淀粉颗粒大小分布的分析和规格
Summary
此处介绍了淀粉颗粒大小分布的可重复和统计有效确定过程,以及使用双参数乘法指定已确定的颗粒日志正常大小分布。它适用于植物和食品科学研究的克级淀粉样品的所有颗粒大小分析。
Abstract
来自所有植物来源的淀粉由颗粒组成,其大小和形状具有不同的发生频率,即显示大小和形状分布。 使用几种类型的颗粒大小技术确定的淀粉颗粒大小数据通常是有问题的,因为一些不可逾越的系统错误导致的可重复性差或缺乏统计意义,包括对颗粒形状的敏感性和颗粒样本大小的限制。我们概述了使用电传感区技术可重复和统计有效确定淀粉颗粒大小分布的程序,以及使用采用的两参数乘法来指定已确定的颗粒日志正常大小分布的程序,提高了准确性和可比性。它适用于克级淀粉样品的所有颗粒大小分析,因此,可以促进淀粉颗粒大小如何由淀粉生物合成装置和机制成型的研究:以及它们如何影响淀粉用于食品和工业用途的特性和功能。通过使用概述的程序对甜品淀粉样品的颗粒大小分布进行复制分析,可以呈现具有代表性的结果。我们进一步讨论了该过程的几个关键技术方面,特别是颗粒日志正常大小分布的倍增规格,以及克服颗粒聚合频繁孔径堵塞的一些技术手段。
Introduction
淀粉颗粒是植物光合作用和储存组织中的两种主要储备同质聚合物、线性或稀疏分支淀粉样蛋白和高度分支的淀粉样蛋白,与一些小成分(包括脂质和蛋白质)有序地包装在一起的物理结构。来自各种植物物种的淀粉颗粒呈现出许多三维(3D)形状(参考1、2),包括球体、椭圆体、多壳体、血小板、立方体、立方体和不规则的管状物。即使是来自同一植物物种的同一组织或不同组织的组织,也可能有一组不同发生频率的形状。换句话说,来自植物物种的淀粉颗粒可能有一个特征的统计形状分布,而不是一个特定的形状。非均匀和非球形颗粒形状使得难以正确测量和定义淀粉颗粒大小。此外,来自植物物种同一组织的淀粉颗粒具有不同比例的大小,即表现出特征大小分布。这种大小分布使淀粉颗粒大小的分析和描述进一步复杂化。
淀粉颗粒尺寸已使用几类颗粒大小技术进行分析(在参考3中回顾),包括显微镜、沉积/星体场流分馏(Sd/StFFF)、激光衍射和电传感区(ESZ)。然而,这些技术并不同样适合在颗粒形状和大小分布存在的情况下确定淀粉颗粒大小。显微镜,包括光,共生和扫描电子显微镜,是优秀的形态学研究4,5,6,7,结构8,9和发展10,11淀粉颗粒,但很难定义其大小分布由于一些固有的缺陷。光学显微镜数据(IAOM)的微小颗粒图像或软件辅助图像分析的直接测量,这些数据已用于确定来自几个物种的淀粉颗粒大小, 包括玉米12,小麦13,14,土豆15和大麦16,只能测量1D(通常最大长度)或2D(表面积)大小非常有限的数量(数万至几千)淀粉颗粒图像。考虑到淀粉单位重量的巨大颗粒数(假设所有 10 μm 球体的密度为1.5 g/cm3),固有的受技术约束的小颗粒采样尺寸在统计学上很少具有代表性,因此可能导致结果的可重复性差。Sd/StFFF技术可能具有高速和分辨率,淀粉颗粒17的窄尺寸部分,但很少使用,可能是因为它的准确性可能受到损害,不同的形状和淀粉颗粒密度的严重影响。激光衍射技术是应用最广泛的技术,已应用于淀粉颗粒大小分析的所有主要作物品种3,14,16。虽然该技术有许多优点,但它实际上并不适合在颗粒形状分布的情况下确定淀粉颗粒大小。大多数并发激光衍射仪器依靠米光散射理论18来制造均匀的球形粒子,而修改后的Mie理论18依赖于其他一些均匀形状的粒子。因此,该技术本身对颗粒形状非常敏感,甚至不完全适合某些均匀性19的形状,更不用说淀粉颗粒具有一组不同比例的形状了。ESZ 技术测量电场扰动与通过孔径的粒子体积成正比。它以高分辨率提供颗粒体积大小、数量和体积分布信息等。由于ESZ技术独立于粒子的任何光学特性,包括颜色、形状、成分或折射指数,结果非常可重复,因此特别适合确定具有一组形状的淀粉颗粒的大小分布。
淀粉颗粒大小也通过使用许多参数来定义。它们通常用平均直径简单描述,在某些情况下,平均直径是2D图像12、20或等值球直径3平均最大长度的算术手段。在其他情况下,颗粒大小分布使用大小范围21,22,分布平均体积或平均直径(球面等价物,按数字、体积或表面积加权)来指定,假设正常分布 14、23、24、25、26。这些描述的淀粉颗粒大小从各种分析是一个完全不同的性质,并没有严格的可比性。如果直接比较来自不同物种的淀粉颗粒的这些”大小”,甚至同一物种的相同组织,可能会非常具有误导性。此外,在大多数研究中,测量分布宽度(即大小差)的标准偏差σ(或图形标准偏差σg)的公差(或形状)参数被忽略。
为了解决淀粉颗粒大小分析面临的上述关键问题,我们概述了使用 ESZ 技术对淀粉样品颗粒大小分布进行可重复且统计上有效的确定的程序,并使用采用的两参数倍增形式27正确指定已确定的颗粒日体大小分布,提高了准确性和可比性。为了验证和演示,我们使用该程序对甜品淀粉样品进行了复制颗粒大小分析,并使用其图形几何手段 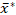 和乘法标准偏差s* 以
和乘法标准偏差s* 以 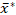 x/(乘以除法) s* 形式指定日志异常差量百分比体积等价球直径分布。
x/(乘以除法) s* 形式指定日志异常差量百分比体积等价球直径分布。
Protocol
Representative Results
Discussion
概述的程序解决了淀粉颗粒大小分析的几种现有方法中的一些关键问题, 包括 3D 颗粒的不当 1D 或 2D 尺寸、由于颗粒形状不均匀而导致尺寸测量失真、由于颗粒样本大小有限而重复性差和统计有效性可疑、颗粒大小不准确或不当(特别是使用平均尺寸)的颗粒大小存在颗粒形状和非正常大小分布。它使用 ESZ 技术测量淀粉颗粒的 3D 大小(体积),对颗粒形状无响应。从具有非常大颗粒样本大小(…
Disclosures
The authors have nothing to disclose.
Acknowledgements
这项工作得到了合作农业研究中心和农业与人文科学学院综合粮食安全研究中心、草原景观A&M大学的部分支持。我们感谢华天的技术支持。
Materials
| Analytical beaker | Beckman Coulter Life Sciences | A35595 | Smart-Technology (ST) beaker |
| Aperture tube, 100 µm | Beckman Coulter Life Sciences | A36394 | For the MS4E, , 1000 µm |
