Analysis of Termination of Transcription Using BrUTP-strand-specific Transcription Run-on (TRO) Approach
Summary
We describe a basic experimental approach for analysis of termination of transcription by RNA polymerase II in vivo using BrUTP by the strand-specific transcription run-on (TRO) approach in budding yeast. This protocol can be extended to study transcription termination by other RNA polymerases both in yeast and higher eukaryotes.
Abstract
This manuscript describes a protocol for detecting transcription termination defect in vivo. The strand-specific TRO protocol using BrUTP described here is a powerful experimental approach for analyzing the transcription termination defect under physiological conditions. Like the traditional TRO assay, it relies on the presence of a transcriptionally active polymerase beyond the 3′ end of the gene as an indicator of a transcription termination defect1. It overcomes two major problems encountered with the traditional TRO assay. First, it can detect if the polymerase reading through the termination signal is the one that initiated transcription from the promoter-proximal region, or if it is simply representing a pervasively transcribing polymerase that initiated non-specifically from somewhere in the body or the 3′ end of the gene. Secondly, it can distinguish if the transcriptionally active polymerase signal beyond the terminator region is truly the readthrough sense mRNA transcribing polymerase or a terminator-initiated non-coding anti-sense RNA signal. Briefly, the protocol involves permeabilizing the exponentially growing yeast cells, allowing the transcripts that initiated in vivo to elongate in the presence of the BrUTP nucleotide, purifying BrUTP-labelled RNA by the affinity approach, reverse transcribing the purified nascent RNA and amplifying the cDNA using strand-specific primers flanking the promoter and the terminator regions of the gene2.
Introduction
The eukaryotic transcription cycle consists of three major steps; initiation, elongation and termination. The termination of transcription by RNA polymerase II consists of two distinct, interdependent steps3. The first step involves cleavage, polyadenylation and release of mRNA from the template, and is immediately followed by the second step, marked by disengagement of polymerase from the template. Proper termination is crucial for the recycling of the polymerase during initiation/reinitiation of transcription, for preventing interference with the transcription of downstream genes, and for keeping aberrant transcription in check by limiting transcription of pervasive non-coding RNA4,5. Despite recent advances, termination is by far the least understood step of the RNA polymerase II transcription cycle. A reliable termination assay is essential for studying the mechanism underlying termination of transcription by RNA polymerase II in vivo.
A number of experimental approaches are used to detect the termination defect in an actively transcribing gene under physiological conditions. These include Northern blot, termination factor ChIP (Chromatin Immunoprecipitation) and the traditional TRO assay. Each of these techniques have some advantages and disadvantages. The traditional TRO assay used to be the most reliable and sensitive approach for detection of a transcription termination defect in vivo1. This assay localizes the transcriptionally active polymerase molecules on different regions of a gene inside the cell. Under normal conditions, the assay shows transcriptionally engaged polymerase molecules strictly distributed between the promoter and terminator region of the gene (Figure 1A). Upon defective termination, however, the polymerase is unable to read the termination signal and is detected in the region downstream of the 3′ end of the gene (Figure 1B).
A transcription termination defect is manifested in the presence of a sense-transcribing polymerase that initiated from the promoter-proximal region, and being unable to read the termination signal, continues transcribing the region downstream of the 3′ end of a gene as shown in Figure 2A. With the realization that the eukaryotic genome exhibits overwhelming pervasive transcription in sense as well as anti-sense directions in and around a gene 6,7,8,9,10, it soon became clear that the traditional TRO assay cannot detect if the polymerase signal downstream of a gene represents a promoter-initiated transcript (Figure 2A) or an aberrant RNA that initiated somewhere in the middle of the gene or downstream of the terminator element (Figure 2B), or the polymerase transcribing in the anti-sense direction from the 3′ end of the gene (Figure 2C). The strand-specific TRO assay using BrUTP described here can distinguish if the observed readthrough polymerase signal represents promoter-initiated extended sense mRNA or an anti-sense transcript that initiated downstream of the gene under examination. We have successfully used this approach to demonstrate the role of Kin28 kinase in termination of transcription in budding yeast2. Employing the approach here we show that Rna14 is required for termination of transcription of ASC1 in budding yeast.
