Dissecting, Fixing, and Visualizing the Drosophila Pupal Notum
Summary
The present protocol details the preparation and visualization of fixed tissue of the Drosophila pupal notum. It can be used for either intact or wounded tissue, and the original architecture of the tissue is preserved. The procedures for dissecting, fixing, and staining are all described in this article.
Abstract
The pupae of Drosophila melanogaster are immobile for several days during metamorphosis, during which they develop a new body with a thin transparent adult integument. Their immobility and transparency make them ideal for in vivo live imaging experiments. Many studies have focused on the dorsal epithelial monolayer of the pupal notum because of its accessibility and relatively large size. In addition to the studies of epithelial mechanics and development, the notum has been an ideal tissue to study wound healing. After an injury, the entire epithelial repair process can be captured by live imaging over 6-12 h. Despite the popularity of the notum for live imaging, very few published studies have utilized fixed notum samples. Fixation and staining are common approaches for nearly all other Drosophila tissues, taking advantage of the large repertoire of simple cellular stains and antibodies. However, the pupal notum is fragile and prone to curling and distortion after removal from the body, making it challenging to complement live imaging. This protocol offers a straightforward method for fixing and staining the pupal notum, both intact and after laser-wounding. With this technique, the ventral side of the pupa is glued down to a coverslip to immobilize the pupa, and the notum is carefully removed, fixed, and stained. The notum epithelium is mounted on a slide or between two coverslips to facilitate imaging from the tissue's dorsal or ventral side.
Introduction
The pupal notum of Drosophila melanogaster has been increasingly used for live imaging studies in the last decade because the animal is both immobile and has a transparent cuticle at this stage1,2,3,4,5,6,7. However, the pupal notum is challenging to dissect and fix, making it difficult to complement live imaging studies with antibody and cell staining. The overall goal of this work is to create a reproducible protocol for dissecting and fixing the pupal notum for antibody and cell staining on new or previously live-imaged samples.
As larvae begin metamorphosis, the epidermis pulls away from the larval cuticle, forming a hard pupal case8. The larval body plan is broken down, and the new adult body plan is developed. During this time, pupae are immobile, making them ideal for live imaging. One commonly imaged tissue is the pupal notum, an adult monolayer epithelium that forms in the dorsal thorax. The notum is visually accessible after a simple dissection to remove the pupal case9. The whole animal can then be mounted, and the notum can be live imaged for hours or days, making it an ideal tissue to study epithelial cell behaviors during development, homeostasis, and following wounding10,11,12,13,14. However, the notum is challenging to dissect and fix because it is fragile and covered with a thin transparent adult cuticle that is hydrophobic. This hydrophobic cuticle makes it prone to curling in aqueous solutions when removed from the rest of the body. Thus, notum dissection and fixation has been reported only rarely and the dissection is often not described15,16,17,18. Without a detailed protocol in the literature, it is prohibitively difficult for a Drosophila researcher to complement live imaging with staining of pupae.
This technique aims to reproducibly dissect and fix samples that have been previously live-imaged, including those that have been laser-wounded. Because live imaging requires removal of the pupal case, this dissection technique begins by removing the anterior pupal case, unlike previous protocols that pin down or bisect pupae within the pupal case4,19,20. The notum is a fragile tissue, and wounding may exacerbate its fragility. Thus, to support this delicate tissue, the integument (the epithelium and attached transparent adult cuticle) of the notum and part of the head and abdomen are dissected away from the rest of the pupa while always submerged in an aqueous environment. This method reduces the likelihood of the tissue curling and being unusable. This technique has successfully stained wounded notum tissue as early as 30 min post wounding (Figure 1E–H) and at 3 h post wounding (Figure 1I–L). This protocol is expected to be effective for the duration of notum development or wound repair. The current technique will be helpful for researchers wishing to unite the live-imaging capabilities of the pupal notum with the abundance of available immunohistochemistry reagents.
Protocol
Drosophila melanogaster (fruit flies) were maintained at 25 °C on a standard cornmeal-molasses medium. The studies were conducted on EGFP-tagged histone H2A pupae (w[*]; P{w+mC=His2Av-EGFP.C}2/SM6a). The flies were obtained from a public stock center (see Table of Materials).
1. Pupae immobilization
- Apply a 2" strip of double-sided tape to a microscope slide.
- Identify white prepupae in vials raised at 25 °C, and use a marker to indicate their location outside the vial. Return the vials to 25 °C.
NOTE: White prepupae are characterized by their immobility, white color, and everted spiracles. These form 0-1 h after puparium formation (APF), or stage P18. - 12-15 h later, carefully remove 3-4 of the indicated pupae (without popping them) using a dissection scope and collect them on the microscope slide next to the tape.
