Evaluation of Capnography Sampling Line Compatibility and Accuracy when Used with a Portable Capnography Monitor
Summary
The goal of this study was to evaluate the accuracy of capnography sampling lines used in conjunction with a portable bedside capnography monitor. Sampling lines from 7 manufacturers were evaluated for tensile strength, rise time, and ETCO2 accuracy as a function of respiratory rate or supplemental oxygen flow rate.
Abstract
Capnography is commonly used to monitor patient’s ventilatory status. While sidestream capnography has been shown to provide a reliable assessment of end-tidal CO2 (ETCO2), its accuracy is commonly validated using commercial kits composed of a capnography monitor and its matching disposable nasal cannula sampling lines. The purpose of this study was to assess the compatibility and accuracy of cross-paired capnography sampling lines with a single portable bedside capnography monitor. A series of 4 bench tests were performed to evaluate the tensile strength, rise time, ETCO2 accuracy as a function of respiratory rate, and ETCO2 accuracy in the presence of supplemental O2. Each bench test was performed using specialized, validated equipment to allow for a full evaluation of sampling line performance. The 4 bench tests successfully differentiated between sampling lines from different commercial sources and suggested that due to increased rise time and decreased ETCO2 accuracy, not all nasal cannula sampling lines provide reliable clinical data when cross-paired with a commercial capnography monitor. Care should be taken to ensure that any cross-pairing of capnography monitors and disposable sampling lines is fully validated for use across respiratory rates and supplemental O2 flow rates commonly encountered in clinical settings.
Introduction
Capnography is a commonly used technology designed to assess the integrity of a patient’s ventilatory status by measuring the patient’s end-tidal CO2 (ETCO2) and respiratory rate1. When used in combination with pulse oximetry, a more comprehensive assessment of respiratory function can be achieved2,3. Capnography is frequently used in the post-anesthesia care unit, in intubated or deeply sedated patients4, in the intensive care unit (ICU), and in the emergency department5. In fact, the American Society of Anesthesiologists (ASA)6,7 recommends continuous capnography during all general anesthesia procedures8 and during moderate and deep sedation, which included an estimated 106 million procedures in the United States from January 2010-December 20149,10.
Inherent in the use of capnography is reliance on a device that provides the clinician with an accurate assessment of a patient’s ventilatory status. Capnography monitoring can be either sidestream, in which exhaled breath is diverted to a monitor by a nasal cannula and tubing, or mainstream, in which exhaled breath is measured at the source without diverting the sample11. Mainstream capnography is most often used in intubated patients, whereas sidestream capnography is used for both intubated and non-intubated patients12. One important component of sidestream capnography is the sampling line, which delivers CO2 from a patient’s exhaled breath to the detector, where breath analysis occurs1,13. Commercial sampling line designs vary significantly, with differences in sampling line connection points, nasal cannula shapes, and tubing volumes, all of which can affect sampling line performance13,14. For example, nasal cannula sampling lines can have up to 10 connections between the nasal cannula, humidifier, ETCO2 sampling line, and O2 delivery tubes (Figure 1). Each of these connections represents a potential weak point in the monitoring system.
The performance of nasal cannula sampling lines can be evaluated by a variety of tests such as the overall weak point and rise time. In addition, they can be tested to determine the impact of respiratory rate and the delivery of supplemental oxygen on ETCO2 readings. Although previous studies have reported ETCO2 accuracy on a limited number of sampling lines15,16,17,18,19,20,21,22,23, there are no known studies that have evaluated nasal cannula capnography sampling line performance using a combination of tests, such as identification of the overall weak point, measurement of rise time, and determination of ETCO2 accuracy.
The overall weak point of a sampling line can be measured using a tensile strength test, in which each connection point is tested for how much force is exerted on the connection before it reaches a breaking point. The tensile strength test can identify the weakest connection point for a medical device, allowing direct comparisons between unique device designs. This style of strength test is often performed on medical devices, ranging from pacing leads to catheters24,25. Since capnography sampling lines have a large number of tubing connection points, the weakest connection point can differ depending on the device design. The tensile strength of connection points is particularly important in mobile environments such as ambulances, where sampling lines can be pulled apart unintentionally due to space constraints. Capnography sampling lines can also become unintentionally disconnected in hospital rooms, where multiple monitoring systems are often simultaneously connected to a patient, and the equipment lines can become tangled and pulled on by either a mobile patient or a healthcare provider. In both scenarios, the tension applied to the sampling line can result in a loss of capnography data and in some instances, interruption of supplemental O2 delivery.
Another critical element of sidestream capnography monitoring affected by sampling line design is rise time, defined as the time required for a measured CO2 value to increase from 10% to 90% of the final value14. The rise time is a direct indicator of the system resolution, defining how well individual breaths are separated from one another during sampling (Figure 2A). In practice, a shorter rise time is preferable to a long rise time. This is due to the potential mixing of multiple breath samples in capnography systems with long rise times, resulting in inaccurate ETCO2 measurements14. Importantly, rise time is affected by both breath flow and sampling line design, due to the friction of air moving along the tubing, the presence of filters, and the volume of dead space within the sampling line. Sampling lines with more dead space have reduced breath sample resolution, resulting in mixed breath ETCO2 waveforms, and as a result, inaccurate ETCO2 readings13,14. These poorly differentiated breath samples occur most often in patients with a rapid respiratory rate, including infants and children14,15,16.
ETCO2 measurements can also be impacted by respiratory rate and the delivery of supplemental oxygen15,26,27,28. Although changes in minute ventilation and presence of respiratory depression can be easily detected with a capnograph27,28, there is scarce data on specific performance of nasal cannula capnography sampling lines at different respiratory rates. A recent study found that during steady breathing, respiratory rate measured by a respiratory volume monitor and capnograph were strongly correlated (R = 0.98 ± 0.02) and consistent for all breathing rates, including normal, slow, and fast breathing rates28. Regarding use of supplemental oxygen, a separate study compared ETCO2 readings in healthy volunteers in the presence of pulsed or continuous oxygen flow, using between 2 and 10 L/min oxygen17. While the pulsed oxygen flow had a limited impact on measured ETCO2 (median 39.2 mmHg), continuous oxygen flow, which is standard in clinical settings, resulted in a wide range of ETCO2 measurements (median 31.45 mmHg, range 5.4 to 44.7 mmHg) that were clinically different from ETCO2 readings in the absence of supplemental oxygen17. In addition, differences in ETCO2 measurements in the presence of supplemental oxygen flow have been compared across nasal cannula designs15,18. In contrast to nasal cannulas with oral scoops, one study found that some cannulas failed to deliver exhaled CO2 to the capnometer in the presence of 10 L/min O218. Another study reported that while ETCO2 readings with supplemental oxygen during simulated normal ventilation were normal, ETCO2 readings were reduced in the presence of supplemental oxygen during simulated hypoventilation and hyperventilation15. This is consistent with evidence that ETCO2 accuracy is more difficult to achieve when the flow rate of CO2 in exhaled breath is similar to the flow rate of supplemental oxygen, due to dilution of the exhaled CO2 (Figure 2B)20.