Protocol
NOTE: This method was used in the research article reported in Medler, S and Ansari, A.2.
1. Culturing and Harvesting the Cells
- Start a 5 mL culture of cells in YPD medium (10 g/L yeast extract, 20 g/L peptone, 2% dextrose) from a freshly streaked Saccharomyces cerevisiae plate.
- Grow the cells overnight in an orbital shaker at 30 °C and 250 rpm.
- Dilute the overnight grown culture 100 times to a final volume of 100 mL in YPD medium in a 250-mL baffled flask.
NOTE: A total of 100 mL of culture is needed per TRO reaction; if multiple reactions are needed, a larger volume of culture must be used. - Grow the cells to an optical density of 0.8 – 1.0 at 600 nm (A600 ~ 0.8 – 1.0).
- Harvest the cells by centrifugation at 820 x g for 5 min at 4 °C in a sterile 50 mL conical tube.
- Resuspend the cells in 10 mL of ice cold TMN buffer (10 mM Tris-HCl pH 7.5, 5 mM MgCl2, 100 mM NaCl) and incubate on ice for 5 min.
- Centrifuge at 820 x g for 5 min at 4 °C to pellet the cells. Gently remove the supernatant by aspiration.
2. Permeabilizing and Preparing Cells for Run-on Assay
- Resuspend the cells in 1 mL of 0.6% sarkosyl solution by pipetting up and down gently using a truncated P1000 tip. Transfer the cell suspension to a chilled 1.5 mL microcentrifuge tube.
- Shake the cells gently at 4 °C for 25 min on a platform shaker.
NOTE: Microcentrifuge tubes should be packed into a 50 mL conical tube with ice to maintain a cold temperature, avoiding the possibility of any new initiation events occurring at the promoter at this stage. - Centrifuge the cells at 1,200 x g for 6 min at 4 °C using a tabletop refrigerated centrifuge.
- Gently aspirate the supernatant to remove any excess liquid from the permeabilized cell pellet.
3. Transcription Run-on Reaction
- Add 150 µL of transcription run-on buffer (50 mM Tris-HCl pH 7.5, 100 mM KCl, 10 mM MgCl2, 2 mM DTT (dithiothreitol), 0.75 mM each of ATP, CTP, GTP, and BrUTP, 200 U RNase inhibitor) to the permeabilized cell pellet.
- Mix gently by pipetting up and down using a truncated P200 tip.
NOTE: If processing multiple samples, keep everything on ice until all samples are ready and then proceed to the next step. - Incubate for 5 min at 30 °C in a water bath.
NOTE: Adjust the time of incubation between 1 to 5 min to ensure optimal incorporation of BrUTP into the nascent RNA. - Stop the reaction by adding 500 µL of cold ready-to-use RNA isolation reagent. Incubate for 5 min at room temperature.
4. RNA Extraction
- Add 200 µL of acid-washed glass beads to the cell suspension (step 3.4), and shake vigorously for 20 min at 4 °C in a vortex mixer.
- Puncture the bottom of the microcentrifuge tube using a hot 22 G needle and place it on the top of a 15-mL sterile conical tube.
- Centrifuge at 300 x g for 1 min at 4 °C to collect the cell lysate. Transfer the cell lysate to a 1.5 mL microcentrifuge tube.
- Add 500 µL of cold RNA isolation reagent and 200 µL of chloroform to the cell lysate. Mix the contents on a vortex mixer and centrifuge at 16,168 x g for 20 min at 4 °C.
- Transfer the upper aqueous phase to a new microcentrifuge tube.
- Add an equal volume of cold phenol/chloroform/isoamyl alcohol (125:24:1) pH 4-5. Shake vigorously on a vortex mixer and centrifuge at 16,168 x g for 15 min at 4 °C.
- Transfer the upper aqueous phase containing RNA to a new microcentrifuge tube. Repeat step 4.6 two more times.
- To the final RNA-containing aqueous phase, add 5 M NaCl stock to get a final concentration of 0.3 M. Add 3 times the volume of cold ethanol to precipitate the RNA.
