Single-cell RNA Sequencing of Fluorescently Labeled Mouse Neurons Using Manual Sorting and Double In Vitro Transcription with Absolute Counts Sequencing (DIVA-Seq)
Summary
This protocol describes the manual sorting procedure to isolate single fluorescently labeled neurons followed by in vitro transcription-based mRNA amplification for high-depth single-cell RNA sequencing.
Abstract
Single-cell RNA sequencing (RNA-seq) is now a widely implemented tool for assaying gene expression. Commercially available single-cell RNA-sequencing platforms process all input cells indiscriminately. Sometimes, fluorescence-activated cell sorting (FACS) is used upstream to isolate a specifically labeled population of interest. A limitation of FACS is the need for high numbers of input cells with significantly labeled fractions, which is impractical for collecting and profiling rare or sparsely labeled neuron populations from the mouse brain. Here, we describe a method for manually collecting sparse fluorescently labeled single neurons from freshly dissociated mouse brain tissue. This process allows for capturing single-labeled neurons with high purity and subsequent integration with an in vitro transcription-based amplification protocol that preserves endogenous transcript ratios. We describe a double linear amplification method that uses unique molecule identifiers (UMIs) to generate individual mRNA counts. Two rounds of amplification results in a high degree of gene detection per single cell.
Introduction
Single-cell RNA sequencing (RNA-seq) has transformed transcriptomic studies. While large-scale single-cell RNAseq can be performed using a variety of techniques, such as droplets1,2, microfluidics3, nanogrids4, and microwells5, most methods cannot sort defined cell types that express genetically encoded fluorophores. To isolate a select cell population, fluorescence-activated cell sorting (FACS) is often used to sort labeled cells in a single-cell mode. However, FACS has some restrictions and requires meticulous sample processing steps. First, a large number of input cells are typically needed (often several million cells per mL), with a significant fraction (>15–20%) containing the labeled population. Second, cell preparations may require multiple rounds of density gradient centrifugation steps to remove glial fraction, debris, and cell clumps that might otherwise clog the nozzle or flow cell. Third, FACS usually employs staining and destaining steps for live/dead staining (e.g., 4′,6-diamidino-2-phenylindole (DAPI), propidium iodide (PI), and Cytotracker dyes), which take up additional time. Fourth, as a rule of thumb for two-color sorting (such as DAPI and green/red fluorescent protein (GFP/RFP)), usually two samples and one control are needed, requiring an unlabeled sample to be processed in addition to the desired mouse strain. Fifth, filtering is often performed multiple times before and during sample sorting to proactively prevent clogged sample lines in an FACS machine. Sixth, time must be allotted in most commonly used FACS setups to initialize and stabilize the fluid stream and perform droplet calibration. Seventh, control samples are typically run in sequence prior to the actual sample collection to set up compensation matrices, doublet rejection, setting gates, etc. Users either perform steps six and seven themselves ahead of time or require the assistance of a technician in parallel. Finally, post-FACS, there are often steps to ensure that only labeled single cells are present in each well; for example, by checking samples in a high-content screening setup such as a fast plate imager.
To circumvent the steps outlined above and facilitate a relatively quick, targeted sequencing of a small population of single fluorescently labeled neurons, we describe a manual sorting procedure followed by two rounds of a highly sensitive in vitro amplification protocol, called double in-vitro transcription with absolute counts sequencing (DIVA-Seq). The RNA amplification and cDNA library generation are adapted from Eberwine et al.6 and Hashimshony et al.7, with certain modifications to suit mouse interneurons that have smaller cellular volumes; furthermore, we have also found that it is equally useful for excitatory pyramidal neurons.
Protocol
All the procedures including animal subjects have been approved by IACUC at Cold Spring Harbor Laboratory, NY (IACUC #16-13-09-8).
1. Manual Sorting of Fluorescently Labeled Mouse Neurons
- Pull glass microcapillaries (see Table of Materials) to 10–15 µm exit diameter using a capillary puller with the following settings: heat: 508, pull: blank, vel: blank, time: blank.
- Attach 120–150 cm flexible silicone tubing (~0.8 mm inside diameter) to a 0.2 µm polyvinylidene difluoride (PVDF) membrane syringe filter and a two-way tubing valve using suitable tubing connectors.