The accuracy of ETCO2 readings has been evaluated in multiple independent studies, all of which concluded that capnography offered a reliable measure of ventilation status16,18,19,20,21,22. However, few studies have compared the accuracy of different sidestream capnography systems, and although capnography sampling lines are used with a variety of commercial capnography monitors, the accuracy of these cross-paired devices is not well-described23. Thus, determining whether alternative commercial sampling lines are compatible with capnography monitors and provide accurate data is important for healthcare providers who use this equipment to monitor patient ventilation.
The purpose of this study was to determine the compatibility and accuracy of commercially available sidestream capnography sampling lines used in conjunction with a portable capnography monitor. A series of four bench tests were performed using specially designed, validated systems to compare the performance of a series of capnography sampling lines with a single respiratory monitor. The four major outcomes of the study included (1) tensile strength and identification of the weak connection point for each capnography sampling line; (2) rise time; (3) ETCO2 accuracy as a function of respiratory rate; and (4) ETCO2 accuracy in the presence of supplemental oxygen.
Protocol
The capnography sampling lines used in these bench tests included 16 adult, pediatric, and neonatal capnography sampling lines from 7 commercial sources. Among the 16 sampling lines included in the bench tests, 5 sampling lines were from the same manufacturer as the capnography monitor utilized for the bench tests (‘matched’), and 11 sampling lines were from alternate manufacturers (‘cross-paired’) (Table of Materials). All of the nasal cannula sampling lines share a similar design, with up to 10 connection points between the cannula, humidifier, O2 connector, CO2 connector, 4-way, O2 tube, and CO2 tube (Figure 1).
1. Measure sampling line tensile strength
- Calibrate the tensile testing jig.
- In the tensile testing jig software, set the load cell selection to 100.00 kg and the load parameter to 10.00 kg.
- Attach sampling line components (example: O2 connector with O2 tube) to the calibrated tensile testing jig.
- Starting with a mass of 0 kg, initiate tension on the sampling line component and observe whether the sampling line connection remains intact.
- If the sampling line connection remains intact, automatically increase the mass in a continuous manner, and observe when the subparts break or disconnect.
NOTE: The resolution of the jig is limited to 10 g increments. - Record the maximum tension (kg) exerted before the sampling line break occurred.
- Repeat the tensile strength test for all 10 potential sampling line subparts: O2 connector with O2 tubing; O2 tubing with 4-way; 4-way with O2 tubing; O2 tubing with cannula; cannula with CO2 tubing; CO2 tubing with 4-way; 4-way with CO2 tubing; CO2 tubing with CO2 connector; humidifier with tubing; tubing with cannula.
- Repeat the tensile strength test on 16 sampling lines from 7 commercial sources.
2. Measure rise time and sampling line accuracy
- Calibrate the rise time measurement device.
- Cut standard 0.95 mm internal diameter CO2 PVC tube into ten 15 cm pieces.
- Operate the jig using the following steps:
- Turn on the air compressor, jig controller, and power supply.
- Open the CO2 gas flow.
- Attach the sampling channel directly to the measurement chamber without the sample.
- Calibrate the air and CO2 flow to 10 L/min and the gas sampling rate to 50 mL/min using a mass flow meter and a dedicated restrictor.
NOTE: The maximum sampling rate of the capnography monitor is 50 mL/min. - Open the jig software and define the test parameters as follows: Air:CO2 ratio 1:1; Air time = 3 seconds, CO2 time = 3 seconds, 10 cycles, rise time measurement length: none.
- Open the CO2 valve.
- Select the Finish Calibration button on the Measurement tab and make sure it turns green.
- Select the Measure button and wait for the gas flow cycles to end.
- Close the CO2 valve.
- Record the background rise time and ensure the result is less than 60 ms. If it is larger, clean the optical chamber with air flow and re-connect the y-piece/airway adapter properly.
- Take 10 measurements and calculate the average rise time value.
- Compare the rise time value to the margins and confirm it is inside the specification limits, pre-defined as rise time background < 60 ms and rise time of a control sample, a 15 cm PVC tube, 0.95 mm internal diameter, equal to 39 ± 5 ms.
- Compare the delivery time to the margins and confirm it is inside the specification limits, predefined as background delivery time <100 ms and delivery time of a control sample, a 15 cm PVC tube, 0.95 mm internal diameter, equal to 152 ± 5 ms.
- Open a new commercial sampling line.
- Connect the sampling line to the rise time measurement device.
- Click on the Start button in the rise time measurement device software and wait for the device to measure the rise time.
NOTE: The device repeats the measurement 10 times and automatically averages the repeats to report the rise time mean and standard deviation.- Copy the rise time result to the report.
- Disconnect the sampling line from the rise time measurement device.
- Calculate maximum respiratory rate for inhalation:exhalation time ratios of 1:1 and 1:2, in breaths per minute (BPM).
- Calculate the maximum respiratory rate using the measured rise time for the sampling line and a 1:1 breath ratio, using the following equation:
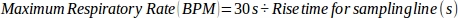
where 30 s represents the cumulative time used to exhale during 1 min (1:1 inhalation:exhalation time).
NOTE: For a 1:1 breath ratio, the maximum respiratory rate represents the fastest allowed respiratory rate without impacting ETCO2 accuracy when the time required for inhalation and exhalation is the same. - Calculate the maximum respiratory rate using the measured rise time for the sampling line and a 1:2 breath ratio, using the following equation:
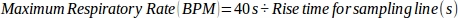
where 40 s represents the cumulative time used to exhale during 1 min (1:2 inhalation:exhalation time).
NOTE: For a 1:2 breath ratio, the maximum respiratory rate represents the fastest allowed respiratory rate without impacting ETCO2 accuracy when the time used to exhale is twice as long as the time used to inhale.
- Calculate the maximum respiratory rate using the measured rise time for the sampling line and a 1:1 breath ratio, using the following equation:
- Calculate exhalation time for inhalation:exhalation time ratios of 1:1 and 1:2.