| Disposable transfer pipettor, | Fisher Scientific (Fishersci.com) | 13-711-9AM | Other disposable transfer pipettors with similar orifice can also be used. |
| Fisherbrand Conical Polypropylene Centrifuge Tubes, 50 ml | Fisher Scientific (Fishersci.com) | 05-539-13 | Any other similar types of tubes can be used. |
| Glass beakers, 150 to 250 ml | Fisher Scientific (Fishersci.com) | 02-540K | These beakers are used to contain methanol for washing the aperture tube and stirer between runs. |
| LiCl | Fisher Chemical | L121-100 | |
| Methanol | Fisher Chemical | A412-500 | Buy in bulk as the analysis uses a large quantity of methanol. |
| Mettler Toledo ML-T Precision Balances | Mettler Toledo | 30243412 | Any other precision balance with a readablity 0.01 g to 1 mg will work. |
| Multisizer 4e Coulter Counter | Beckman Coulter Life Sciences | B23005 | The old model, Multisizer 3 can also be used with slight adjustment of parameters. The 4e model comes with a 100 μm aperture tube. Other aperture tubes of different diameter can be purchased separately from the company. |
| Ultrasonic processor UP50H | Hielscher Ultrasound Technology | UP50H | Other laborator sonicator having a low-power (<50 Watt) output can be also used. Both MS1 and MS2 sonotrodes for the particular sonicator can be used to disperse starch granules in 5 ml methanol. Always use the lowest setting first, 20% amplitude and 0.1 or 0.2 cycle, and raise the setting if aggregates persist in suspension. |
References
- Shannon, J. C., Garwood, D. L., Boyer, C. D., BeMiller, J., Whistler, R. . Starch:Chemistry and Technology Food Science and Technology. , 23-82 (2009).
- Singh, N., Singh, J., Kaur, L., Singh Sodhi, N., Singh Gill, B. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chemistry. 81 (2), 219-231 (2003).
- Lindeboom, N., Chang, P. R., Tyler, R. T. Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: a review. Starch – Stärke. 56 (34), 89-99 (2004).
- Baldwin, P. M., Davies, M. C., Melia, C. D. Starch granule surface imaging using low-voltage scanning electron microscopy and atomic force microscopy. International Journal of Biological Macromolecules. 21 (1-2), 103-107 (1997).
- Jane, J. L., Kasemsuwan, T., Leas, S., Zobel, H., Robyt, J. F. Anthology of starch granule morphology by scanning electron microscopy. Starch-Stärke. 46 (4), 121-129 (1994).
- Matsushima, R., Nakamura, Y. . Starch: Metabolism and Structure. , 425-441 (2015).
- Wang, S. -. q., Wanf, L. -. l., Fan, W. -. h., Cao, H., Cao, B. -. s. Morphological analysis of common edible starch granules by scanning electron microscopy. Food Science. 32 (15), 74-79 (2011).
- Baldwin, P. M., Adler, J., Davies, M. C., Melia, C. D. Holes in starch granules: confocal, SEM and light microscopy studies of starch granule structure. Starch-Stärke. 46 (9), 341-346 (1994).