- Incubate for 1 h or overnight at -20 °C.
NOTE: Perform steps 4.9 – 4.11 while the anti-BrdU beads are being incubated for blocking (see step 5.3).
- Incubate for 1 h or overnight at -20 °C.
- Centrifuge at 16,168 x g for 20 min at 4 °C. Remove the supernatant and resuspend the RNA pellet in 100 µL of DEPC (diethylpyrocarbonate) water.
- Use a RNA isolation mini kit to separate the RNA from the unincorporated BrUTP nucleotides. Elute the RNA from the column with 100 µL of RNase-free water.
- Incubate the RNA in a 65 °C water bath for 5 min and then transfer to ice for at least 2 min.
5. Affinity Purification of BrUTP-labeled RNA
- To 25 µL of anti-BrdU settled beads, add 500 µL of 0.25x SSPE (Sodium Saline Phosphate EDTA) binding buffer (20x SSPE: 3 M NaCl, 0.2 M NaH2PO4, 0.02 M EDTA, pH 7.4, 1 mM EDTA, 0.05% Tween, 37.5 mM NaCl). Centrifuge at 200 x g for 30 s at 4 °C and remove the supernatant.
- Repeat step 5.1 three times.
- Add 500 µL of blocking buffer (1x binding buffer, 0.1% polyvinylpyrrolidone, 1 mg ultra-pure bovine serum albumin (BSA)) to the beads and shake gently for 1 – 2 h at 4 °C on a platform shaker.
- Wash the beads two more times with 500 µL of binding buffer, as described in step 5.1.
- Add 400 µL of binding buffer to the beads.
- Transfer 100 µL of RNA (from step 4.11) directly to the beads. Incubate with gentle shaking for 1 – 2 h at 4 °C on a platform shaker.
- Wash the beads sequentially with 500 µL of binding buffer, 500 µL of low salt buffer (0.2x SSPE, 1 mM EDTA, 0.05% Tween), 500 µL of high salt buffer (0.25x SSPE, 1 mM EDTA, 0.05% Tween, 100 mM NaCl), and twice with 500 µL of TET buffer (1x TE, 0.05% Tween), spinning at 200 x g for 30 s at 4 °C in between each wash.
- Elute the BrUTP-labeled RNA from beads sequentially, twice with 150 µl and once with 200 µl of elution buffer (20 mM DTT, 150 mM NaCl, 50 mM Tris-HCl pH 7.5, 1 mM EDTA, 0.1% SDS), by incubating for 4 min at 42 °C in a water bath followed by a short spin to separate beads from the supernatant.
6. Precipitation of BrUTP Labeled RNA
- Add 500 µL of cold phenol/chloroform/isoamyl alcohol (125:24:1) pH 4 – 5 to the affinity purified BrUTP labeled RNA. Mix on a vortex mixer and centrifuge at 16,168 x g for 15 min at 4 °C.
- Transfer the upper aqueous phase to a new microcentrifuge tube.
- Add 5 M NaCl stock to get a final concentration of 0.3 M and 3 times the volume of cold ethanol to precipitate the RNA.
- Incubate at -20 °C overnight.
- Collect the precipitated RNA by centrifugation at 16,168 x g for 30 min at 4 °C.
- Remove the supernatant and allow the pellet to air dry for 10 min.
- Resuspend the RNA pellet in 26 µL of DEPC water.
- Determine the final RNA concentration by measuring the absorbance at 260 nm using a spectrophotometer. Also, determine the 260/280 ratio. A ratio ~2 is indicative of a pure RNA sample.
- Store the RNA samples in aliquots at -80 °C until needed for cDNA synthesis.
7. Reverse Transcription of BrUTP-labeled RNA
- Adjust the RNA concentration to 100 ng/µL using DEPC water. Use 0.5 µg of RNA for each cDNA synthesis reaction.
NOTE: The concentration of RNA template may vary from 0.5 to 1.0 µg to achieve optimal cDNA synthesis. - Reverse transcribe the RNA using multiple reverse primers designed downstream of the poly(A) site (termination site) as shown in Figure 3A.