- For a 1:1 breath ratio, use the following equation:
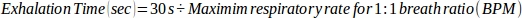
where 30 s represents the cumulative time used to exhale during 1 min (1:1 inhalation:exhalation time). - For a 1:2 breath ratio, use the following equation:
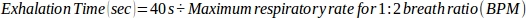
where 40 s represents the cumulative time used to exhale during 1 min (1:2 inhalation:exhalation time).
- For a 1:1 breath ratio, use the following equation:
- Determine the accuracy of each sampling line at 150 BPM for 1:1 and 1:2 breath ratios by evaluating the maximum respiratory rate.
NOTE: If the maximum respiratory rate is ≥150 BPM, then the sampling line is considered accurate for the breath ratio, but if the maximum respiratory rate is <150 BPM, then the sampling line is not considered accurate at 150 BPM. - Repeat steps 2.2-2.8 for all 16 sampling lines tested.
- Perform statistical analysis using statistical software.
- Compare mean and standard deviation using Student’s t-test, with a two-sided significance level of 0.05, for all capnography monitor matched sampling lines vs. all capnography monitor cross-paired sampling lines.
- Repeat statistical analysis to compare all capnography monitor matched pediatric sampling lines to all capnography monitor cross-paired pediatric sampling lines.
- Repeat statistical analysis to compare all capnography monitor matched adult sampling lines to all capnography monitor cross-paired adult sampling lines.
3. Measure ETCO2 accuracy as a function of respiratory rate
- Prepare the manikin by placing in a supine position and connect the sampling line to the manikin per manufacturer instructions.
- Attach the sampling line to the capnography monitor and change the capnography monitor setting to accept sampling lines from all manufacturers by selecting Settings and Cancel Gold Ring Identification.
- Prepare and calibrate the breath simulator jig, to control the simulated respiratory rate.
NOTE: The breath simulator jig is composed of a 2-way electrical operating valve, allowing for precise control of the flow of CO2 and N2 to the manikin, to simulate human breathing.- Use a flow meter to measure the gas flow and calibrate it to 10 L/min.
- Open the breath simulator jig software and set the duty cycle to 50%.
- Test for leaks in the system using a leak testing jig.
- Connect the sampling line to the CO2 port on the leak testing jig.
- Create a kink in the sampling line to prevent CO2 from exiting the end of the sampling line.
- Using a flow rate of 50 mL/min CO2, allow the pressure in the sampling line to increase to 300 mmHg, and then stop adding CO2.
- Observe if the pressure in the sampling line remains the same or decreases. If the pressure decreases, this confirms a leak in the system, and a new sampling line should be applied in Step 4.2.
- Connect the breath simulator jig to the manikin.
- Increase the 5% CO2 flow rate to 10 L/min and the N2 flow rate to 10 L/min using the breath simulator jig. Keep flow rates constant throughout the test.
- Wait 30 seconds to allow a steady capnography waveform to be established, then record the ETCO2 value (mmHg).
- Measure a total of 10 ETCO2 values over 180 seconds.
- Change the respiration rate using the breath simulator jig, allow the capnography waveform to normalize for 30 seconds, and record 10 ETCO2 readings over 180 seconds.
- Repeat readings for each respiratory rate examined: 10, 20, 40, 60, 80, 100, 120, and 150 BPM.
- Determine the average and standard deviation of the 10 measured readings at each respiratory rate.
- Repeat steps 4.1-4.8 for all 16 sampling lines tested.
- Perform statistical analysis using Bland-Altman graphical plots to evaluate sampling line bias.
4. Measure ETCO2 accuracy in the presence of supplemental O2
- Prepare the manikin and breath simulator jig as described in Steps 4.1-4.3. Set the breath simulator jig to 10 BPM.
- Connect the O2 line to 100% O2.
- Increase the CO2 flow rate to 6 L/min and the O2 flow rate to 0 L/min, to use as a reference measurement.
- To allow the capnography waveform to stabilize, wait 30 seconds before recording the ETCO2 value.
- Read the ETCO2 value 10 times over 180 seconds.
- Change the flow rate of the CO2 and O2, allow the capnography waveform to normalize for 30 seconds, and repeat the 10 ETCO2 measurements over 180 seconds. To capture common clinical scenarios, use the following combinations of CO2 and O2 flow rates:
- Use a combination of 2 L/min CO2 and 2 L/min O2.
- Use a combination of 4 L/min CO2 and 2 L/min O2.
- Use a combination of 4 L/min CO2 with 4 L/min O2.
- Use a combination of 6 L/min CO2 with 4 L/min O2.
- Use a combination of 6 L/min CO2 with 6 L/min O2.
- Use a combination of 8 L/min CO2 with 6 L/min O2.
- Repeat the test as described in 5.1-5.6 for each sampling line.
- Perform statistical analysis using Bland-Altman graphical plots to evaluate sampling line bias.
Representative Results
Tensile strength
Sixteen capnography sampling lines from 7 manufacturers were tested to determine the tensile strength of each major sampling line joint (Figure 1, Table of Materials). Due to differences in sampling line design, not all joints exist in all sampling lines. The capnography monitor matched sampling lines 8, 9, 14, 15, and 16 had minimum overall tensile strengths between 3.55 kg and 5.94 kg. Most cross-paired sampling lines exhibited similar overall tensile strengths (Table 1). Sampling line 6 had the weakest tensile strength, with tensile strength equal to 1.33 kg at the connection between the CO2 tube and the 4-way. Common weak points among all sampling lines included the connection between the CO2 tubing and the 4-way, and the connection between the cannula and the CO2 tube.
Rise time
The rise time, defined as time required for the measured CO2 value to increase from 10% to 90% of the final value (Figure 2), was determined for the same 16 capnography sampling lines (Table of Materials). Comparison of capnography monitor matched vs cross-paired sampling lines found that the rise time for all cross-paired sampling lines was significantly higher (147 ± 23 ms vs. 201 ± 66 ms, respectively; p<0.001). A significant difference was also present between adult matched and cross-paired sampling lines (135 ± 13 ms vs. 214 ± 61 ms; p<0.001) but not between pediatric matched and cross-paired sampling lines (156 ± 25 ms vs. 169 ± 69 ms; p=0.395). Based on the measured rise time for each sampling line, the maximum respiratory rate (BPM), and exhalation time, using an inhalation: exhalation ratio of 1:1 and 1:2, the accuracy of each sampling line at 150 BPM was determined. While a majority of the sampling lines exhibited accuracy at 150 BPM for both breathing ratios, sampling lines 2, 3, 6, 7, 12, and 13 each failed to maintain accuracy at 150 BPM, whereas sampling lines 1, 4, 5, 8, 9, 10, 11, 14, 15, and 16 maintained accuracy in all tested conditions (Table 2). In particular, sampling lines 3, 6, and 13 all failed to meet the accuracy standard at 150 BPM in both the 1:1 and 1:2 inhalation:exhalation ratios.
