A 3D Culture Method of Spheroids of Embryonic and Liver Zebrafish Cell Lines
Summary
Here, we present an effective, easy, and fast 3D culture protocol for the formation of spheroids of two zebrafish (Danio rerio) cell lines: ZEM2S (embryo) and ZFL (normal hepatocyte).
Abstract
Fish cell lines are promising in vitro models for ecotoxicity assessment; however, conventional monolayer culture systems (2D culture) have well-known limitations (e.g., culture longevity and maintenance of some in vivo cellular functions). Thus, 3D cultures, such as spheroids, have been proposed, since these models can reproduce tissue-like structures, better recapturing the in vivo conditions. This article describes an effective, easy, and fast 3D culture protocol for the formation of spheroids with two zebrafish (Danio rerio) cell lines: ZEM2S (embryo) and ZFL (normal hepatocyte). The protocol consists of plating the cells in a round-bottom, ultra-low attachment, 96-well plate. After 5 days under orbital shaking (70 rpm), a single spheroid per well is formed. The formed spheroids present stable size and shape, and this method avoids the formation of multiple spheroids in a well; thus, it is not necessary to handpick spheroids of similar sizes. The ease, speed, and reproducibility of this spheroid method make it useful for high-throughput in vitro tests.
Introduction
Spheroids are small spheres of cells formed when cells are cultured in close cell-to-cell contact in 3D culture. The capacity of spheroids to mimic the in vivo tissue environment has already been studied in a variety of cell lines and primary cells1,2. However, although spheroids are well developed for mammalian toxicity studies, the development of spheroids for toxicity studies with non-mammalian vertebrates (e.g., fish) is still in progress3. For fish cell lines, spheroids have been developed by a variety of different methods, such as orbital shaking (OS) using different types of well-plates3,4,5,6,7, and the method of magnetic levitation using magnetic nanoparticles8. However, some of these culture methods for spheroids may have more disadvantages than others.
For example, gyratory methods in large microplates (24-well plates) may generate a high number of spheroids differing in size and shape; indeed, multi-spheroid structure formation has been demonstrated7. This requires intense efforts to handpick spheroids with a similar size and shape for an experiment. The hanging drop 3D culture method is commonly used for generating spheroids of mammalian cell lines1,2,9,10,11, whereby single spheroids per drop can be generated, avoiding the problems described above. However, although a modified hanging drop method (hanging drop + orbital shaking) is able to generate ZFL spheroids using an inexpensive method, it has its disadvantages12. The cellular aggregates formed cannot be maintained for long periods in the drops; thus, they need to be transferred to well plates. This process requires intense handling and long periods of work in a laminar flow hood, since it is performed dropwise using a micropipette12. In addition, this method requires 10 days to fully form the ZFL spheroids (5 days in hanging drop + 5 days in OS)12. These disadvantages can limit the application of 3D fish spheroids for toxicity testing, especially considering potential applications for chemical prioritization and product sustainability.
Thus, this article describes a 3D culture protocol able to generate single spheroids of ZFL (D. rerio normal hepatocyte) and ZEM2S (D. rerio blastula phase embryo) cell lines based on the combined use of 96-well, ultra-low attachment plates (ULA-plates) and an orbital shaker (22 mm rotational diameter). The method applied is simple and reproducible, and can generate high numbers of spheroids of similar size and shape in a short period (5 days). The advantages of this method can support the application of fish 3D models for aquatic toxicity studies in both industry and academia, as well as the progress of implementing alternative methods for ecotoxicity testing.
Protocol
The key steps to generate 3D spheroids of ZFL and ZEM2S cell lines in a round-bottom 96-well plate are presented in Figure 1.
NOTE: See the Table of Materials for details related to all materials used in this protocol and Table 1 for solutions and culture media used in this protocol.
1. Cell culture medium and monolayer cultures
- Grow both cell lines (ZFL, ZEM2S) as monolayers in an incubator at 28 °C without CO2, and subculture them at a subcultivation ratio of 1:3 when they reach ~80% confluence.
- Start with a T75 flask of zebrafish cells at ~80% confluence, cultured as described above.
- Remove the complete medium and wash the cells by adding 1x phosphate-buffered saline (PBS) (0.01 M) to the culture flask with the aid of a pipette.
