrodes are indicated in red. Scale bar = 50 µm. Please click here to view a larger version of this figure.
Finally, the MEA setup allows for studying possible electrode surface modification that may increase recording sensitivity. Two commercially available MEA electrodes were used in this study with two different platinum surfaces: Grey Platinum (Grey Pt), consisting of a 150 nm thick Pt layer (impedance: 400 KΩ/1 KHz) and Black Platinum, (Black, Pt), obtained by electrochemical deposition of Pt at the end of the micro-fabrication process (impedance: 20 KΩ/1 KHz). See Materials Table (point 6) for details.
Six independent MEA experiments were performed per electrode type. Black Pt MEA allows for detection of neuronal activity on a higher number of electrodes per MEA (Figure 3A) and reduction of the stimulus amplitude needed to elicit a response (Figure 3B). We analyzed for 30-35 independent electrode pairs, in 6 different experiments, the current threshold needed to achieve a response. Black Pt electrodes performed significantly better showing a threshold of 31.09 µA +/-2.4 compared to Grey Pt electrodes 47.57 µA +/- 1.97.
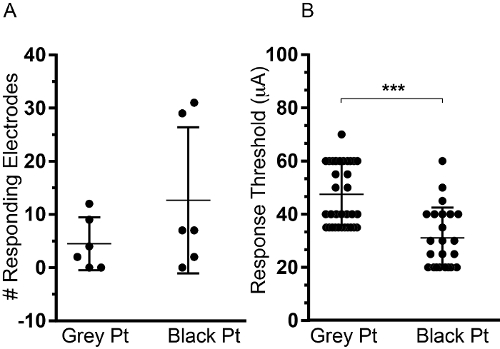
Figure 3. Comparison of Grey vs Black Pt electrodes. Two types of electrodes (Grey and Black Pt) were compared side by side using 12 independent MEA cultures, 6 on each MEA type. (A) The total number of responding electrodes per experiment is shown. (B) Amplitude threshold to elicit a response, measured using 30-35 independent electrode pairs in 6 independent MEA experiments is shown. Data are shown as Mean+/-SD. (Student t Test p <0.001) Please click here to view a larger version of this figure.
Discussion
The protocol described here shows how to culture SGN explants on MEA and assess SGN activity by extracellular non-invasive recordings. This platform and the protocol we have recently developed 5 allow for identification of novel stimulation protocols and electrode materials resulting in reduced energy requirements to activate SGN, with potential interest for further implementation of cochlear implants and other neuro-prosthetics. Several precautions in the presented procedure are fundamental for the accomplishment of accurate and reproducible experiments.
Careful dissection of the spiral ganglion and further handling of the primary tissue requires particular caution. These experiments have been performed using C57/Bl6 mice between 5 and 7 days old. Similar results were obtained using Wistar rats in the same age range, (data not shown). We believe this is the best age for fine dissection as the cochlear bone is still soft enough for easy removal by forceps, and the spiral ganglion and organ of Corti can be easily separated without rupturing dendrite processes or SGN somas. At earlier ages the tissue is too soft and the chances to rip the SGN somas together with the organ of Corti is higher, while at later stages, the hardening of the bony capsule of the cochlea increases the risk of damaging the tissue while dissecting. Proper isolation of the SG as well as careful cutting of the explants is critical. These should be in the range of 200-500 µm in size to maximize attachment to the MEA surface and to have sufficient number of SG somas in the explants, from which neurites will regrow. To increase neurotrophic support in the initial days of culture, fresh BDNF is added daily and the organ of Corti is placed in co-culture.
The speed of dissection is also important. All steps should be performed swiftly and using ice cold solutions in order to minimize tissue deterioration. The time between euthanasia and placing the explant on the MEA should be between 10-15 min and is crucial for successful cultures. Have all tools ready, to avoid delays in culture plating.
All steps, including fine dissection, maintenance of the culture and preparation of medium and equipment are performed under sterile conditions. When coating the MEA with the ECM solution it is important to thaw it on ice and to use ice-cold pipette tips and ice-cold medium as the ECM mix gels at higher temperatures. When placing the explants on MEAs, first add the medium to the electrode areas, subsequently place the explants. If the medium is added in a second step, explants tend to detach from the MEA due to shearing stress. Because small volumes of medium are applied to the culture in first 5 days, evaporation of the culture medium has to be minimized. Therefore it is highly recommended to create a humidified chamber using small petri dishes containing PBS in close proximity to the MEAs.