- To each reverse transcription reaction, add 5 µl RNA template (0.5 µg), 1 µL dNTP mix (10 mM each), 2 µL of reverse primer C, D, E, F or G (50 µM each) (see Figure 3A) and nuclease free water. The final reaction volume in each tube is 13 µL.
NOTE: The number of reverse transcription reactions will depend on the number of reverse primers used for cDNA synthesis. For example, for ASC1 five reverse primers were used and therefore five reverse transcription reactions were set up. Label the tubes as C, D, E, F and G on the basis of the reverse primer used for cDNA synthesis. - Incubate the tubes containing RNA template, dNTP mix and primers in a thermocycler at 65 °C for 5 min to remove any secondary structural conformation, and then cool to 4 °C for at least 2 min.
- Add 1 µL reverse transcriptase (200 U/µL), 4 µL buffer and 2 µL of 0.1 M DTT to each tube containing the RNA template and primer (step 7.4). Mix by pipetting gently up and down.
- Perform reverse transcription by incubating the reaction mix in a thermocycler at 42 °C for 60 min.
- Inactivate the reverse transcriptase at 65 °C for 20 min.
- Store the cDNA at -20 °C or proceed to the next step.
8. Amplification of cDNA
- Perform the PCR reaction for amplification of each cDNA preparation (labeled C, D, E, F and G for ASC1) from step 7.8 as follows: 3.0 µL cDNA template from step 7.8, 1.0 µL forward primer B (10 µM), 1.0 µL reverse primer C or D or E or F or G (10 µM), 2.5 µL 10x polymerase PCR buffer, 0.5 µL dNTP mix (10 mM each), 1.0 µL MgCl2 (2.5 mM), 15.5 µL PCR grade water, and 0.5 µL thermostable polymerase enzyme.
NOTE: Different dilutions of cDNA template ranging from 1:5 to 1:100 should be tested to optimize the PCR product yield. A single promoter-proximal forward primer will be used to amplify cDNA obtained using different reverse primers. For example, a single forward primer B located near the promoter was used in combination with five reverse primers from C to G for ASC1. B-C, B-D, B-E, B-F and B-G were used in tubes labeled as C, D, E, F and G respectively as shown in Figure 3A. - Run the PCR using the following thermal cycling parameters: 1 cycle 95 °C for 2 min (hot start for activation of polymerase), 30 – 40 cycles 95 °C for 30 s (denaturation), 54 °C for 15 s (annealing), 68 °C for 1 min (extension), and 1 cycle 68 °C for 5 min (final extension).
NOTE: The annealing temperature will vary depending on the specific primers being used and the extension time will vary depending on the expected product size. - Separate the PCR product by electrophoresis on 0.8%, 1.5%, or 2% agarose gels depending on the expected size of the product.
- Quantify the PCR product as described in El Kaderi et al., 201211.
NOTE: Alternatively, use real time RT-qPCR strategy to quantify the amount of nascent transcripts.
Representative Results
To demonstrate the applicability of the BrUTP-strand-specific TRO procedure in detecting a transcription termination defect, we used a termination-defective, temperature-sensitive mutant of RNA14 called rna14-1. The role of Rna14 in termination of transcription by RNA polymerase II was demonstrated using the traditional TRO assay that detected RNA in the terminator-proximal region of selected genes1. The detection of RNA signal beyond the terminator region in the rna14-1 mutant at the non-permissive temperature was construed as the termination defect. The observed signal, however, could be ascribed to the pervasively transcribing polymerase that initiated transcription from somewhere near the 3′ end of the gene as shown in Figure 2B. To conclusively demonstrate the role of Rna14 in termination, we performed BrUTP-strand-specific TRO in the rna14-1 mutant and the isogenic wild type strain at 37 °C. We expected that in the wild type strain, the TRO signal will be constrained between the promoter and terminator regions as shown in Figure 1A. In the rna14-1 mutant, however, the polymerase will read through the termination signal and the TRO signal will be detected beyond the 3′ end of the gene as shown in Figure 1B and 2A.