- With the aid of a pipette, add 3 mL of 1x trypsin-0.5 mM EDTA (0.05% [v/v]) to the culture flasks, and incubate at 28 °C for 3 min for the detachment of cells from the flask.
- Gently tap the flask to release the cells, and then stop the trypsin digestion by adding 3 mL of complete culture medium to the flask.
- Using a pipette, transfer the cell suspension to a 15 mL conical centrifuge tube, and centrifuge at 100 × g for 5 min.
- After the pellet formation, carefully remove the supernatant, add 1 mL of the complete medium for the respective cell line in use (ZFL or ZEM2S), and resuspend using a micropipette. Take an aliquot for cell counting.
2. Cell counting with trypan blue dye exclusion test
- Add 10 µL of the cell suspension and 10 µL of trypan blue dye to a microtube to count the cells and evaluate their viability. Mix the cell suspension and dye using a pipette.
- Then, transfer 10 µL of this mixture (cell suspension + trypan blue) to a Neubauer chamber and count the cells in the four large squares (quadrants: Q) placed at the corners of the chamber, considering cells that do not take up trypan blue as viable. Calculate the number of viable cells using equation (1):
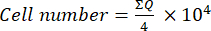 (1)
(1) - To calculate the final cell number in the cell suspension, multiply the cell number determined using equation (1) by 2 (the dilution factor due to the use of trypan blue).
NOTE: Alternatively, an automated cell counting system can be used.
3. Cell plating in ULA-plates
- After calculating the cell number, adjust the cell suspension to plate 200 µL of this suspension per well of a 96-well round-bottom ULA-plate with the number of cells required for each cell line, as indicated below:
- Plate 7,000 viable ZFL cells/well; thus, for the entire ULA-plate, use 700,000 cells in 20 mL of complete medium.
- Plate 3,500 viable ZEM2S cells/well; thus, for the entire ULA-plate, use 350,000 cells in 20 mL of complete medium.
- Prepare the cell suspension with the adjusted concentration of cells in a medium reservoir and mix it using a multichannel micropipette, taking care not to form foam or bubbles. Using the multichannel micropipette, add 200 µL of the adjusted cell suspension to each well of the ULA-plate.
NOTE: The plate must be sealed with parafilm or adhesive sealing foil to avoid culture medium evaporation from the 96-well plate.
4. Spheroid formation
- Incubate the ULA-plate on an orbital shaker at 70 rpm for 5 days in a 28 °C incubator. Allow the spheroids to form over 5 days of orbital shaking (Figure 2), reaching an average size of ~225 µm in diameter (ZFL spheroids) and ~226 µm in diameter (ZEM2S spheroids)12.
NOTE: After 5 days of incubation (maximum circularity), the spheroids are ready to be used. - To maintain the spheroids in culture for more than 5 days, remove 100 µL of the spent medium every 5 days, and add 100 µL of fresh complete culture medium using a multichannel micropipette.
NOTE: Take care not to aspirate the spheroids during this process.
5. Measuring size (diameter) and shape (circularity index) of spheroids
- Acquire the images.
- Under an inverted light microscope with an imaging capture system, obtain an image of a defined scale.
NOTE: Use a microscope stage calibration slide or a Neubauer slide (in which the quadrant sizes are known) to obtain the scale. - Under the microscope and using the same objective lens used to obtain the scale's picture, obtain images of the fully formed spheroids (i.e., 5-day-old spheroids).
NOTE: All images must be taken using the same imaging capture system, as image resolution is important to determine the spheroids' size and shape, and it may differ among types of systems.
- Under an inverted light microscope with an imaging capture system, obtain an image of a defined scale.
- Set the scale.
- Using ImageJ software, open the image of the defined scale (click File | open).
- Select the straight-line selector from the toolbar and, using the mouse, drag out a line across the extension of the defined scale in the image.
- Set the scale by selecting Analyze | Set scale, and wait for the Set Scale window to open.
- In the Set Scale window, fill the blank of Known distance with the known distance (µm) corresponding to the straight line; fill the Unit of length with um for µm. Click OK.
NOTE: The scale in pixels/µm is displayed at the bottom of the window.
- Set the measurement parameters.
- In the ImageJ software, select Analyze | Set measurements to open the Set measurements window.