- Prepare 500 mL of chilled (4 °C) artificial cerebrospinal fluid (ACSF, Table 1), and oxygenate by bubbling 5% carbon dioxide balanced oxygen through an airstone for 15 min or until the solution clears completely.
- Prepare 100 mL of ACSF with a cocktail of activity blockers in a 150 mL beaker (Table 1).
- Prepare 100 mL of ACSF with 1 mg/mL Streptococcus fraction IV protease in a 150 mL beaker (Table 1).
- Prepare 100 mL of ACSF with 1% fetal bovine serum (FBS) solution in a 150 mL beaker and oxygenate with 5% carbon dioxide balanced oxygen through an airstone.
- Dissect the brain from a euthanized mouse by opening the cranium with a small scissor and extracting the fresh brain using fine forceps without damaging the cortex.
NOTE: Mice of any strain, age, and gender can be used as desired. Do not freeze the brain or perform manual sorting on mice injected with viruses or other hazardous/infectious agents. - Keep the brain in chilled and oxygenated ACSF, leaving an airstone attached to 5% carbon dioxide balance oxygen during the entire duration of the sectioning.
- Position the brain on the vibratome chuck and cut coronal or sagittal sections at 300 µm thickness. Collect as many sections as needed to obtain a minimum of ~10–50 labeled cells up to several hundred cells. Put slices on a cotton meshed slice holder, placed inside a beaker so they are bathed in oxygenated ACSF.
- Move fresh slices into a beaker containing ACSF with activity blockers and block for 15–20 min at room temperature. Keep bubbling oxygen using airstone.
- Move the slices to a beaker containing ACSF with the protease solution to perform mild digestion at room temperature: 20–30 min for P4-14 animals or up to 45–60 min for P28-56 animals. Keep bubbling oxygen.
- Wash out ACSF with protease by moving slices back to the beaker containing ACSF with activity blockers solution for 5–10 min at room temperature. Keep bubbling oxygen.
- Move individual sections to a 100 mm Petri dish containing ACSF with 1% FBS at room temperature. Under a fluorescent dissection scope, microdissect areas and layers of interest having a minimum of 10–50 cells (up to a few hundred). Perform the microdissection with a pair of fine forceps or by attaching 22–28 G injection needles onto wooden holders (skewers).
- Using a Pasteur pipette, move the microdissected pieces to a 2 mL microfuge tube containing ~0.8 mL of 1% FBS in ACSF solution.
- Triturate the dissected tissue in the microfuge tube at room temperature. Make three long-stemmed Pasteur pipettes with decreasing exit diameters by rolling them over an open flame. Perform ~10 strokes with each pipette, starting with the biggest and ending with the smallest.
- Dispense the dissociated cells into a 100 mm Petri dish containing oxygenated ACSF (Petri dish can be thinly coated with 5 mm of 1% agar or clear silicone compound at 1:10 ratio, if desired). Keep at room temperature.
- Wait ~5-7 min for the cells to gradually settle, then observe the GFP/RFP signal under a dissection microscope.
NOTE: Single cells, debris, and small clumps will be visible in bright-field (BF). - Pick 10–15 GFP/RFP cells at a time using a capillary pipette (from step 1.1) attached to flexible silicone tubing (0.4–0.8 mm inner diameter) and dispense the cells into a 100 mm Petri dish containing fresh oxygenated ACSF.
- By blocking the end of the tubing valve with the tongue, position the capillary close to the cell of interest. Capture cells using capillary action upon relieving the block, and quickly block again to prevent excess fluid from entering the pipette. Dispense the cells onto a 100 mm Petri dish with fresh oxygenated ACSF by gently blowing, while observing the capillary tip under fluorescence optics of the dissection microscope. Aim to collect ~100–150 cells total.
- Repeat step 1.19 once or twice more (depending on debris) and transfer a total of ~50–75 cells to a new 100 mm Petri dish, making sure that contaminants such as debris are minimal in bright-field differential interference contrast (DIC) optics.