ETCO2 accuracy as a function of respiratory rate
Accuracy of ETCO2 was measured using respiration rates between 10 and 150 BPM for 16 sampling lines from 7 manufacturers (Table of Materials). The expected ETCO2 in the presence of 5% CO2 was 34 mmHg at ambient pressure, and the range predefined as acceptable accuracy was ±2 mmHg for readings between 0-38 mmHg and ±5% of the reading + 0.08 for every 1 mmHg above 38 mmHg. Among the adult sampling lines tested, at 10 BPM, sampling lines 8 and 9 read ETCO2 equal to 33-34 mmHg (Figure 3A). Sampling lines 2, 5, 6, and 7 also read ETCO2 levels within an acceptable range (31-34 mmHg) at the lowest respiration rates (10-20 BPM). In contrast, sampling lines 3 and 4 reported low ETCO2 levels at the lowest respiration rate (10 BPM), and these readings decreased to 0 mmHg when the respiration rate increased to 80 BPM or higher. Only sampling lines 1, 8, and 9 continued to capture readings at very high respiration rates (120-150 BPM); sampling lines 2, 3, 4, 5, 6, and 7 read ETCO2 values equal to 0 mmHg at very high respiration rates (≥100 BPM). A similar pattern was observed in the pediatric and neonatal sampling lines, in which sampling lines 10, 11, 14, 15, and 16 captured readings across all respiration rates, and sampling lines 12 and 13 reported ETCO2 equal to 0 mmHg at respiration rates ≥100 BPM (Figure 3B). The bias of the ETCO2 readings was confirmed using Bland-Altman plots for capnography monitor matched and cross-paired sampling lines, where a majority of the ETCO2 measurements were within 95% limits, but the matched sampling lines exhibited higher accuracy with a bias toward overestimating ETCO2 at 150 BPM, and the cross-paired sampling lines strongly underestimated ETCO2 measures when respiratory rate was 80 BPM or higher (Figure 4A-B).
ETCO2 accuracy in the presence of supplemental oxygen
In addition to examining the accuracy of ETCO2 values of commercial sampling lines from 7 manufacturers (Table of Materials) as a function of respiratory rate, their accuracy was also evaluated in the presence of 2, 4, or 6 L/min supplemental oxygen (Figure 5), which represent the range of supplemental oxygen flow rates commonly used in clinical settings.3,29 In all cases, the expected ETCO2 was 34 mmHg. In the absence of supplemental oxygen, ETCO2 values were 34 ± 0 mmHg for sampling lines 8 and 9, and as low as 16 ± 0 mmHg for sampling lines 3, 4, and 12 (Figure 5A). Upon the addition of 2 L/min supplemental oxygen, a majority of sampling lines exhibited a decrease in observed ETCO2 values, ranging between 0 ± 0 mmHg and 23 ± 1 mmHg; sampling lines 7, 8, and 9 reported ETCO2 values between 33 ± 0 mmHg and 34 ± 0 mmHg (Figure 5B). The most extreme drop in ETCO2 value occurred in sampling line 2, which measured ETCO2 of 0 mmHg in the presence of as little as 2 L/min supplemental oxygen; this was also observed in sampling lines 2 and 5 in the presence of 4 and 6 L/min supplemental oxygen (Figure 5C-D). Decreased ETCO2 accuracy was also observed in sampling lines 1, 6, 10, 11, and 13 in the presence of 2, 4, or 6 L/min supplemental oxygen (Figure 5B-D). Bland-Altman plots for capnography monitor matched and cross-paired sampling lines indicate that while the matched sampling lines had high precision and limited bias in reading ETCO2 levels in the presence of supplemental oxygen, the cross-paired sampling lines consistently underestimated ETCO2 in the presence of supplemental oxygen (Figure 6A-B).
Table 1: Tensile strength test of capnography sampling lines. Please click here to download this table.
Table 2: Rise time for capnography sampling lines when used in conjunction with a portable capnography monitor. The rise time for each sampling line was measured 10 times to ensure accuracy of results. Please click here to download this table.
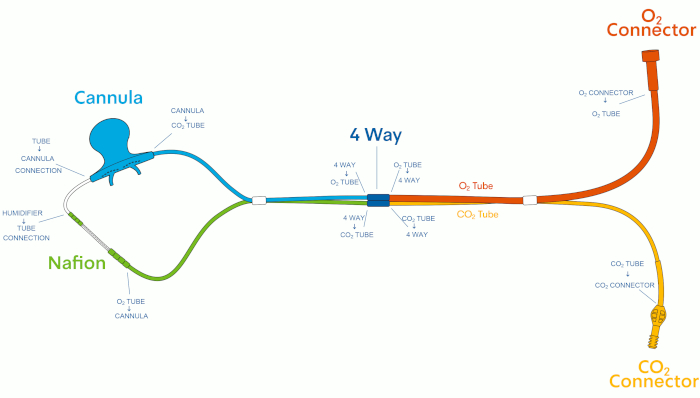
Figure 1: Capnography sampling line design. Please click here to view a larger version of this figure.
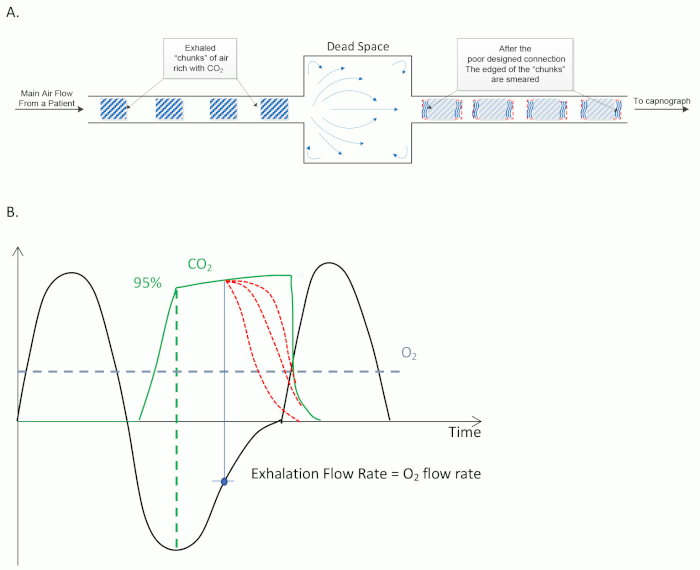
Figure 2: Fundamentals of sidestream capnography. (A) Example design of a sampling line, demonstrating how exhaled CO2 is sampled by the device. (B) Typical correlation between breathing flow rate (black line) and ETCO2 (green line) as function of time. A constant supplemental O2 flow is represented by a blue dashed line. Accurate measurement of ETCO2 occurs when CO2 has peaked (green dashed line). Inaccurate ETCO2 measurements (red dashed lines) can occur later in the breath cycle, when CO2 is diluted with supplemental O2. This occurs most often when the CO2 exhalation flow rate is equal to the flow of supplemental O2. Please click here to view a larger version of this figure.