NOTE: Pupae will now be stage P5 with an everted head sac visible at the pupae's anterior end8. - Place the pupae at least one pupa width apart onto the tape with their ventral sides down.
- Place a drop of adhesive glue on a paraffin film (see Table of Materials) or in a centrifuge tube lid. Dip the end of a 0.1-10 µL pipette tip (no pipette) in the drop of adhesive glue. Tap the pipette tip twice on a 24 mm x 60 mm (1.5 thickness) coverslip, 1 cm x 1 cm away from a corner, creating a line of adhesive glue ~1/2 the length of the pupa.
- Preset a 0.2-2 µL (P2) pipette to 2 µL, and a 200 µL (P200) pipette to 200 µL, and fit them with tips so they are ready to be filled with 1x PBS + 0.1 mM Ca2+, which will rapidly solidify the adhesive glue on contact.
- Insert forceps (see Table of Materials) near the side of the head and gently remove the case from the anterior to posterior9. Remove as much of the case as possible. Grasp the pupa's developing legs with a pair of blunt forceps and carefully pull the pupa from its case.
NOTE: A small rupture on the ventral portion of the pupae will not be detrimental to this procedure. - Lay the pupa in the corner of the coverslip.
- Grasp the pupa at the posterior abdomen or the developing wing with blunt forceps, lift, and place the ventral side of the pupae down into the line of adhesive glue.
- Quickly fill the P2 pipette with 2 µL of 1x PBS + 0.1 mM of Ca2+, and holding it in the air, expel just enough to form a small bubble at the tip (0.25-0.5 µL).
- Touch the small bubble of the solution to one side of the pupae at the base of the thorax, then repeat on the other side.
NOTE: This will solidify a small amount of the adhesive glue to hold the pupa in place. Generally, all the solutions will not be used. - Fill the P200 pipette with 200 µL of 1x PBS + 0.1 mM of Ca2+, then place the pipette's tip over the thorax and expel the contents to submerge the pupae completely. The remainder of the adhesive glue will solidify immediately.
- Remove ~100 µL of the PBS solution, so the pupa is barely submerged before proceeding immediately to the next step.
NOTE: For unwounded samples, start from step 1.1. For wounded partially dissected samples, start at step 1.5. Wounding via laser ablation has been described previously14,21. Immobilization, dissection, and mounting steps need to be performed using a dissection microscope.
2. Dissecting the notum
- Grasp a pair of microdissection scissors bracing one side of the handle against the dominant hand's index finger and middle finger, so the thumb of the dominant hand applies the cutting force (Figure 2A,B).
- Stabilize the neck of the scissors against the middle finger of the non-dominant hand while bracing the coverslip with the ring finger of the non-dominant hand.
- Snip at the middle of the dorsal abdomen to create a small hole, ~0.2-0.5 mm. Some hemolymph will usually spill out and is a good indicator of the breach.
- Make small 0.5-0.75 mm cuts through the integument from the posterior to anterior, encircling the dorsal tissue to isolate it. To create a tissue as flat as possible, avoid cutting too ventrally; only the dorsal 'dome' of the thorax should be removed along with small sections of the head and abdomen.
- Repeat posterior to anterior cuts on the other side of the pupae.
- Rotate the dissection stage to allow for a clean cut through the head if necessary.
NOTE: At this stage, the dorsal integument, or notum, will be separated from the rest of the pupa. If it appears separated but is not easily moved, a few cuts below the notum can help dislodge it. - Add ~200 µL of 1x PBS to the center of the coverslip and make a channel connecting it to the original dissection droplet by gently dragging the pipette tip across the cover glass from the new droplet to the original.
- Using a pair of blunt forceps, gently push or drag the isolated notum to the center of the coverslip and rotate, so the interior side faces upward. Never remove the tissue from the droplet.
NOTE: It is essential to move the notum away from the original dissection site to avoid any remnants of the adhesive glue occluding the sample during later imaging. - Hold the notum down with the blunt forceps by pressing into the abdominal or head sections. Using a pair of sharp forceps and/or gentle expulsions of 1x PBS from a 200 µL pipette, remove any remaining fat body, muscle bands, or hemolymph (if present) to fully expose the monolayer epithelium and make the eventual staining more even. The dissected notum should appear as in Figure 3A.
- Once the tissue is clean, use a 200 µL pipette to remove as much of the PBS solution as possible (along with debris and the ventral part of the pupae), monitoring with a dissection scope to avoid aspirating the notum.
- Once most of the liquid has been removed, use an absorbent tissue to carefully wipe away the rest of the adhesive glue and pupa, as well as any other debris that lingers on the coverslip.