Briefly, we grew the rna14-1 mutant and its isogenic wild type cells at 25 °C to mid-log phase and then shifted cells to 37 °C for three hours. Transcription run-on assay was performed to incorporate BrUTP into nascent RNA synthesized by an actively transcribing RNA polymerase as described above. The BrUTP labeled RNA was purified and reverse transcribed using primers C, D, E, F, and G shown in Figure 3A. The corresponding cDNA was PCR amplified using primer pairs B-C, B-D, B-E, B-F, and B-G and fractionated on agarose gels (Figure 3B). Quantification of gels is shown in Figure 3C. The presence of PCR amplified products from the primer pairs B-E, B-F, and B-G in the rna14-1 mutant reflects a termination read through phenotype (Figure 3B, Lane 11 – 13; and Figure 3C, regions E, F and G). In the wild type cells, however, the TRO signal was limited till the terminator element (Figure 3B, Lanes 2 and 3; and Figure 3C, regions C and D). There was no detectable TRO signal beyond the terminator region (Figure 3B, Lane 4 – 6; and Figure 3C, regions E, F and G). Thus, we could detect the promoter-initiated nascent transcripts that read through the termination signal in the mutant but not in the wild type cells, thereby confirming that Rna14 is a termination factor.
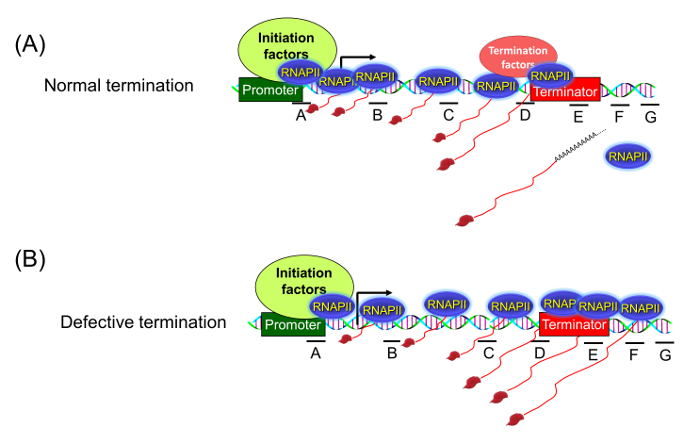
Figure 1: Transcription Run-on (TRO) Assay Detects the Presence of Transcriptionally Active RNA Polymerase in Different Regions of a Gene. (A) When termination is normal, there is no polymerase signal beyond the terminator region. (B) Upon defective termination, the polymerase is unable to read the termination signal and can be detected beyond the 3′ end of the gene. Please click here to view a larger version of this figure.
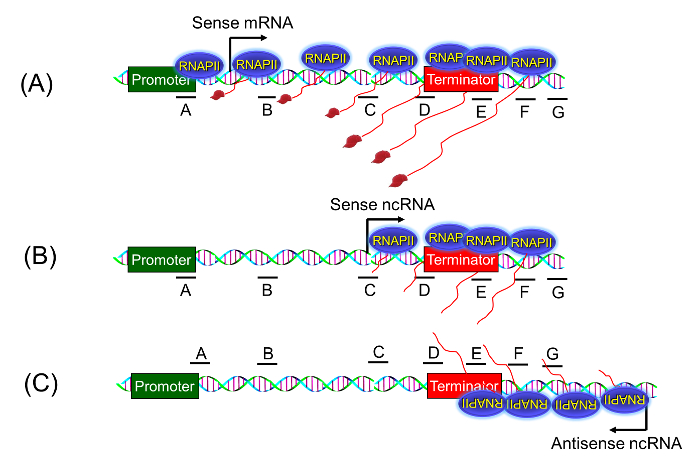
Figure 2: Strand-specific TRO Assay. The strand-specific TRO assay can detect if the presence of a transcriptionally active polymerase beyond the 3′ end of the gene is a true termination defect due to a promoter-initiated polymerase reading through the termination signal (A), or it is simply a pervasively transcribing polymerase transcribing in sense direction (B), or it is the anti-sense ncRNA transcribing polymerase initiating from the 3′ end (C). Please click here to view a larger version of this figure.