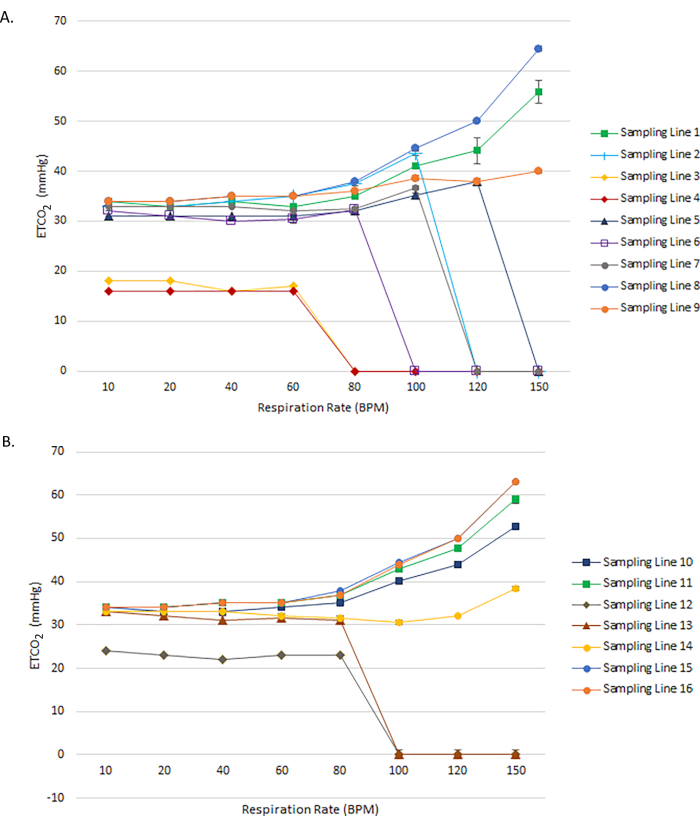
Figure 3: ETCO2 accuracy of adult and pediatric capnography sampling lines as a function of respiration rate. Measured ETCO2 values for (A) Adult and (B) Pediatric and Neonatal capnography sampling lines across a range of respiratory rates from 10 to 150 BPM. In all cases, the expected ETCO2 value is 34 mmHg. Please click here to view a larger version of this figure.
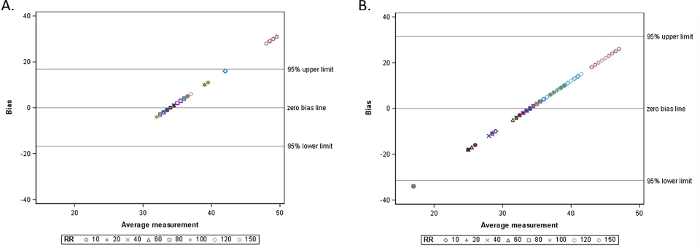
Figure 4: Bland-Altman plot for ETCO2 measures by (A) Matched sampling lines as a function of increasing respiratory rate and (B) Cross-paired sampling lines as a function of increasing respiratory rate. Please click here to view a larger version of this figure.
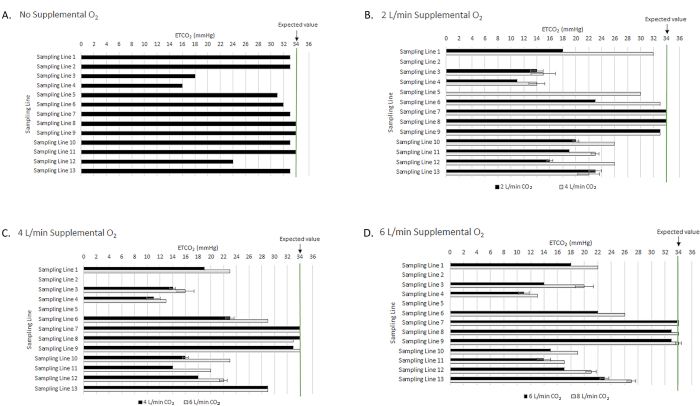
Figure 5: ETCO2 accuracy of capnography sampling lines in the presence of increasing supplemental oxygen. ETCO2 accuracy is reported for (A) No supplemental oxygen; (B) 2 L/min supplemental oxygen; (C) 4 L/min supplemental oxygen; and (D) 6 L/min supplemental oxygen. The green line at 34 mmHg represents the expected ETCO2 value across all measurements. Please click here to view a larger version of this figure.
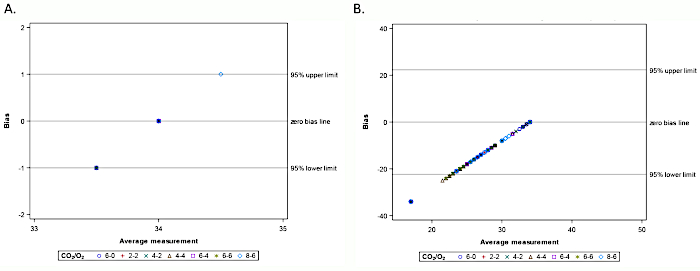
Figure 6: Bland-Altman plot for ETCO2 measures by (A) Matched sampling lines as a function of increasing supplemental O2 flow rate; (B) Cross-paired sampling lines as a function of increasing supplemental O2 flow rate. Please click here to view a larger version of this figure.