- In the Set measurements window, select the boxes for the desired measurements (i.e., Area and Shape descriptors). Click OK.
- Obtain the spheroids' diameter and circularity.
- Open the picture of a spheroid (File | Open).
- Select the freehand selection tool in the toolbar and, using the mouse, select the outer side of the spheroid, as demonstrated in Figure 3A.
NOTE: To zoom in or out on the image, press the Ctrl key and use the mouse to scroll down or up, or press the Ctrl key and use the up or down arrow keys on the keyboard. - Select Analyze | Measure to open the Results window, in which the measured values are displayed.
- Using the Area value, calculate the spheroids' size (diameter) using equation (2):
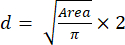 (2)
(2) - The circularity index is given in the Results window as "Circ.", and is automatically calculated by the software using equation (3):
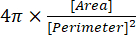 (3)
(3)
NOTE: The circularity index of 1.0 represents a perfect spheroidal shape, while a value close to 0.0 indicates an elongated shape13.
Representative Results
Single spheroids per well with a stable size and shape are formed by this method. Figure 2 illustrates the formation process of single spheroids of ZFL and ZEM2S cells in a well of a ULA-plate under orbital shaking (70 rpm). The ZFL and ZEM2S cell lines have different behaviors in 3D culture. The ZEM2S cell line presents features that confer the ability to readily form a spheroidal shape since the first day of the orbital shaking (Figure 2E), while the ZFL cell line requires 5 days to reach the desirable spheroidal shape (Figure 2A–C). The spheroids generated by this method also showed stability in terms of shape in longer periods of culture (periods >5 days), as demonstrated in Figure 2D (16-day-old ZFL spheroid) and Figure 2H (10-day-old-ZEM2S spheroid). The spheroids' diameter and circularity were determined by measuring their projected area in the obtained images using software for processing and analyzing scientific images13 (Figure 3). The Table of Materials indicates the software used for image processing and analysis. ZFL cells tend to form large cell aggregates (average size 319 ± 23.33 µm) on the first day of orbital shaking, which decreases over time until day 5 (225.62 ± 19.20 µm) (Figure 4A), enhancing the circularity over time in culture (Figure 4B). Unlike ZFL cells, the ZEM2S cell line forms small spheroids from the first day (202.64 ± 5.78 µm), which progressively increase until day 5 (226.63 ± 4.80 µm) (Figure 4C). Figure 4E shows the different growth patterns in the ZFL and ZEM2S cell lines in 3D culture. We recommend using the ZFL and ZEM2S spheroids on the fifth day because they reach a higher spheroidal shape (circularity index of 0.80 ± 0.01 and 0.83 ± 0.00, respectively) (Figure 4B,D), which indicates a greater stability of this 3D model.
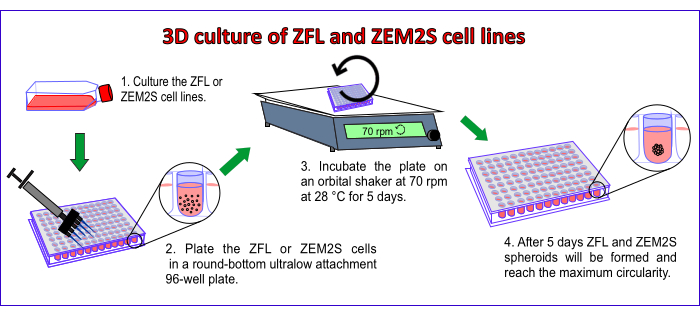
Figure 1: The key steps to generate 3D spheroids of ZFL and ZEM2S cell lines in a round-bottom 96-well plate. Please click here to view a larger version of this figure.