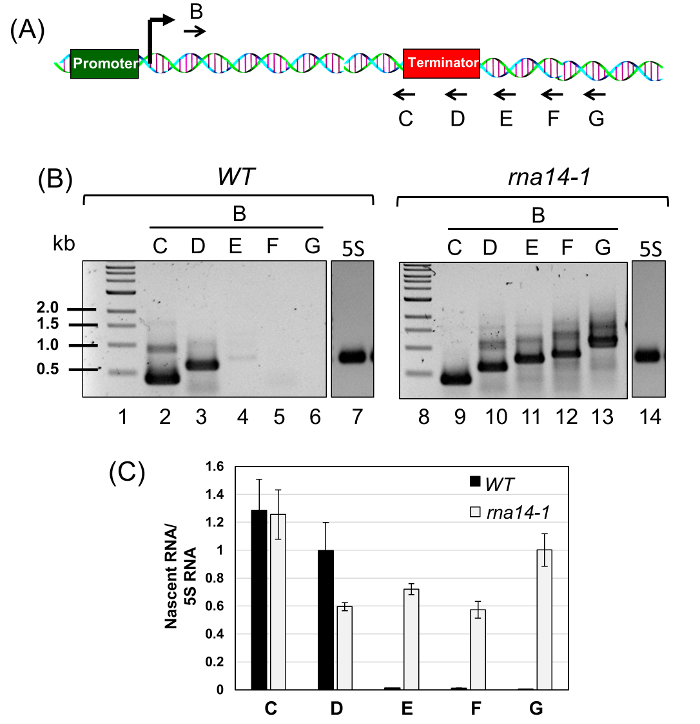
Figure 3: Rna14 is a Termination Factor as Polymerase Reads through the Termination Signal in the rna14-1 Mutant. (A) Schematic depiction of ASC1 gene showing the position of the forward primer B and five reverse primers, C, D, E, F, and G used for cDNA synthesis of purified BrUTP-labeled RNA. (B) cDNA made from reverse primers C, D, E, F and G was PCR amplified using the primers pairs B-C, B-D, B-E, B-F and B-G respectively. (C) The quantitative analysis of TRO data shown in (B) above in wild type and rna14-1 cells at 37 °C. 5S RNA was used as the normalization control. The error bars represent one full unit of standard deviation based on a minimum of three trials. Please click here to view a larger version of this figure.
Discussion
The strand-specific TRO protocol used here was adapted from the protocol used for GRO-Seq (Global Run On-Sequencing) analysis in mammalian cells12. We successfully modified the protocol to study nascent transcription in budding yeast. To specifically analyze the transcription termination defect, we adjusted the protocol further by getting rid of the RNA hydrolysis step. This allowed us to specifically detect the full length nascent transcripts that initiated from the transcription start site near the promoter region.
There are several critical steps in the protocol that should be performed with utmost caution to get a meaningful result. First, the protocol must be performed with exponentially growing yeast cells. The cells in the log phase (A600 ~ 0.8 – 1.0) give the best results as the majority of cells at this stage are actively growing and exhibit robust transcription. Second, the RNA isolation step should be performed as quickly as possible at a temperature between 2 – 4 °C. Third, before affinity purification of Br-UMP labelled RNA, unincorporated BrUTP should be removed from the RNA preparation. We used a RNA kit to get rid of BrUTP from the purified RNA preparation. Fourth, the use of a robust reverse transcriptase is critical for successful accomplishment of the protocol.
The strand-specific TRO protocol used here has advantages over the Northern blot technique in detecting the termination defect as it is more sensitive, faster and exhibits higher resolution. This protocol is also better than the traditional TRO protocol as it can distinguish the sense transcripts from the anti-sense transcripts and if the observed result is due to promoter-initiated transcription or aberrant transcription starting from a promoter downstream region. The strand-specific TRO protocol has some limitations. It is more laborious and time consuming than steady state mRNA detection techniques. It requires large number of cells. Therefore, the analysis of transcription of low transcribing genes might be a problem.