Discussion
A series of four bench tests were performed to compare the accuracy and compatibility of matched and cross-paired capnography sampling lines with a portable capnography monitor. These calibrated tests measured average rise time and ETCO2 levels across 10 independent repeat measures for each of the 16 sampling lines tested, and identified minimal variation in the results. While the tensile strength of the commercial sampling lines remained within the product specifications, the rise time differed significantly between capnography monitor matched and cross-paired sampling lines (p<0.001), and ETCO2 accuracy as a function of respiratory rate and in the presence of supplemental O2 was higher in capnography monitor matched sampling lines as opposed to cross-paired sampling lines. In particular, several of the cross-paired adult and pediatric sampling lines had rise times considered inaccurate at a maximum respiratory rate 150 BPM. The same sampling lines exhibited poor ETCO2 accuracy at high respiratory rate or in the presence of supplemental oxygen.
The tensile strength test utilized a calibrated tensile testing jig to successfully measure tension across capnography sampling line components ranging from 1.33 to 26.6 kg. Although tensile strength tests are often performed on other types of medical devices24,25, our method was unique in that it examined the tensile strength of each segment of the capnography sampling line. Therefore, in addition to determining the tensile strength of each sampling line component, it also allowed for identification of the overall weak point of the complete sampling line. The test results confirmed that nearly all of the sampling lines do meet product specifications, pre-defined as withstanding a force of 2 kg. One limitation of this testing system is the continuous, gradual increase in force applied to the sampling line, as opposed to a sudden strong force, which could be encountered in clinical settings. Importantly, as a validated instrument, the jig used to measure the tensile strength of the capnography sampling lines could be used for other applications, such as measuring the tensile strength of other sampling tubes and medical devices that have the potential to experience tension in a clinical setting.
Rise time is an important technical feature of sidestream capnography sampling lines and determines their ability to provide a precise, high resolution reading of CO2 in exhaled breath1,14. Due to the importance of this technical feature, we sought to measure the rise time using a validated rise time measurement device, so that the maximum respiratory rate and exhalation time could be calculated. We needed to modify the rise time measurement parameters to remove the upper time limit on the rise time jig, so that the rise time could be collected for all sampling lines before the measurement period ended. The long rise time observed for some capnography sampling lines could reflect an increased volume of dead space in these sampling lines. Importantly, as part of this method, we determined the maximum respiratory rate and exhalation time for two unique breathing patterns, defined by inhalation:exhalation ratios equal to 1:1 and 1:2. This unique aspect of the analysis allowed evaluation of the accuracy of measured CO2 in circumstances that represent patients whose breathing pattern is uniform or whose exhalation time lasts longer than their inhalation time. In sampling lines in which the calculated maximum respiratory rate was >150 BPM, we concluded that the sampling line was accurate. Although a rapid breathing rate of 150 BPM is unlikely to be encountered clinically, we determined the accuracy of each sampling device at this high breath rate because it is considered the technical upper limit for many capnography sampling lines. While a respiratory rate of 150 BPM is non-physiologic, the bench test highlights that while some capnography sampling lines were accurate across the full technical range of respiratory rates, other sampling lines failed to achieve the same accuracy standard. Compared to the capnography monitor matched sampling lines, some of the cross-paired sampling lines, including sampling lines 2 and 7, failed to achieve accuracy at 150 BPM for the 1:1 inhalation:exhalation ratio, and sampling lines 3, 6, and 13 failed to achieve the accuracy standard at 150 BPM for both inhalation:exhalation ratios. This could be due to a larger dead space within the sampling lines, which results in a longer rise time and a mixing of breath samples.
To apply the rise time findings to a clinical setting, we performed two tests to examine ETCO2 accuracy when sampling lines were connected to a portable capnography monitor via a manikin. For both tests, we needed to modify the default capnography monitor settings to allow the monitor to recognize cross-paired sampling lines. First, similar to a previous study, we controlled respiratory rate using a respiratory rate controller, and monitored the resulting ETCO2 measurements for each sampling line18. A key component of this test was the use of a pre-defined set of respiratory rates ranging from 10 to 150 BPM, to determine ETCO2 accuracy across respiratory patterns that patients could exhibit. While the expected ETCO2 level was 34 mmHg in all circumstances, we observed many instances in which, as respiratory rate increased, sampling lines no longer reported accurate ETCO2 readings, but instead, dropped to 0 mmHg, which is not a clinically meaningful result. In fact, only sampling lines 1, 8, 9, 10, 15, and 16 did not measure ETCO2 values of 0 mmHg at any respiratory rate. This accuracy could be due to the design of the sampling lines, such that those with higher friction or larger dead space volume result in lower resolution breath samples at increased respiratory rate, similar to what we observed in the rise time test. While the sampling lines with high ETCO2 readings may contain less dead space that enable them to deliver discrete breath samples, the error of ETCO2 readings above 38 mmHg was pre-defined as ±5% of the reading + 0.08 for every 1 mmHg above 38 mmHg. This could partially explain why the ETCO2 readings were increased above 34 mmHg during high respiratory rate in some sampling lines. In contrast, the sampling lines with low or zero ETCO2 readings may contain more dead space, resulting in mixed breath samples that the capnography monitor does not recognize as valid breaths, and thus reports as no breath. Importantly, 3 of the cross-paired sampling lines from one manufacturer did not exhibit accurate ETCO2 readings at any respiratory rate tested between 10 and 150 BPM, suggesting that it does not provide clinically reliable ventilatory information when cross-paired with the capnography monitor used in the test (Table of Materials). Together, these observations suggest that devices with a longer rise time have a lower maximum accurate respiration rate and exhibit low ETCO2 accuracy at the maximum accurate respiration rate.
In the second test of ETCO2 accuracy using a manikin, we maintained a constant respiratory rate but introduced the flow of supplemental oxygen to the system. This test mimics a common occurrence in hospital settings in which patients being monitored by sidestream capnography receive supplemental oxygen, and where ETCO2 accuracy is key in understanding a patient’s respiratory function, as supplemental oxygen can mask ventilation challenges due to high oxygen saturation readings from pulse oximetry30,31. Similar to the ETCO2 accuracy test with varying respiratory rate, in this test, a key step in the protocol was to measure ETCO2 accuracy across multiple supplemental oxygen flow rates. The main limitation of the ETCO2 tests is that the tests are performed using a manikin and a controlled breathing system, as opposed to a human subject, in which breathing patterns vary between individuals. In a control reading without supplemental O2, we observed that sampling lines 3, 4, and 12, all from the same manufacturer, failed to report the expected ETCO2 value of 34 mmHg, and only sampling lines 8, 9, and 11 reported this value. In the presence of 2, 4, or 6 L/min supplemental O2, a majority of the sampling lines exhibited reduced ETCO2 accuracy, with the exception of the matched sampling lines 8 and 9 and the cross-paired sampling line 7. In particular, similar to our observations upon increase of the respiratory rate, the ETCO2 readings for sampling lines 2 and 5 dropped to 0 mmHg in the presence of supplemental O2, suggesting that their ETCO2 accuracy when cross-paired with a capnography monitor is very low. This may be due to the design of the sampling lines, and in particular, the nasal cannula design, which is designed to both deliver oxygen to a patient and collect breath samples from a patient. If the nasal cannula contains a large amount of dead space, mixing of the supplemental oxygen and the exhaled breath can occur, resulting in low amplitude, mixed breaths that the capnography monitor does not detect as exhaled breath. In such a case, the ETCO2 measurement would drop to zero, as we observed with some of the cross-paired sampling lines tested.