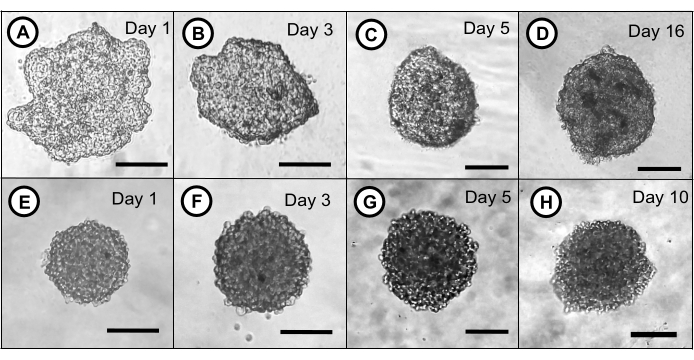
Figure 2: Single spheroids of ZFL and ZEM2S cell lines formed in round-bottom 96-well ULA-plates under orbital shaking. A ZFL cell aggregate is formed on the first day of OS (A). The cell aggregate increases its circularity on the third day (B) and reaches an adequate spheroidal shape on day 5 (C). (D) A 16-day-old ZFL spheroid in ULA-plate. The ZEM2S cell line forms spheroids from the first day of OS (E). The ZEM2S spheroid maintains its shape and increases in size on the third day (F), and its maximum circularity is reached on day 5 (G). (H) A 10-day-old ZEM2S spheroid in a ULA-plate. Scale bars = 100 µm. Abbreviations: OS = orbital shaking; ULA = ultra-low attachment. Please click here to view a larger version of this figure.
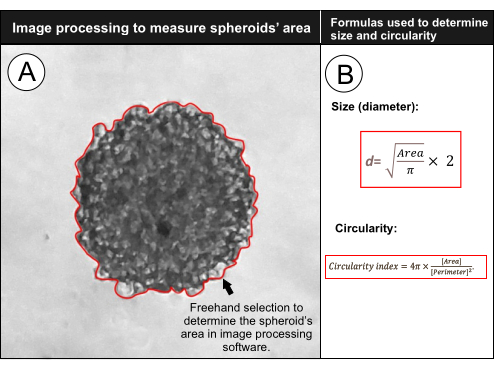
Figure 3: Determining spheroids' size and circularity using software for processing and analyzing scientific images. After setting the software to measure a selected area based on a known scale, select the outer side of the spheroid to determine its area and circularity (A). The spheroid's area is used to determine its size by calculating the diameter d, and the software provides the spheroid's circularity by a formula that uses the area and perimeter selected (B). Please click here to view a larger version of this figure.
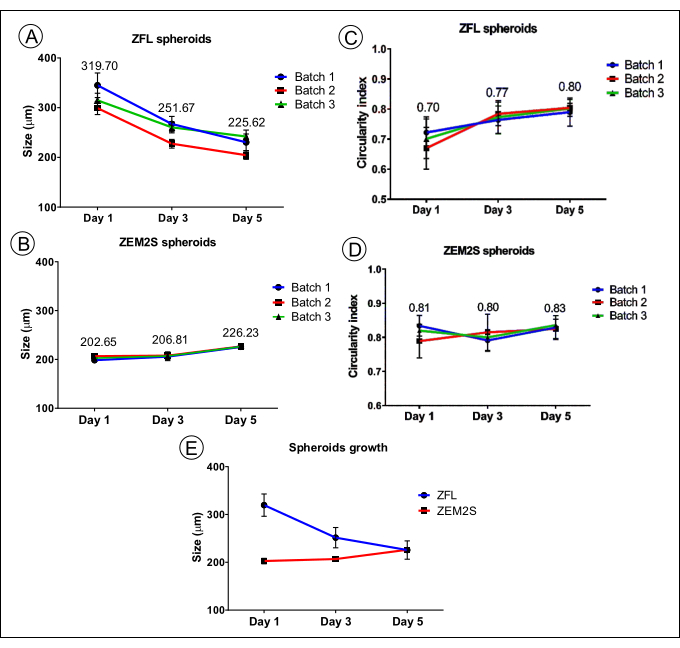
Figure 4: Reproducibility of the 3D spheroid method showed by uniform-sized spheroids (diameter) and circularity (spheroidal shape). The measured size of spheroids of the ZFL (A) and ZEM2S (B) cell lines. The measured circularity index of ZFL (C) and ZEM2S (D) spheroids. The growth pattern of ZFL and ZEM2S spheroids (E). Data are representative of three technical replicates from different cell batches. The numbers above each point indicate the mean of the triplicates for days 1, 3, and 5. Data showed as mean ± standard deviation (n = 10). This figure has been modified from Souza et al.12. Please click here to view a larger version of this figure.