We have successfully used this technique to study the termination defect in the RNA polymerase II transcription cycle in budding yeast2. The approach can be extended to study termination of transcription by RNA polymerase I and RNA polymerase III in yeast as well. With appropriate modifications, the approach can also be applied to study the transcription termination defect in higher eukaryotes. Overall, the technique is relatively inexpensive, highly reproducible and requires standard equipment used in a molecular biology laboratory.
Declarações
The authors have nothing to disclose.
Acknowledgements
Research in Ansari lab was supported by the research grant (No. MCB 1020911) from National Science Foundation.
Materials
| Phenol -chloroform-isoamyl alcohol mixture | Sigma-Aldrich | 77619 | pH 4-5 |
| TRIzol reagent | Life technologies | 15596-026 | |
| RNase Inhibitor, human placenta | New England Biolabs | M0307S | |
| Anti-BrdU beads | Santa Cruz Biotechnology | sc-32323AC | |
| Protoscript II | New England Biolabs | M03368L | or an equivalent enzyme |
| Advantage 2 polymerase Mix | Clontech | 639201 | or an equivalent enzyme |
| 5-Bromouridine 5' triphosphate sodium salt | Santa Cruz Biotechnology | sc-214314A | |
| RNeasy Mini kit | Qiagen | 74104 | |
| ATP | New England Biolabs | N0451AA | |
| CTP | N0454AA | ||
| GTP | N0452AA | ||
| Ultra-pure bovine serum albumin (BSA) | MC Labs | UBSA-100 | |
| Asc1 B | AGATTCGTCGGTCACAAGTCC | ||
| Asc1 C | GAACTTTATACATATTCTTAGTT AGCAGTC |
||
| Asc1 D | TGTACATATGTATTTTCGCAGCA | ||
| Asc1 E | GCCAAGGAGACTGAATTTAATG | ||
| Asc1 F | CTATGGAATGGGGGTTTTAAG | ||
| Asc1 G | GGTTATGGCAGACATGCCAC | ||
| 5s cDNA | AGATTGCAGCACCTGAGT | ||
| 5’ 5s reverse | GGTTGCGGCCATATCTAC | ||
| 3’ 5s reverse | TGAGTTTCGCGTATGGTC |
Referências
- Birse, C. E., Minvielle-Sebastia, L., Lee, B. A., Keller, W., Proudfoot, N. J. Coupling termination of transcription to messenger RNA maturation in yeast. Science. 280 (5361), 298-301 (1998).
- Medler, S., Ansari, A. Gene looping facilitates TFIIH kinase-mediated termination of transcription. Sci Rep. 5, 12586 (2015).
- Richard, P., Manley, J. L. Transcription termination by nuclear RNA polymerases. Genes Dev. 23 (11), 1247-1269 (2009).
- Mischo, H. E., Proudfoot, N. J. Disengaging polymerase: terminating RNA polymerase II transcription in budding yeast. Biochim Biophys Acta. 1829 (1), 174-185 (2013).
- Porrua, O., Libri, D. Transcription termination and the control of the transcriptome: why, where and how to stop. Nat Rev Mol Cell Biol. 16 (3), 190-202 (2015).
- Jacquier, A. The complex eukaryotic transcriptome: unexpected pervasive transcription and novel small RNAs. Nat Rev Genet. 10 (12), 833-844 (2009).
- Xu, Z., et al. Bidirectional promoters generate pervasive transcription in yeast. Nature. 457 (7232), 1033-1037 (2009).
- Neil, H., et al. Widespread bidirectional promoters are the major source of cryptic transcripts in yeast. Nature. 457 (7232), 1038-1042 (2009).
- Clark, M. B., et al. The reality of pervasive transcription. PLoS Biol. 9 (7), e1000625 (2011).
- Goodman, A. J., Daugharthy, E. R., Kim, J. Pervasive antisense transcription is evolutionarily conserved in budding yeast. Mol Biol Evol. 30 (2), 409-421 (2013).
- El Kaderi, B., Medler, S., Ansari, A. Analysis of interactions between genomic loci through Chromosome Conformation Capture (3C). Curr Protoc Cell Biol. Chapter 22, Unit22 15 (2012).
- Core, L. J., Waterfall, J. J., Lis, J. T. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 322 (5909), 1845-1848 (2008).