NOTE: If some adhesive glue remains on the coverslip, it will not cause an issue so long as it is thinner than the dorsal tissue itself. - Add 150-200 µL of 4% PFA (in 1x PBS) and fix for 20 min at room temp. Depending on the dissection speed, 1 or more more pupae can be dissected during the first pupa's fixation.
- Remove the PFA and replace it with 1x PBS to wash the notum once for 30 s.
- If proceeding with antibody staining, perform 5 min washes (3 times) in 1x PBS or 1x PBST (Supplementary File 1) to permeabilize the tissue if the antigen is intracellular.
- Store the sample in 1x PBS + 0.02% NaN3 overnight in a humidified chamber, or if planning to stain the tissue, incubate overnight in Blocking solution (Supplementary File 1).
3. Staining the notum
NOTE: For staining using antibodies or cellular stains follow the steps below. -The notum must not be removed from the solution, as this will likely cause the tissue to curl. Thus, adapt staining protocols to be conducted entirely on the coverslip and keep in a humidified chamber for any steps longer than 5 min. Monitoring the samples under a dissection microscope can help to prevent accidental aspiration of the tissue during washes.
- To visualize the cell borders, incubate in 200 µL of anti-FasIII primary Mouse IgG2a antibody (see Table of Materials) at 1:8 concentration diluted in Blocking Buffer + 0.02% NaN3 overnight at 4 °C.
- Wash out excess primary antibody (3 times) with 200 µL of 1x PBS + 0.02% NaN3 for 1 h per wash at room temperature.
- Perform secondary antibody incubation with 200 µL of 1:200 concentration anti-mouse IgGa2 in Cy3 in Blocking Buffer + 0.02% NaN3 for 2 h at room temp.
- Wash out excess secondary antibody (3 times) with 200 µL of 1x PBS + 0.02% NaN3 for 1 h per wash at room temperature.
- To visualize the nuclei, incubate samples in 1 µg/mL of DAPI for 45 min to allow sufficient time for the stain to penetrate through muscle bands; fixing in a DAPI-containing mounting medium is not effective with this tissue.
- Wash out excess DAPI (3 times) with 200 µL of 1x PBS + 0.02% NaN3 for 5 min per wash at room temperature, then leave in 200 µL of 1x PBS + 0.02% NaN3 overnight at 4 °C, or mount immediately.
4. Mounting and visualizing the notum
- Following staining, prepare a new 24 × 60 coverslip (topper) with supports.
NOTE: Because the notum is dome-shaped, flattening it completely results in distorted wrinkled tissue. Creating a gap between the two coverslips allows the notum to retain its normal shape. - Create a gap of ~200 µm by using spacers made from 22 x 22 coverslips (No. 0 thickness, ~100 µm thick), adhered ~1 cm apart with nail polish in the middle of the topper.
- To adhere, place the spacers on the topper and paint the distal edges of the spacers with a thin layer of nail polish. Let dry.
NOTE: Only use a nail polish that is thin and runny; thick nail polish will add unnecessary additional space between the coverslip and topper. - Remove as much of the aqueous solution as possible from the sample.
- Immediately apply two drops (~100 µL) of anti-fade mounting medium (see Table of Materials) to the sample.
- If necessary, use clean, sharp forceps to position the notum in the center of the anti-fade mounting medium droplet.
- Place the coverslip with the notum onto a ~10 x 40 mm support, such as a piece of thin foam (cut from the packing material inside coverslip boxes), to elevate the sample so it will not adhere to the work surface.
- Under a dissection scope, slowly lower the topper onto the sample. Once the anti-fade mounting medium meets the topper, gently release and allow capillary action to pull the topper down.
NOTE: Within the first few seconds, minor adjustments can be made to the coverslip position without damaging the notum. - Place another piece of foam onto the topper and use a standard microscope slide as a weight to gently coax the anti-fade mounting medium between the sample coverslip, topper, and spacers.
- After 5-10 min, use an absorbent tissue to wick away any excess anti-fade mounting medium by gently touching the edges of the coverslip.
- Gently apply nail polish to each corner of the coverslips to adhere them together. Once dry, paint all edges of the coverslips to seal. Avoid coating all edges first, as this can often shift the coverslip and damage the dorsal tissue.
- Visualize the notum under fluorescence microscopy (see Table of Materials) through the dorsal and/or the ventral side.