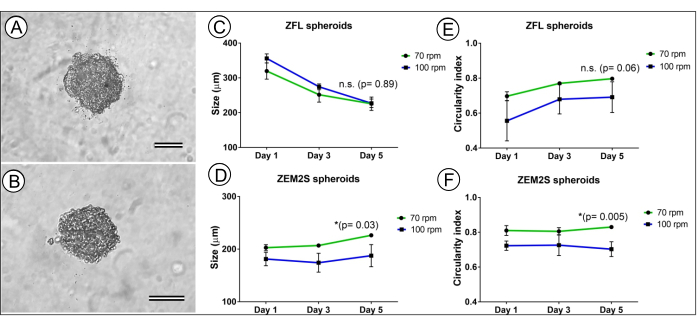
Figure 5: Comparison between the formation of ZFL and ZEM2S spheroids performed at two rotational speeds (70 and 100 rpm). A ZFL spheroid (A) and ZEM2S spheroid (B) formed after 5 days of orbital shaking at 100 rpm (scale bars represent 100 µm). The growth pattern of ZFL spheroids (C) and ZEM2S spheroids (D) formed at 70 and 100 rpm. The circularity index of ZFL spheroids (E) and ZEM2S spheroids (F) formed at 70 and 100 rpm. Data are shown as mean ± standard deviation (n = 10). *indicates statistical significance (p < 0.05). n.s.: non-significant difference (t-test) between spheroids formed on day 5 at 70 and 100 rpm. This figure has been modified from Souza et al.12. Please click here to view a larger version of this figure.
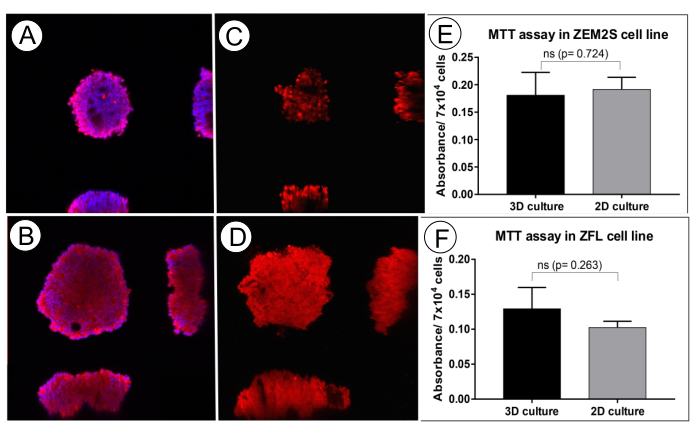
Figure 6: The cellular integrity and viability of ZFL and ZEM2S spheroids (5 days old) formed by orbital shaking in round-bottom ultra-low attachment well-plate. Staining of cell membranes by Lectin Alexa Fluor 594 (red) and nuclei by DAPI (blue) demonstrate the cellular integrity in the core of a ZEM2S spheroid (A) and ZFL spheroid (B). Fluorescence of resorufin (red) formed by a reduction of Alamarblue demonstrates viable cells in the core of both the ZEM2S (C) and ZFL (D) spheroids. The images represent orthogonal planes captured using a confocal microscope. MTT viability assay in 3D culture (spheroids) and 2D culture of the ZEM2S (E) and ZFL cell line (F). Data showed as mean of absorbance measured at 570 nm ± standard deviation. ns: non-significant difference by Welch's t-test. This figure has been modified from Souza et al. (2021)12. Please click here to view a larger version of this figure.
Table 1: Solutions for ZFL and ZEM2S cultivation. Please click here to download this Table.
Table 2: Comparison of the 3D spheroid methods used to form single ZFL spheroids. * The best result for a tested parameter. a The selected parameter from the best results verified in the conventional hanging drop method. b The selected parameter from Baron et al.3 for forming a fish spheroid with orbital shaking. c Variations equally suitable to a tested parameter. Abbreviations: HD = hanging drop; OS = orbital shaking; ULA = ultra-low attachment; poly-HEMA = poly(2-hydroxyethyl methacrylate). This table has been modified from Souza et al.12. Please click here to download this Table.