- Finally, using a fresh microcapillary pipette each time, choose a single cell in no more than 0.5 µL volume and expel it into a single 0.2 mL microfuge tube (or one strip of eight 0.2 mL microfuge tubes) containing 1 µL of sample collection buffer with primers N10B1-N10B96 (Table 2). Break the pipette tip in the microfuge tube to ensure that the cell stays in the collection buffer. Discard the pipettes.
- Freeze the cells by putting tubes on dry ice and storing them long-term in a -80 °C freezer until they are ready to be processed for RNA amplification.
2. First Round RNA Amplification
NOTE: The following procedure is for single strip of eight 0.2 mL microfuge tubes. Scale the reactions as needed.
- Synthesize the first strand of the cDNA (first round).
- Heat strips from step 1.22 at 70 ˚C for 5 min followed by 4 ˚C for 5 min; repeat twice.
- Assemble on ice reverse transcriptase (RT) master mix/first-strand synthesis master mix using the following ingredients (see Table of Materials): 10x first-strand buffer (2 µL), dNTP mix (4 µL), RNase inhibitor (1 µL), and enzyme (1 µL).
- Briefly centrifuge the strip on a tabletop centrifuge to collect everything at the bottom. Keep on ice.
- Add 1 µL of RT master mix/first-strand synthesis master mix to the above tubes (total volume of 1 µL of sample buffer from cell collection + 1 µL of RT = 2 µL/tube). Mix well by pipetting. Briefly spin to collect the entire content at the bottom and keep it on ice.
- Incubate the above tube/s at 42 °C for 2 h in an air oven. Put the tubes on ice immediately to terminate the reaction and proceed to second-strand synthesis step.
- Synthesize the second strand of the cDNA (first round).
- Make second strand master mix (80 µL) on ice in the following order in a 1.5 mL tube: nuclease-free water (63 µL), 10x second strand buffer (10 µL), dNTP mix (4 µL), DNA polymerase (2 µL), and RNaseH (1 µL). Mix the ingredients well by vortexing, then spin to collect at the bottom and keep on ice.
- Start the thermal cycler, run the following program to pre-cool the unit, be ready to start step 2.2.3 (lid heat = off; 16 °C for 2 h, 4 °C hold), and pause at 16 °C.
- Add an 80 µL/strip (or 10 µL/tube) of second strand master mix to each tube of the strip and keep on ice. Transfer the strip to the thermal cycler and resume the 16 °C step initiated above. Incubate the strip for 2 h at 16 °C.
- After the second-strand synthesis step, place the tubes on ice to proceed to the next cDNA purification step.
- Purify double stranded cDNA (first round).
NOTE: All centrifugations are performed at ~8,000 x g at room temperature. Never exceed 16,000 x g to avoid damage to the filter cartridge.- Start heating 100 µL of nuclease-free water to 50–55 °C for later use in a dry heating block. Never exceed 58 °C to prevent partial denaturation of cDNA.
- Add 250 µL of cDNA binding buffer to a 1.5 mL tube. Check for precipitates in the buffer, then warm the buffer solution to 37 °C for 10 min to re-dissolve precipitates.
- Transfer cDNAs from a single 8-tube strip to the cDNA binding buffer. Combine the cells in this step for further downstream processes (8 cells in 1 tube). Mix thoroughly by pipetting 2-3 times and flicking 3-4 times. Spin to collect the content at the bottom.
- Put the cDNA filter cartridge into wash tubes (from an in vitro transcription (IVT) kit) firmly. Add the above mix to the center of the filter. Centrifuge at 8,000 x g for ~1 min or until it is through the filter. Discard the flow-through and replace the wash tube.
- Add 500 µL of wash buffer from the IVT kit to the column.
NOTE: Make sure that ethanol has been added to the wash buffer previously. - Centrifuge at 8,000 x g for ~1 min or until it is through the filter. Discard the flow-through and centrifuge again at 8,000 x g for ~1 min to empty the cartridge.
- Transfer the cDNA filter to a cDNA elution tube (1.5 mL nuclease-free tube). Apply 8.5 µL of pre-warmed nuclease-free water to the center of the filter. Wait for 2 min and centrifuge at 8,000 x g for ~1.5 min.
- Elute again with 8.5 µL of pre-warmed nuclease-free water and proceed immediately to the first round IVT.
NOTE: Double-stranded cDNA recovery volume will be ~16 µL.