The experiments described here were performed using commercially available MEA electrodes with specific electrode dimensions, coating materials and intra electrode distance (as described in point 2, Note). The culture conditions have been optimized here in order to maximize neuronal coverage of the specific electrode grid design. It is possible that other configurations of electrode spacing, geometries and surfaces may require different coatings or cell densities. These steps may require initial troubleshooting in order to achieve high-density cultures.
Concerning the electrophysiological recordings, before starting the recordings the culture is transferred from culture medium at 37 °C to extracellular solution at RT. In order to avoid unstable recordings, wait for approximately 10 min to allow the culture to stabilize. When stimulating the culture, particular caution should be used in selecting the stimulus as large amplitudes (>3 V) and durations may cause damage to the culture. For a reference concerning stimulation pulse shapes and duration see reference 5.
For data acquisition and processing homemade hardware (recording chamber, connections to the amplifiers and amplifiers) were used and specific analysis programs were selected (see Material Table, point 7). However, other commercial MEA setups and other software packages are suited as well for these operations. We have previously assessed the responses of our cultures at 3 different time points and showed an increased activity with prolonged culture time 5. Here we suggest 18 days in vitro for recordings. MEA systems allowing for continuous recordings in sterile, humidified chambers, could facilitate the identification of the optimal time points for each culture type.
A major drawback of this bioassay is the high variation in the number of electrodes per culture that show responses to stimulation. This rate of responses to stimulation mainly depends on four factors: the density of the neurites in the culture, the contacts between the neurites and the electrodes, the diameter of the neurites or neurites bundles, and the impedance of the electrodes. Regarding the neurite density, only neurites that grow over at least two electrodes can be used for stimulation experiments. The contact between neurites and electrodes depends on the surrounding tissue that can on the one hand isolate the neurites from the electrode, and thus worsen the contact, or, on the other hand, isolate the neurites and the electrode from the surrounding bath and thus improve the contact. Tissue density, as well as neurite size, are defined by the explant used and the culture conditions. Dissociated neuronal cultures have also been successfully tested but neuronal density in these cultures is much lower, resulting in a reduced number of recording electrodes. Therefore the cell culture should be adapted to the specific scientific question to be addressed.
Finally, the impedance of the electrodes is mainly given by the size and the surface of the electrodes. Materials, creating a large surface such as black platinum, as shown in Figure 3, decreasing the impedance of the electrodes may also improve the coupling between electrodes and neurites 7-9.
Strategies to improve the success rate of stimulation therefore include the optimization of the culture conditions, the increase of the number of total electrodes or electrodes density and the modulation of the electrode surface 10.
Up to now, electrophysiological characterization of SG neurons had been performed using patch clamp techniques. This allows for intracellular recordings of action potentials and detailed analysis of intracellular ionic currents from single neurons. Here we present an in vitro bioassay that can be used to study activity profiles of spiral ganglion neurons by analyzing spontaneous activity or responses to extracellular stimulation of many neurons simultaneously. Additionally, the interaction between electrode and spiral ganglion neurons can be studied and optimized by application of modified or new materials. Finally, even if not shown here, this platform can be used in combination with an external electrode, mounted on a micromanipulator as recently shown by our group 5, in order to study the relationship between the distance of the stimulating electrode and culture activity. All these novel aspects allow for the mimicking of key features of cochlear implant electrodes may lead to the design of novel prosthetic devices.
Our model is a very useful in vitro tool to investigate strategies to improve the efficacy of stimulation of auditory neurons and further optimize CI technology. Once the technique is mastered, one could envision screening for modification of a number of variables: a) different neuronal populations, b) different electrode materials/size/impedances c) perform chronic experiments to test material toxicity or electrode-stimulation induced toxicity, which could shed light on safer and more effective stimulation protocols by in vivo electrode arrays.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Ruth Rubli at the Physiology Department of the University of Bern, Switzerland for valuable technical help with the experiments. This work was supported in part by the EU-Fp7-NMP programme (grant agreement no. 281056; project NANOCI – www.nanoci.org).