Representative Results
The presented technique works well on the unwounded notum (Figure 1A–D), allowing for investigation of the development and homeostasis of the tissue, e.g., the formation of the polyploid mechanosensory bristle cells18, or the anterior to the posterior flow of epithelial cells10. This protocol is also applicable to a laser-ablated notum (Figure 1E–L), where the cellular response to injury can be analyzed live with endogenous fluorophores such as Histone2-EGFP (Figure 1B,F,J). Post-staining with immunohistochemistry (step 3) can reveal many features, such as Fasciclin III, which labels cell borders (Figure 1C,G,K). Additionally, quantitative stains such as DAPI (Figure 1A,E,I) can be used to assess DNA content changes, including wound-induced polyploidy22.
The current protocol is particularly beneficial as it can be used following long-term live imaging experiments. As pupae are immobile, they can be imaged for hours (Figure 4A,B). Importantly, this protocol does not cause considerable changes to the wound epithelium's overall architecture or morphology following dissection (Figure 4B,C). Thus, features within the tissue could be imaged long-term and then further investigated with immunohistochemistry or cellular stains.
Imaging deep within tissues is complex due to their opacity, and Drosophila are coated in a waxy cuticle8 making deep imaging even more difficult. However, with this technique, a dissected notum can be placed between two coverslips allowing for imaging of the epithelial monolayer from either side: the apical side through the cuticle (Figure 5A–D) and/or the basal side of the epithelium which faces the body cavity (Figure 5E–H). These different views are ideally suited to visualize different structures within the tissue. For instance, the apical view is ideal for visualizing epithelial cell borders and nuclei that lie just below the cuticle (Figure 5A–D). With the basal view, these apical signals are less visible (Figure 5E–H). However, basal structures are observed at the wound margin (Figure 5J,L yellow, white arrows). These basal structures are much brighter as there is less occlusion in the basal view than in the apical view.
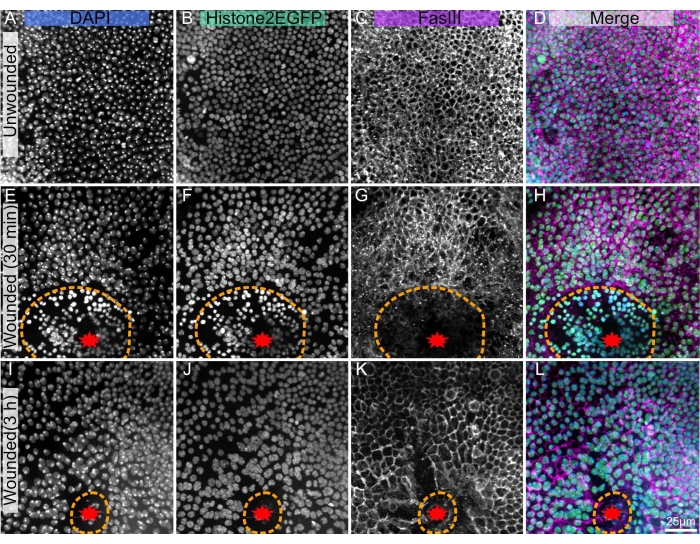
Figure 1: Dissected, fixed, and stained Drosophila pupal nota. (A–D) Unwounded notum. (E–H), Wounded notum 30 min after laser ablation. (I–L) Wounded notum 3 h after laser ablation. (A,E,I) DAPI stain shows nuclei. (B,F,J) Transgenic Histone2-EGFP, used in live imaging, is visible after fixing and staining. (C,G,K) The anti-FasIII antibody shows that antibody stains work well on the fixed notum. (D,H,L) Merged image. The images are captured with 40x objective using spinning disc microscopy, maximum intensity projections of Z-stacks are shown with 0.3 µm Z-slices. A-D represents 263 Z-slices. E-H represents 195 slices. I-L represents 53 Z-slices. The orange dashed line denotes wound margin. Scale bar in L is 25 µm, applicable to A-L. Please click here to view a larger version of this figure.
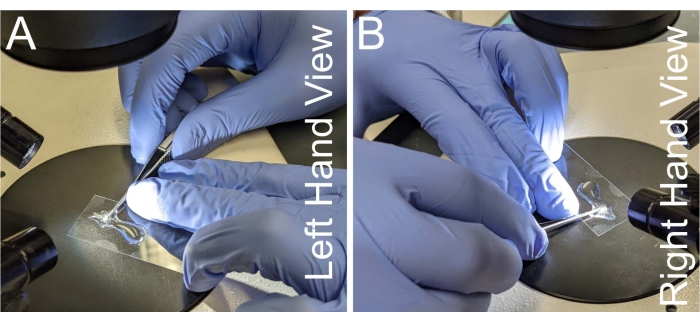
Figure 2: Hand placement for notum dissection. (A–B) Left and right views of hand placement for holding the microdissection scissors. To prevent hand shaking, the neck of the scissors is placed against the middle finger of the non-dominant hand. Scissors need to be parallel to the microscope slide to avoid the warping of the notum tissue. Please click here to view a larger version of this figure.