- Chakraborty, I., Pallen, S., Shetty, Y., Roy, N., Mazumder, N. Advanced microscopy techniques for revealing molecular structure of starch granules. Biophysical Reviews. 12 (1), 105-122 (2020).
- Bechtel, D. B., Wilson, J. D. Amyloplast formation and starch granule development in hard red winter wheat. Cereal Chemistry. 80 (2), 175-183 (2003).
- Evers, A. Scanning electron microscopy of wheat starch. III. Granule development in the endosperm. Starch-Stärke. 23 (5), 157-162 (1971).
- Wang, Y. J., White, P., Pollak, L., Jane, J. L. Characterization of starch structures of 17 maize endosperm mutant genotypes with Oh43 inbred line background. Cereal Chemistry. 70, 171-179 (1993).
- Peng, M., Gao, M., Abdel-Aal, E. S. M., Hucl, P., Chibbar, R. N. Separation and characterization of A-and B-type starch granules in wheat endosperm. Cereal Chemistry. 76, 375-379 (1999).
- Wilson, J. D., Bechtel, D. B., Todd, T. C., Seib, P. A. Measurement of wheat starch granule size distribution using image analysis and laser diffraction technology. Cereal Chemistry. 83 (3), 259-268 (2006).
- Liu, Q., Weber, E., Currie, V., Yada, R. Physicochemical properties of starches during potato growth. Carbohydrate Polymers. 51 (2), 213-221 (2003).
- Chmelik, J., et al. Comparison of size characterization of barley starch granules determined by electron and optical microscopy, low angle laser light scattering and gravitational field-flow fractionation. Journal of the Institute of Brewing. 107 (1), 11-17 (2001).
- Moon, M. H., Giddings, J. C. Rapid separation and measurement of particle size distribution of starch granules by sedimentation/steric field-flow fractionation. Journal of Food Science. 58 (5), 1166-1171 (1993).
- Wriedt, T., Wolfram, H., Wriedt, T. . The Mie Theory: Basics and Applications. , 53-71 (2012).
- Schuerman, D. W., Wang, R. T., Gustafson, B. &. #. 1. 9. 7. ;. S., Schaefer, R. W. Systematic studies of light scattering. 1: Particle shape. Applied Optics. 20 (23), 4039-4050 (1981).
- Goering, K. J., Fritts, D. H., Eslick, R. F. A study of starch granule size and distribution in 29 barley varieties. Starch-Stärke. 25 (9), 297-302 (1973).
- Chen, Z., Schols, H. A., Voragen, A. G. J. Starch granule size strongly determines starch noodle processing and noodle quality. Journal of Food Sciences. 68 (5), 1584-1589 (2003).
- Dai, Z. M. Starch granule size distribution in grains at different positions on the spike of wheat (Triticum aestivum L.). Starch-Starke. 61 (10), 582-589 (2009).
- Edwards, M. A., Osborne, B. G., Henry, R. J. Effect of endosperm starch granule size distribution on milling yield in hard wheat. Journal of Cereal Science. 48 (1), 180-192 (2008).
- Karlsson, R., Olered, R., Eliasson, A. C. Changes in starch granule size distribution and starch gelatinization properties during development and maturation of wheat, barley and rye. Starch – Starke. 35 (10), 335-340 (1983).
- Li, W. -. Y., et al. Comparison of starch granule size distribution between hard and soft wheat cultivars in Eastern China. Agricultural Sciences China. 7 (8), 907-914 (2008).
- Park, S. H., Wilson, J. D., Seabourn, B. W. Starch granule size distribution of hard red winter and hard red spring wheat: Its effects on mixing and breadmaking quality. Journal of Cereal Science. 49 (1), 98-105 (2009).
- Limpert, E., Stahel, W. A., Abbt, M. Log-normal distributions across the sciences: keys and clues. Bioscience. 51 (5), 341-352 (2001).
- Gao, M., et al. Self-preserving lognormal volume-size distributions of starch granules in developing sweetpotatoes and modulation of their scale parameters by a starch synthase II (SSII). Acta Physiologiae Plantarum. 38 (11), 259 (2016).
- Wattebled, F., et al. STA11, a Chlamydomonas reinhardtii locus required for normal starch granule biogenesis, encodes disproportionating enzyme. Further evidence for a function of alpha-1,4 glucanotransferases during starch granule biosynthesis in green algae. Plant Physiology. 132 (1), 137-145 (2003).
- Ji, Y., Seetharaman, K., White, P. J. Optimizing a Small-Scale Corn-Starch Extraction Method for Use in the Laboratory. Cereal Chemistry. 81 (1), 55-58 (2004).
- Halloy, S., Whigham, P. The lognormal as universal descriptor of unconstrained complex systems: a unifying theory for complexity. Proceedings of the 7th Asia-Pacific Complex Systems Conference. , 309-320 (2004).
- Furusawa, C., Suzuki, T., Kashiwagi, A., Yomo, T., Kaneko, K. Ubiquity of log-normal distributions in intra-cellular reaction dynamics. Biophysics (Nagoya-shi). 1, 25-31 (2005).