Discussion
This is a simple, easy, and fast method for generating spheroids of zebrafish liver and embryo cell lines. This method was developed by this group based on modifications of existing 3D spheroid methods to overcome problems reported in scientific studies related to spheroid formation, as well as uncertainties in data accuracy from 3D spheroid assays. For instance, the problems reported lie in difficulties of handling, the time-consuming nature of generating spheroids, the necessity of selecting spheroids of a similar size and shape to perform an assay, as well as difficulties in the process of transferring spheroids from the drops to microplates in the hanging drop method3,7,12. Further, this protocol recommends the culture of ZFL and ZEM2S cell lines in a CO2-free condition. The culture medium composition and the CO2-free environment proposed in this protocol for both cell lines are widely reported in the literature. The ZFL cell line is usually cultured in L-15 and RPMI media with or without the addition of sodium bicarbonate and without CO214,15,16,17,18,19,20. The ZEM2S cell line is cultured according to instructions of the bioresource center, and its culture media was formulated for CO2-free cultures; thus, CO2 and air mixture can be detrimental to cells when using this type of culture media21.
The present fish spheroid protocol was developed after evaluating several parameters (i.e., different cell densities, speed of orbital shaking [70 or 100 rpm], and the use of different 96-well plates [flat bottom or round-bottom]) to determine the best parameters to form ZFL spheroids. Additionally, a comparison with other 3D culture methods (i.e., hanging drop method and hanging drop method combined with orbital shaking method) was made, indicating that the orbital shaking of round-bottom ULA-plates had the best performance (i.e., easy, fast, and reproducible method). Table 2 shows the parameters and performance of other 3D culture methods evaluated to form ZFL spheroids.
Round-bottom ULA-plates have already been reported to form single spheroids of human cells followed by centrifugation22 or orbital shaking23,24. In this protocol, we are proposing the use of the same plate, except under orbital shaking to generate single spheroids of zebrafish cell lines (i.e., one spheroid per well of a 96-well plate). This is particularly advantageous because it can easily and rapidly form a good quantity of spheroids, and the formed spheroids can be maintained in culture on the same plate. Thus, chemical exposure can be done directly on the ULA-plate to perform different types of toxicity assays.
In order to determine the adequate orbital shaking speed to form ZFL and ZEM2S spheroids, we compared the spheroids' formation at 70 and 100 rpm. The results show that this method performs better at 70 rpm, and a higher rotational speed can significantly impact ZEM2S spheroids' size and shape (Figure 5D,F). ZFL spheroids did not show significant differences in size and shape at these rotational speeds; however, lower circularity indexes were obtained at 100 rpm, showing a negative impact in spheroidal shape at a higher rotational speed (Figure 5C,E).
The initial cell density of 3,500 ZEM2S cells and 7,000 ZFL cells generate spheroids with similar sizes on the fifth day in culture (226.63 and 225.62 µm, respectively). Given that oxygen and nutrients are transported by diffusion in spheroids25, their diameter may strongly influence the nutrition and cellular integrity in spheroids' core; generally, spheroids up to 100 µm are recommended to avoid necrotic core26. To verify the cellular integrity in the spheroids' core, we evaluated the nuclear and membrane integrity by immunostaining with DAPI and Lectin Alexa Fluor 594 by confocal microscopy (Figure 6A,B), and the spheroids' integrity was demonstrated. The cellular viability in the spheroids' core was also verified by exposing them to resazurin (Alamarblue) and analyzing the fluorescence of resorufin by a confocal microscope (Figure 6C,D). When the cells are viable, the resazurin is reduced to resorufin (fluorescent substance) by cellular metabolic function. The performance of the MTT assay also demonstrated no significant difference in cell viability comparing ZFL and ZEM2S spheroids with monolayer culture (2D culture) (Figure 6E,F). Although the spheroids are greater than 100 µm, the results show that this protocol generates viable spheroids of ZFL and ZEM2S cell lines with adequate nutrition even in their inner portion.
The cell densities of ZFL and ZEM2S cells and the speed of orbital shaking applied in this protocol allowed the formation of very stable spheroids in size and shape, with low variability among the wells (Figure 4), avoiding the need to select adequate spheroids as a previous step of performing an assay. If the assay procedure requires transferring the spheroids to other plates or tube/microtubes, they can be easily transferred with a micropipette without disaggregation problems.