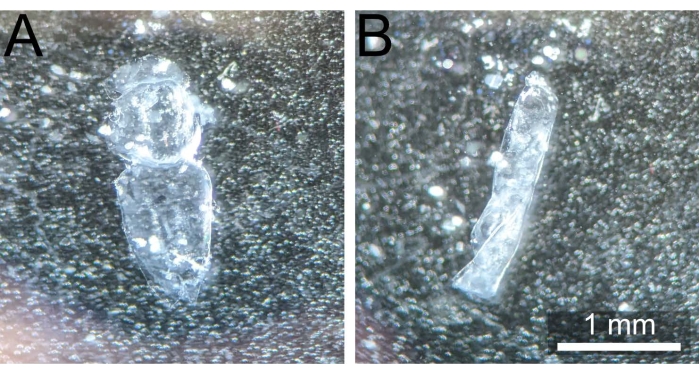
Figure 3: Correctly dissected notum vs. curled notum. (A) Pupal notum post dissection and cleaning uncurled and ready for fixation. (B) Pupal notum curled on itself after being removed from 1x PBS, thus rendering it unusable. Please click here to view a larger version of this figure.
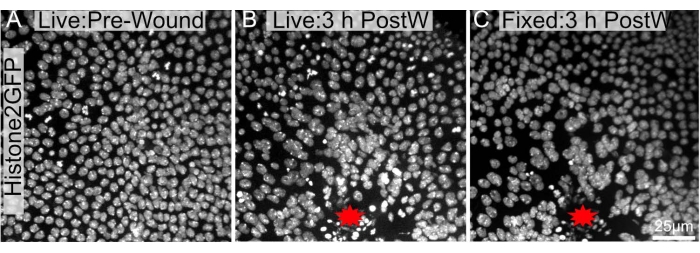
Figure 4: Pre and post-fixed images are largely similar. A pupal notum expressing Histone2-EGFP in the nuclei was imaged (A) live before ablation and (B) 3 h after laser-ablation. (C) The notum was retrieved, dissected, and fixed as mentioned in the protocol and re-imaged after fixing. The wound site is labeled with a red star. The images were captured with 40x objective using spinning disc microscopy, Z-slices were taken every 0.3 µm. Maximum intensity projections of 34 Z-slices pre-wounding (A), 48 Z-slices 3 h after wounding (B), and 103 Z-slices post dissection, fixation, and staining (C) are shown. Scale bar in C is 25 µm, applicable to A-C. Please click here to view a larger version of this figure.
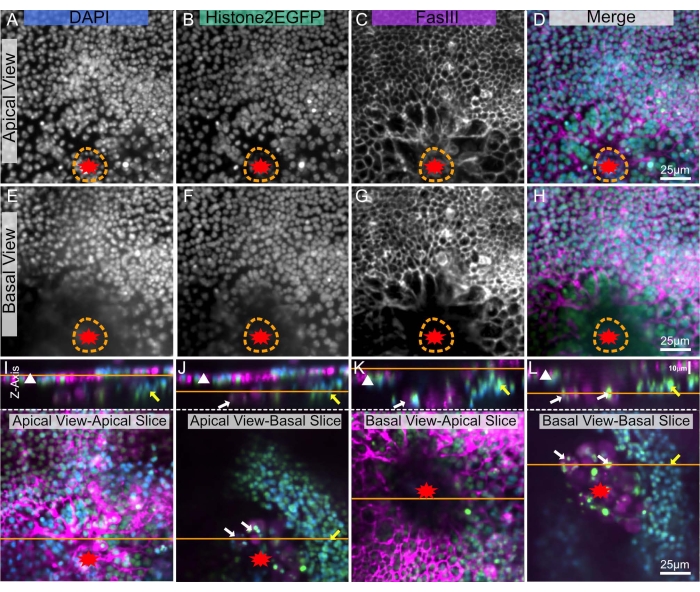
Figure 5: Imaging apical and basal sides of a wounded notum. The same wounded pupal notum is shown in all panels. The wound is marked with a red star. The orange dotted line indicates the wound margin. (A–D) The notum is imaged from the apical side, through the cuticle. (E–H) The notum is imaged from the basal interior side. (I–L) The top panels show an X-Z image of the notum, apical side up, with the epithelial sheet designated by a white triangle. The orange line denotes the apical-basal plane of the X-Y slice, shown in the lower panels. Within the lower panels, the orange line shows the plane of the above X-Z image. (I) Apically imaged notum – apical slice. The top X-Z view shows precise imaging of the apical epithelial sheet (white triangle). This view is equivalent to a live imaging view. (J) Apically imaged notum – basal slice, partially obstructed by apical tissue. Other tissues denoted by yellow arrow (possible muscle band) and white arrows (possible blood cells) are visible on the basal side. (K) Basally imaged notum – apical slice, partially obstructed by basal tissue and wound scab (dark central area). (L) Basally imaged notum – basal slice. This view best shows the basal tissues denoted with yellow and white arrows. A,E: DAPI stain, B,F: Histone-EGFP, C,G: FasIII staining. The images were captured with 20x objective with spinning disc microscopy. Z-slices were taken every 0.9 µm. A-D shows the maximum intensity projection of 19 slices. E-H represents the maximum intensity projection of 17 slices. 25 µm scale bars in D,H,L apply to A-D, E-H, I-L, respectively. Please click here to view a larger version of this figure.