Materials
| culture medium | |||
| Neurobasal medium | Invitrogen | 21103-049 | 24 ml (for 25 ml) |
| HEPES | Invitrogen | 15630-080 | 250 μl (for 25 ml) |
| Glutamax | Invitrogen | 35050-061 | 250 μl (for 25 ml) |
| B27 | Invitrogen | 17504-044 | 500 μl (for 25 ml) |
| FBS | GIBCO | 10099-141 | 10% (for 25 ml) |
| BDNF | R&D Systems | 248-BD-025/CF | final 5 ng/ml (for 25 ml) |
| Name | Company | Catalog Number | Comments |
| extracellular solution (pH 7.4) | |||
| NaCl | 145mM | ||
| KCl | 4mM | ||
| MgCl2 | 1mM | ||
| CaCl2 | 2mM | ||
| HEPES | 5mM | ||
| Na-pyruvate | 2mM | ||
| Glucose | 5mM | ||
| Name | Company | Catalog Number | Comments |
| blocking solution | |||
| PBS | Invitrogen | 10010023 | |
| BSA | Sigma | A4503-50G | 2% |
| Triton X-100 | Sigma | X100 | 0.01% |
| Name | Company | Catalog Number | Comments |
| Immunostaining solutions | |||
| TuJ | R&D Systems | MAB1195 | dil 1:200 |
| DAPI | Sigma | D9542 | |
| Paraformaldehyde | Sigma | 158127 | 4% |
| Fluoreshild | Sigma | F6057 | |
| Name | Company | Catalog Number | Comments |
| plastic/tools | |||
| petri dish 35 mm | Huberlab | 7.627 102 | |
| petri dish 94 mm | Huberlab | 7.633 180 | |
| Dumont #5 tweezer | WPI | 14098 | |
| Dumont #55 tweezer | WPI | 14099 | |
| Name | Company | Catalog Number | Comments |
| Materials | |||
| Enzymatic solution: Terg-a-Zyme | Sigma | Z273287-11KG | |
| Extracellular Matrix (ECM) mix: Matrigel TM | Corning | 356230 | |
| MEA electrodes | Qwane Biosciences | (Lausanne, Switzerland) | |
| Name | Company | Catalog Number | Comments |
| Software | |||
| Labview | National Instruments | Switzerland | |
| IgorPro | WaveMetrics | Lake Oswega, USA |
References
- O’Donoghue, G. Cochlear implants–science, serendipity, and success. N Engl J Med. 369 (13), 1190-1193 (2013).
- Shepherd, R. K., Hatsushika, S., Clark, G. M. Electrical stimulation of the auditory nerve: the effect of electrode position on neural excitation. Hear Res. 66 (1), 108-120 (1993).
- Tykocinski, M., et al. Comparison of electrode position in the human cochlea using various perimodiolar electrode arrays. Am J Otol. 21 (2), 205-211 (2000).
- Wilson, B. S., Dorman, M. F. Cochlear implants: current designs and future possibilities. J Rehabil Res Dev. 45 (5), 695-730 (2008).
- Hahnewald, S., et al. Response profiles of murine spiral ganglion neurons on multi-electrode arrays. J. Neural Eng. 13, (2015).
- Tscherter, A., Heuschkel, M. O., Renaud, P., Streit, J. Spatiotemporal characterization of rhythmic activity in rat spinal cord slice cultures. Eur J Neurosci. 14 (2), 179-190 (2001).
- Heim, M., Yvert, B., Kuhn, A. Nanostructuration strategies to enhance microelectrode array (MEA) performance for neuronal recording and stimulation. J Physiol Paris. 106 (3-4), 137-145 (2012).
- Kim, R., Nam, Y. Novel platinum black electroplating technique improving mechanical stability. Conf Proc IEEE Eng Med Biol Soc. , 184-187 (2013).
- Cellot, G., et al. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat Nanotechnol. 4 (2), 126-133 (2009).
- Spira, M. E., Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nat Nano. 8 (2), 83-94 (2013).