Here, we demonstrated a protocol developed based on the best parameters to form viable ZFL and ZEM2S spheroids. The ZFL and ZEM2S cell lines may be useful in aquatic toxicity testing to estimate chemical effects on fish23,24,27,28,29,30. Additionally, developmental endpoints may also be studied using fish embryo cell lines, such as the ZEM2S cell line (fibroblast cells of the blastula phase), which retain a degree of pluripotency28. These make these zebrafish spheroids potentially useful for fish toxicity testing. Furthermore, as an easy fish spheroid method, it can be used in high-throughput ecotoxicity testing, supporting its application in industry and academia.
Disclosures
The authors have nothing to disclose.
Acknowledgements
In memory of Dr. Márcio Lorencini, a coauthor of this work, an excellent researcher in the field of cosmetics and devoted to promoting cosmetic research in Brazil. The authors are grateful to the Multi-user Laboratory in the Physiology Department (UFPR) for equipment availability and for the financial support of the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil) (Finance Code 001) and the Grupo Boticário.
Materials
| 96-well Clear Round Bottom Ultra-Low Attachment Microplate, Individually Wrapped, with Lid, Sterile | Corning | 7007 | |
| DMEM, powder, high glucose, pyruvate | Gibco | 12800-017 | |
| Ham's F-12 Nutrient Mix, powder | Gibco | 21700026 | |
| HEPES (1M) | Gibco | 15630080 | |
| Image Processing and analysis in Java (ImageJ) 1.52p software | National Institutes of Health, USA |
Available at: https://imagej.nih.gov/ij/index.html | |
| Leibovitz's L-15 Medium, powder | Gibco | 41300021 | |
| Orbital shaker | Warmnest | KLD-350-BI | 22 mm rotation diameter |
| Dulbeccos PBS (10x) with calcium and magnesium | Invitrogen | 14080055 | |
| Penicillin-Streptomycin (10,000 U/mL) | Gibco | 15140122 | |
| RPMI 1640 Medium | Gibco | 31800-014 | |
| FBS – Fetal Bovine Serum, qualified, USDA-approved regions | Gibco | 12657-029 | |
| Sodium bicarbonate, powder, bioreagent for molecular biology | Sigma-Aldrich | S5761 | |
| Trypan blue stain (0,4%) | Gibco | 15250-061 | |
| Trypsin-EDTA (0.5%), no phenol red | Gibco | 15400054 | |
| ZEM2S cell line | ATCC | CRL-2147 | This cell line was kindly donated by Professor Dr. Michael J. Carvan (University of Wisconsin, Milwaukee, USA) |
| ZFL cell line | BCRJ | 256 |
References
- Elje, E., et al. The comet assay applied to HepG2 liver spheroids. Mutation Research. Genetic Toxicology and Environmental Mutagenesis. 845, 403033 (2019).
- Kelm, J. M., Timmins, N. E., Brown, C. J., Fussenegger, M., Nielsen, N. K. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnology and Bioengineering. 83 (2), 173-180 (2003).
- Baron, M. G., Purcell, W. M., Jackson, S. K., Owen, S. F., Jha, A. N. Towards a more representative in vitro method for fish ecotoxicology: morphological and biochemical characterisation of three-dimensional spheroidal hepatocytes. Ecotoxicology. 21 (8), 2419-2429 (2012).
- Alves, R. F., Rocha, E., Madureira, T. V. Fish hepatocyte spheroids – A powerful (though underexplored) alternative in vitro model to study hepatotoxicity. Comparative Biochemistry and Physiology. Toxicology & Pharmacology. 262, 109470 (2022).
- Baron, M. G., et al. Pharmaceutical metabolism in fish: using a 3-D hepatic in vitro model to assess clearance. PloS One. 12 (1), 0168837 (2017).
- Langan, L. M., et al. Spheroid size does not impact metabolism of the β-blocker propranolol in 3D intestinal fish model. Frontiers in Pharmacology. 9, 947 (2018).
- Lammel, T., Tsoukatou, G., Jellinek, J., Sturve, J. Development of three-dimensional (3D) spheroid cultures of the continuous rainbow trout liver cell line RTL-W1. Ecotoxicology and Environmental Safety. 167, 250-258 (2019).