Supplementary File 1: Preparation of solutions and buffers. Recipes for the following solutions are detailed: 1xPBS, 1x PBST, blocking solution, and paraformaldehyde fixative. Please click here to download this File.
Discussion
Critical steps
Optimizing three steps will dramatically increase the success of this protocol. First, in step 1.5, be sparing with the adhesive glue applied to the coverslip. If too much adhesive glue is added, the pupae can become entombed in a thick layer of solidified adhesive glue, which will make dissection impossible, and if it covers the notum itself, the adhesive glue will occlude the light from the sample. Second, during steps 2.3-2.6, ensure to remove only the top dome of the notum, excluding as much of the lateral tissue as possible. If included, the lateral tissue will become compressed during mounting and cause the middle of the notum to buckle inward, often placing it outside of the working distance of high numerical aperture objectives. Third, during the cleaning step 2.9, extreme care must be taken not to damage the monolayer epithelium. If the tissue has large portions missing or no signal can be detected, this step is likely to blame.
Troubleshooting
Issue 1: Following mounting, the pupa comes away from the double-sided tape during dissection. This is a common issue, especially for beginners. The best remedy is to ensure that the outside of the pupae case is completely dry/free of food debris. Removing food with a pair of blunt forceps and allowing the case to air dry for 10-15 min will help adhere to the tape. Alternatively, use the non-dominant hand and a pair of blunt forceps to hold the pupae down against the tape during dissection. If the problem persists, applying a small, 5-10 µL drop of nail polish to the base of the posterior end of the case and allowing it to harden generally provides enough adhesion for even the unruliest of pupae.
Issue 2: Notum collapses during step 2.3, or the initial breach through the integument is not smooth. If the integument is difficult to breach, there may be too much adhesive glue from the immobilization steps. Placing the dissected pupae into less adhesive glue will improve this issue. Further, ensure that the microdissection scissors are sharp. Blunt scissors will not be able to cut into the integument and tend to cause it to collapse inwards. Once hemolymph spills out of the pupae, ensure one of the cutting blades can enter the pupa without causing the notum to deform. If the notum collapses and the blade does not enter the pupa, continue snipping until a blade enters the pupae.
Issue 3: During step 2.4, scissors catch or drag the integument. If the scissors begin to catch or drag the integument, it often helps to switch to the opposite side of the pupae and proceed to 'loosen' the integument. Further blunt microdissection scissors will make it difficult to achieve clean cuts through the integument, and sharpened scissors must be used.
Issue 4: The sample is accidentally aspirated during staining (steps 3.1-3.6). The dissected notum is challenging to see because it is transparent. It can be helpful to place a dark blue or black sheet below the coverslip to provide contrast (an old pipette tip holder rack works well.) Additionally, all solution changes can be performed under a dissection microscope.
Issue 5: No signal is detected/patchy signal is detected. After ruling out stain-specific problems, if no signal is detected or it is patchy, step 2.9 (cleaning) is likely the culprit. An absent or patchy signal can originate from damage and removal of the epithelial tissue during cleaning. Conversely, a poor signal can be caused by occlusion from the muscle bands/fat body cells if they are not removed, as they can limit the diffusion of stains and antibodies into the notum relative to the surrounding tissue. If the tissue is damaged, being gentler during cleaning is the best solution. If, instead, the stain is visible but patchy, increasing the vigor/time dedicated to the cleaning step is recommended to remove as much of the muscle and fat body as possible. Further, increasing the stain duration can help resolve this problem with better cleaning.