Similar to previous studies examining the accuracy of capnography, we successfully identified circumstances where the ETCO2 accuracy using a variety of sampling lines was acceptable, including cases in which there was a moderate respiratory rate or when no supplemental O2 was used19,20,21,22,23,32. Importantly, many of the sampling lines failed to maintain ETCO2 accuracy upon an increase in respiratory rate or upon the introduction of supplemental O2, which is consistent with previous assessments of capnography accuracy15,18,20,23. Together, the findings are consistent with previous bench tests that successfully measure the accuracy of capnography sampling lines15,18. Given that many of the sampling lines cross-paired to the capnography monitor exhibited reduced ETCO2 accuracy in clinically relevant circumstances, care should be taken to ensure that any cross-paired commercial sampling lines and monitors are validated before being used to monitor patient ventilation status.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by Medtronic. Marco Scardapane (Medtronic Study and Scientific Solutions MC2, Rome, Italy) performed statistical analysis.
Materials
| Adult CO2/O2 Nasal Cannula | Respironics | M2750A | Sampling Line 1 |
| Adult Dual Nasal Cannula, Female Luer | Flexicare | 032-10-126U | Sampling Line 2 |
| Divided Adult Capnograpy Cannula, Female Luer | Salter Labs | 4707FTG-7-7 | Sampling Line 3 |
| Divided Adult Capnograpy Cannula, Female Luer | Salter Labs | 4797F-7-7 | Sampling Line 4 |
| Hudson RCI Softech Bi-Flo EtCO2/O2 Cannula, Female Luer | Hudson | 1845 | Sampling Line 5 |
| CO2/O2 Adult Cannula, Female Luer | Westmed | 539 | Sampling Line 6 |
| Adult ETCO2 Cannula | Ventlab | 4707 | Sampling Line 7 |
| O2/CO2 Nasal FilterLine sampling line, Adult, Female Luer | Medtronic | 6912 | Sampling Line 8 https://www.medtronic.com/covidien/en-us/products/capnography/filterline-etco2-sampling-lines.html |
| Smart CapnoLine Plus sampling line, Adult, Female Luer | Medtronic | 9822 | Sampling Line 9 https://www.medtronic.com/covidien/en-us/products/capnography/filterline-etco2-sampling-lines.html |
| Pediatric CO2/O2 Nasal Cannula | Respironics | M2751A | Sampling Line 10 |
| Pediatric CO2/O2 Oral/Nasal Cannula | Respironics | M2761A | Sampling Line 11 |
| Divided Pediatric Capnograpy Cannula, Female Luer | Salter Labs | 4703F-7-7 | Sampling Line 12 |
| Hudson RCI Softech Plus Pediatric Divided Nasal Cannula | Hudson | 2850 | Sampling Line 13 |
| FilterLine H Set sampling line, Infant/Neonate | Medtronic | 6324 | Sampling Line 14 https://www.medtronic.com/covidien/en-us/products/capnography/filterline-etco2-sampling-lines.html |
| O2/CO2 Nasal FilterLine sampling line, Pediatric, Female Luer | Medtronic | 6913 | Sampling Line 15 https://www.medtronic.com/covidien/en-us/products/capnography/filterline-etco2-sampling-lines.html |
| Smart CapnoLine sampling line, Pediatric, Female Luer | Medtronic | 7269 | Sampling Line 16 https://www.medtronic.com/covidien/en-us/products/capnography/filterline-etco2-sampling-lines.html |
| Breathing simulator | Medtronic | T-158 | |
| Capnostream 35 portable respiratory monitor | Medtronic | PM35MN | https://www.medtronic.com/covidien/en-us/products/capnography/capnostream-35-portable-respiratory-monitor.html |
| Flow/Leak Tester | Emigal Electronic test solutions LTD | N/A | |
| Flow Meter | Omega | FMA1823A | |
| Gas: 100% N2 | Airgas | GR04930 | |
| Gas: 100% O2 | Airgas | 10133692 | |
| Gas: 5%CO2, 21%O2, 74% N2 | Airgas | HPE400 | |
| Manikin | Tru Corp-AirSim Advance | S/N: AA3617A29092017C | |
| Rise Time Jig | Medtronic | T-547 | |
| Tensile Testing Machine | MRC Lab | B1/E | |
| Statistical software | SAS Institute Inc | v9.4 |
References
- Siobal, M. S. Monitoring Exhaled Carbon Dioxide. Respiratory Care. 61 (10), 1397-1416 (2016).
- Lam, T., et al. Continuous Pulse Oximetry and Capnography Monitoring for Postoperative Respiratory Depression and Adverse Events: A Systematic Review and Meta-analysis. Anesthesia and Analgesia. 125 (6), 2019-2029 (2017).
- Chung, F., Wong, J., Mestek, M. L., Niebel, K. H., Lichtenthal, P. Characterization of respiratory compromise and the potential clinical utility of capnography in the post-anesthesia care unit: a blinded observational trial. Journal of Clinical Monitoring and Computing. , 00333-00339 (2019).
- Merchant, R. N., Dobson, G. Special announcement: Guidelines to the Practice of Anesthesia – Revised Edition 2016. Canadian Journal of Anaesthesia. 63 (1), 12-15 (2016).
- Whitaker, D. K., Benson, J. P. Capnography standards for outside the operating room. Current Opinion in Anaesthesiology. 29 (4), 485-492 (2016).
- American Society of Anesthesiologists Task Force on Neuraxial Opeiods, et al. Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid adminstration. Anesthesiology. 110 (2), 218-230 (2009).