- Jeong, Y., et al. Differential effects of CBZ-induced catalysis and cytochrome gene expression in three dimensional zebrafish liver cellculture. Journal of Environmental and Analytical Toxicology. 6, 2161 (2016).
- Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. Journal of Visualized Experiments. (51), e2720 (2011).
- Lee, W. G., Ortmann, D., Hancock, M. J., Bae, H., Khademhosseini, A. A hollow sphere soft lithography approach for long-term hanging drop methods. Tissue Engineering. Part C, Methods. 16 (2), 249-259 (2010).
- Timmins, N. E., Nielsen, L. K. Generation of multicellular tumor spheroids by the hanging-drop method. Methods in Molecular Medicine. 140, 141-151 (2007).
- de Souza, I. R., et al. Development of 3D cultures of zebrafish liver and embryo cell lines: a comparison of different spheroid formation methods. Ecotoxicology. 30 (9), 1893-1909 (2021).
- Ferreira, T., Rasband, W. ImageJ user guide. ImageJ/Fiji. 1, 151-161 (2012).
- Guidony, N. S., et al. ABC proteins activity and cytotoxicity in zebrafish hepatocytes exposed to triclosan. Environmental Pollution. 271, 116368 (2021).
- da Silva, N. D. G., et al. Interference of goethite in the effects of glyphosate and Roundup® on ZFL cell line. Toxicology In Vitro. 65, 104755 (2020).
- Yang, Y., et al. Temperature is a key factor influencing the invasion and proliferation of Toxoplasma gondii in fish cells. Experimental Parasitology. 217, 107966 (2020).
- Lopes, F. M., Sandrini, J. Z., Souza, M. M. Toxicity induced by glyphosate and glyphosate-based herbicides in the zebrafish hepatocyte cell line (ZF-L). Ecotoxicology and Environmental Safety. 162, 201-207 (2018).
- Lachner, D., Oliveira, L. F., Martinez, C. B. R. Effects of the water soluble fraction of gasoline on ZFL cell line: Cytotoxicity, genotoxicity and oxidative stress. Toxicology In Vitro. 30, 225-230 (2015).
- Morozesk, M., et al. Effects of multiwalled carbon nanotubes co-exposure with cadmium on zebrafish cell line: Metal uptake and accumulation, oxidative stress, genotoxicity and cell cycle. Ecotoxicology and Environmental Safety. 202, 110892 (2020).
- Dognani, G., et al. Nanofibrous membranes for low-concentration Cr VI adsorption: kinetic, thermodynamic and the influence on ZFL cells viability. Materials Research. , 24 (2021).
- ZEM2S (ATCC®CRL-2147™). American Type Culture Collection Available from: https://www.atcc.org/products/crl-2147 (2022)
- Bell, C. C., et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Scientific Reports. 6, 25187 (2016).
- Gajski, G., et al. Genotoxic potential of selected cytostatic drugs in human and zebrafish cells. Environmental Science and Pollution Research International. 23 (15), 14739-14750 (2016).
- Meng, Q., Yeung, K., Chan, K. M. Toxic effects of octocrylene on zebrafish larvae and liver cell line (ZFL). Aquatic Toxicology. 236, 105843 (2021).
- Mueller-Klieser, W. Method for the determination of oxygen consumption rates and diffusion coefficients in multicellular spheroids. Biophysical Journal. 46 (3), 343-348 (1984).
- Glicklis, R., Merchuk, J. C., Cohen, S. Modeling mass transfer in hepatocyte spheroids via cell viability, spheroid size, and hepatocellular functions. Biotechnology and Bioengineering. 86 (6), 672-680 (2004).
- Ho, R. K., Kimmel, C. B. Commitment of cell fate in the early zebrafish embryo. Science. 261 (5117), 109-111 (1993).
- Biswas, S., Emond, M. R., Jontes, J. D. Protocadherin-19 and N-cadherin interact to control cell movements during anterior neurulation. The Journal of Cell Biology. 191 (5), 1029-1041 (2010).
- Bradford, C. S., Sun, L., Collodi, P., Barnes, D. W. Cell cultures from zebrafish embryos and adult tissues. Journal of Tissue Culture Methods. 16 (2), 99-107 (1994).
- He, S., et al. Genetic and transcriptome characterization of model zebrafish cell lines. Zebrafish. 3 (4), 441-453 (2006).