Issue 6: The notum tissue has a warped/wrinkled appearance during imaging. Warping and wrinkling of the tissue come from two sources. First, compressing the notum during mounting will cause it to buckle and warp. The best solution is to remove as much of the lateral tissues as possible so the dome is as short as possible and can fit between the coverslip spacers. Second, if the notum is bent during dissection, this bend will not straighten out during mounting, so extra care must be taken not to warp the notum during dissection. Accidental bending of the notum is most common when cutting the integument away from the rest of the pupae. It is tempting to have the dissection scissors at an angle relative to the pupae instead of keeping them in the sample plane as the pupae. However, angled scissors cause the integument to buckle upwards when cut instead of remaining flat.
Existing methods, limitations, and future applications
Wang et al.20 reported a comparable dissection protocol for the isolation of pupal epithelium. This technique requires that the pupa remains within its case and be rapidly bisected with a scalpel. This protocol is incompatible with previously live-imaged samples, as live imaging requires removing a large section of the pupal case. Because pupae lack rigidity, pupal bisection outside of the case mangled the tissue, inspiring the creation of this protocol. The technique detailed here allows for isolation and fixation of the notum, and it could be used as the first step for a wide array of other methods such as cryosectioning, in situ hybridization, or electron microscopy.
This technique has some limitations. First, dissecting, fixing, and staining the notum is more time-consuming than live-imaging fluorescently tagged proteins in the notum, which requires only a simple dissection to remove the pupal case9,23. Secondly, compared to dissections of other Drosophila tissues, this dissection is more difficult because of the thin, fragile tissue and hydrophobic cuticle. For simply visualizing proteins in Drosophila epithelia, immunohistochemistry on fixed embryos, larval wing discs, or ovaries is easier. However, this technique allows the power of live imaging to be paired with fixation and staining, making it a powerful tool once mastered.
A dissection/fixation technique has some advantages over live imaging. Basal (interior) structures can be better resolved with a basal view (Figure 5L,J). Most importantly, live imaging is limited to fluorophores that must be genetically supplied, often requiring lengthy genetic crossing schemes. In contrast, the present protocol allows the application of stains, immunohistochemistry, and other techniques which require dissection and fixation. This dramatically increases the number of signals probed in the tissue while potentially decreasing the time to experimental results.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Dr. M. Shane Hutson for establishing the laser ablation system used for pupal wounding and his contributions to and feedback on developing the protocol. This work was supported by 1R01GM130130 to APM and M. Shane Hutson. JW was supported by 2T32HD007502-21.
Materials
| ½” Scotch Permanent Double-Sided tape | Scotch 3M | 665 | |
| 0.1-10 µL uTIP pipette tip | Biotix | M-0011-9FC | |
| 22 x 22 mm No. 0 thickness coverslips | Thomas Scientific | 1207Z28 | |
| 24 x 60 mm coverslip | Corning | 2980-246 | |
| 3M VetBond | 3M | 1469SB | Called "adhesive glue" in protocol |
| Anti-FasIII primary Mouse IgG2a | Developmental Studies Hybridoma Bank | 7G10 | |
| Calcium Chloride | Fisher Chemical | c79-500 | |
| Cy3-conjugated AffiniPure Goat Anti-Mouse IgG2a | Jackson ImmunoResearch | 115-165-206 | |
| DAPI | Sigma Aldrich | D9542-1mg | |
| Dumont #5 Fine Tip Forceps | Fine Science Tools | No. 11254-20 | |
| Dumont #5 Fine Tip Forceps: Blunt | Fine Science Tools | No. 11254-20 | Heavily used and unsharpened forceps, or dulled with a whetstone |
| Fisherbrand Double frosted microscope slides | Fisher Scientific | 22-034-486 | |
| Fluorescent Light Source | Lumencor | Celesta 90-10512 | |
| Fly Stock: EGFP-tagged Histone H2A | Bloomington Drosophila Stock Center | 24163 | |
| Fly Vial Foam Plugs "Flugs" | Genesee Scientific | 49-101 | |
| Humidified Chamber: Foil-wrapped small container lined with filter paper saturated with water | Cole-Parmer | 759075D | A great humidified chamber can be made from the styrofoam box containing Cole-Parmer cuvettes, 759075D, filled with 50 ml water. |
| KimWipe | KimTech | 34155 | Called "Absorbent Tissue" in protocol |
| Nikon Ti2 Eclipse | Nikon | Eclipse Ti2-E | |
| NIS Elements Software | Nikon | AR | |
| Parafilm | Pechiney Plastic Packaging | PM-996 | |
| Paraformaldehyde (PFA) 16% | Ted Pella, Inc | 18505 | |
| Plastic Vials | Genesee Scientific | 32-114 | |
| Sodium Azide (NaN3) | Fisher Scientific | 19038-1000 | |
| Stereo Microdissection Scope | Carl Zeiss | STEMI 2000 | |
| Vannas Spring Scissors | Fine Science Tools | 15000-00 | New or freshly sharpened scissors |
| Vecta Shield | Vector Laboratories | H-1000 | Called " antifade mounting medium" in protocol |
| Vecta Shield with DAPI | Vector Laboratories | H-1200 | Not ideal for pupal notum. |
| X-Light V2 Spinning Disc | Crest Optics | V2 L-FOV |
References
- Valon, L., et al. Robustness of epithelial sealing is an emerging property of local ERK feedback driven by cell elimination. Developmental Cell. 56 (12), 1700-1711 (2021).