- American Society of Anesthesiologists Task Force on Neuraxial Opeiods, et al. Practice Guidelines for the Prevention, Detection, and Management of Respiratory Depression Associated with Neuraxial Opioid Administration: An Updated Report by the American Society of Anesthesiologists Task Force on Neuraxial Opioids and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 124 (3), 535-552 (2016).
- American Society of Anesthesiologists Committee on Standards and Practice Parameters. Standards for Basic Anesthetic Monitoring. American Society of Anesthesiologists Committee on Standards and Practice Parameters. , (2015).
- American Society of Anesthesiologists Task Force on Moderate Procedural Sedation and Analgesia, the American Association of Oral and Maxillofacial Surgeons, American College of Radiology, American Dental Association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Practice Guidelines for Moderate Procedural Sedation and Analgesia 2018: A Report by the American Society of Anesthesiologists Task Force on Moderate Procedural Sedation and Analgesia, the American Association of Oral and Maxillofacial Surgeons, American College of Radiology, American Dental Association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Anesthesiology. 128 (3), 437-479 (2018).
- Nagrebetsky, A., Gabriel, R. A., Dutton, R. P., Urman, R. D. Growth of Nonoperating Room Anesthesia Care in the United States: A Contemporary Trends Analysis. Anesthesia and Analgesia. 124 (4), 1261-1267 (2017).
- Jaffe, M. B. Respiratory Gas Analysis-Technical Aspects. Anesthesia and Analgesia. 126 (3), 839-845 (2018).
- Richardson, M., et al. . Capnography for Monitoring End-Tidal CO2 in Hospital and Pre-hospital Settings: A Health Technology Assessment. 142, (2016).
- Anderson, C. T., Breen, P. H. Carbon dioxide kinetics and capnography during critical care. Critical Care. 4 (4), 207-215 (2000).
- Schmalisch, G. Current methodological and technical limitations of time and volumetric capnography in newborns. Biomedical Engineering Online. 15 (1), 104 (2016).
- Phillips, J. S., Pangilinan, L. P., Mangalindan, E. R., Booze, J. L., Kallet, R. H. A Comparison of Different Techniques for Interfacing Capnography With Adult and Pediatric Supplemental Oxygen Masks. Respiratory Care. 62 (1), 78-85 (2017).
- Fukuda, K., Ichinohe, T., Kaneko, Y. Is measurement of end-tidal CO2 through a nasal cannula reliable. Anesthesia Progress. 44 (1), 23-26 (1997).
- Burk, K. M., Sakata, D. J., Kuck, K., Orr, J. A. Comparing Nasal End-Tidal Carbon Dioxide Measurement Variation and Agreement While Delivering Pulsed and Continuous Flow Oxygen in Volunteers and Patients. Anesthesia and Analgesia. , (2019).
- Chang, K. C., et al. Accuracy of CO(2) monitoring via nasal cannulas and oral bite blocks during sedation for esophagogastroduodenoscopy. Journal of Clinical Monitoring and Computing. 30 (2), 169-173 (2016).
- Takaki, S., Mihara, T., Mizutani, K., Yamaguchi, O., Goto, T. Evaluation of an oxygen mask-based capnometry device in subjects extubated after abdominal surgery. Respiratory Care. 60 (5), 705-710 (2015).
- Takaki, S., et al. Deep Breathing Improves End-Tidal Carbon Dioxide Monitoring of an Oxygen Nasal Cannula-Based Capnometry Device in Subjects Extubated After Abdominal Surgery. Respiratory Care. 62 (1), 86-91 (2017).
- Mason, K. P., Burrows, P. E., Dorsey, M. M., Zurakowski, D., Krauss, B. Accuracy of capnography with a 30 foot nasal cannula for monitoring respiratory rate and end-tidal CO2 in children. Journal of Clinical Monitoring and Computing. 16 (4), 259-262 (2000).
- Zhang, C., Wang, M., Wang, R., Wang, W. Accuracy of end-tidal CO2 measurement through the nose and pharynx in nonintubated patients during digital subtraction cerebral angiography. Journal of Neurosurgical Anesthesiology. 25 (2), 191-196 (2013).
- Ebert, T. J., Novalija, J., Uhrich, T. D., Barney, J. A. The effectiveness of oxygen delivery and reliability of carbon dioxide waveforms: a crossover comparison of 4 nasal cannulae. Anesthesia and Analgesia. 120 (2), 342-348 (2015).
- Chan, C. W., Chan, L. K., Lam, T., Tsang, K. K., Chan, K. W. Comparative study about the tensile strength and yielding mechanism of pacing lead among major manufacturers. Pacing and Clinical Electrophysiology. 41 (7), 828-833 (2018).
- Gonzalez Fiol, A., et al. Comparison of Changes in Tensile Strength in Three Different Flexible Epidural Catheters Under Various Conditions. Anesthesia and Analgesia. 123 (1), 233-237 (2016).
- Burton, J. H., Harrah, J. D., Germann, C. A., Dillon, D. C. Does end-tidal carbon dioxide monitoring detect respiratory events prior to current sedation monitoring practices. Academic Emergency Medicine. 13 (5), 500-504 (2006).
- Mehta, J. H., Williams, G. W., Harvey, B. C., Grewal, N. K., George, E. E. The relationship between minute ventilation and end tidal CO2 in intubated and spontaneously breathing patients undergoing procedural sedation. PloS One. 12 (6), e0180187 (2017).
- Williams, G. W., George, C. A., Harvey, B. C., Freeman, J. E. A Comparison of Measurements of Change in Respiratory Status in Spontaneously Breathing Volunteers by the ExSpiron Noninvasive Respiratory Volume Monitor Versus the Capnostream Capnometer. Anesthesia and Analgesia. 124 (1), 120-126 (2017).
- Curry, J. P., Jungquist, C. R. A critical assessment of monitoring practices, patient deterioration, and alarm fatigue on inpatient wards: a review. Patient Safety in Surgery. 8, 29 (2014).
- Fu, E. S., Downs, J. B., Schweiger, J. W., Miguel, R. V., Smith, R. A. Supplemental oxygen impairs detection of hypoventilation by pulse oximetry. Chest. 126 (5), 1552-1558 (2004).
- Gupta, K., et al. Risk factors for opioid-induced respiratory depression and failure to rescue: a review. Current Opinion in Anaesthesiology. 31 (1), 110-119 (2018).
- Casati, A., et al. Accuracy of end-tidal carbon dioxide monitoring using the NBP-75 microstream capnometer. A study in intubated ventilated and spontaneously breathing nonintubated patients. European Journal of Anaesthesiology. 17 (10), 622-626 (2000).