- Levayer, R., Dupont, C., Moreno, E. Tissue crowding induces caspase-dependent competition for space. Current Biology. 26 (5), 670-677 (2016).
- Couturier, L., et al. Regulation of cortical stability by RhoGEF3 in mitotic sensory organ precursor cells in Drosophila. Biology Open. 6 (12), 1851-1860 (2017).
- Couturier, L., Mazouni, K., Corson, F., Schweisguth, F. Regulation of Notch output dynamics via specific E(spl)-HLH factors during bristle patterning in Drosophila. Nature Communications. 10, 3486 (2019).
- Fujisawa, Y., Shinoda, N., Chihara, T., Miura, M. ROS regulate caspase-dependent cell delamination without apoptosis in the drosophila pupal notum. iScience. 23, 101413 (2020).
- Besson, C., et al. Planar cell polarity breaks the symmetry of par protein distribution prior to mitosis in drosophila sensory organ precursor cells. Current Biology. 25 (8), 1104-1110 (2015).
- Koto, A., Kuranaga, E., Miura, M. Apoptosis ensures spacing pattern formation of drosophila sensory organs. Current Biology. 21, 278-287 (2011).
- Bainbridge, S. P., Bownes, M. Staging the metamorphosis of Drosophila melanogaster. Journal of Embryology and Experimental Morphology. 66, 57-80 (1981).
- Moreira, C., Regan, J., Zaidman-Rémy, A., Jacinto, A., Prag, S. Drosophila hemocyte migration: an in vivo assay for directional cell migration. Methods in Molecular Biology. 769, 249-260 (2011).
- Guirao, B., et al. Unified quantitative characterization of epithelial tissue development. Elife. 4, 08519 (2015).
- Cristo, I., Carvalho, L., Ponte, S., Jacinto, A. Novel role for Grainy head in the regulation of cytoskeletal and junctional dynamics during epithelial repair. Journal of Cell Science. 131 (17), 213595 (2018).
- Bellaïche, Y., Gho, M., Kaltschmidt, J. A., Brand, A. H., Schweisguth, F. Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nature Cell Biology. 3 (1), 50-57 (2001).
- Shannon, E. K. Multiple mechanisms drive calcium signal dynamics around laser-induced epithelial wounds. Biophysical Journal. 113 (7), 1623-1635 (2017).
- O’Connor, J. T., et al. Proteolytic activation of Growth-blocking peptides triggers calcium responses through the GPCR Mthl10 during epithelial wound detection. Developmental Cell. 56 (15), 2160-2175 (2021).
- Hartenstein, V., Posakony, J. W. Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development. 107 (2), 389-405 (1989).
- Yeh, E., Zhou, L., Rudzik, N., Boulianne, G. L. Neuralized functions cell autonomously to regulate Drosophila sense organ development. The EMBO Journal. 19 (17), 4827-4837 (2000).
- Loubéry, S., et al. Uninflatable and notch control the targeting of sara endosomes during asymmetric division. Current Biology. 24 (18), 2142-2148 (2014).
- Kawamori, A., Shimaji, K., Yamaguchi, M. Dynamics of endoreplication during Drosophila posterior scutellar macrochaete development. PLoS One. 7 (6), 38714 (2012).
- Couturier, L., Schweisguth, F., Bellen, H. J., Yamamoto, S. . Notch Signaling: Methods and Protocols. , 79-86 (2014).
- Wang, W., Yoder, J. H. Drosophila pupal abdomen immunohistochemistry. Journal of Visualized Experiments. (56), e3139 (2011).
- Kiehart, D. P., et al., Celis, J. E., et al. . Cell Biology (Third Edition). , 87-103 (2006).
- Bailey, E. C., Dehn, A. S., Gjelsvik, K. J., Besen-McNally, R., Losick, V. P. A Drosophila model to study wound-induced polyploidization. Journal of Visualized Experiments. (160), e61252 (2020).
- O’Connor, J. T., S, E. K., Hutson, M. S., Page-McCaw, A. Mounting Drosophila pupae for laser ablation and live imaging of the dorsal thorax. STAR Protocols. , (2022).