- Perform an in vitro transcription (IVT) reaction for amplified RNA (aRNA) production from cDNA (first round).
- Set the hybridization air oven to 37 °C.
- Prepare the master mix for IVT on ice in the following order: to 16 µL of the eluted double-stranded cDNA from step 2.3.8, add 4 µL of T7 ATP solution (75 mM); 4 µL of T7 CTP solution (75 mM); 4 µL of T7 GTP solution (75 mM); 4 µL of T7 UTP solution (75 mM); 4 µL of T7 10x reaction buffer; and 4 µL of T7 enzyme mix. Mix well, spin, and hold on ice.
- Add 24 µL of the IVT master mix to each tube containing 16 µL of cDNA. Mix the contents by pipetting gently and thoroughly. Incubate the tube at 37 °C for 14 h.
NOTE: Incubation for <12 h severely affects yield. - Add 60 µL of non-diethyl pyrocarbonate (non-DEPC) treated RNase-free water to increase the volume to 100 µL and stop the IVT reaction.
- Purify the aRNA.
NOTE: All centrifugations are performed at ~8,000 x g at room temperature. Never exceed 16,000 x g to avoid damage to the filter cartridge.- Start heating ~200 µL of nuclease-free water to 50–55 °C for later use in a dry heating block or PCR machine. Never exceed 58 °C to prevent partial denaturation of aRNA.
- Transfer aRNA to a 1.5 mL nuclease-free tube. Add 350 µL of aRNA binding buffer to each aRNA sample. Add 250 µL of 100% ethanol to each tube and mix by 3 times by pipetting (do not vortex to mix and spin).
- Transfer the mix immediately to the RNA purification column by adding it gently to the center of the filter cartridge. Centrifuge at 8,000 x g for ~1 min or until the mix has passed entirely through the filter. Discard the flow-through and reuse the waste collection tube
NOTE: RNA will start precipitating upon the addition of ethanol. - Add 650 µL of wash buffer to each filter cartridge. Centrifuge for ~1 min at 8,000 x g or until the entire buffer has passed. Discard the flow-through and spin the filters for an additional ~1 min to remove traces of the wash buffer.
- Transfer the filter to a fresh aRNA collection tube. Add 100 µL of pre-heated (50-55 °C) nuclease-free water to the center of the filter. Wait for 2 min, then centrifuge for ~1.5 min at 8,000 x g or until it has passed into the collection tube.
3. Second Round Amplification
- Synthesize the first strand cDNA (second round).
- Transfer aRNA from step 2.5.5 to a 200 µL microfuge tube and vacuum concentrate the 100 µL elutant to 10 µL. Using no heat, run the vacuum concentrator for 65 min. Compare with 10 µL of water in a 200 µL tube to estimate the reduced volume.
- Preheat the hybridization air oven to 42 °C.
- Add 2 µL of random hexamer primers from the IVT kit to aRNA, vortex briefly, and spin to collect the contents.
- Incubate the microfuge tube at 70 °C for 10 min in a thermal cycler, then place it on ice.
- Prepare the following RT master mix: 2 µL of 10x first strand synthesis buffer, 4 µL of dNTP mix, 1 µL of RNase inhibitor, and 1 µL of ArrayScript enzyme. Mix well in a 200 µL microfuge tube by gently vortexing, then spin to collect the contents and place on ice.
- Add 8 µL of the RT master mix (first strand) to each microfuge tube. Place the tubes in a 42 °C air incubator for 2 h.
- Add 1 µL of RNaseH to the above reaction. Mix well by pipetting 2-3 times and flicking 3-4 times, and spin to collect the contents. Incubate at 37 °C for 30 min on a PCR machine. After incubation, proceed to second-strand synthesis step immediately.
- Synthesize the second strand of cDNA (second round).
- Add 3 µL of T7-RA5 primer (at 25 ng/µL working dilution, Table 2) and 2 µL of RNase-free water to each cDNA sample. Mix well, and spin to collect the contents.
- Incubate at 70 °C for 10 min in a thermal cycler. Place the reaction on ice.
- Prepare the RT master mix (second strand) on ice: 58 µL of nuclease-free water, 10 µL of 10x second strand buffer, 4 µL of dNTP mix, and 2 µL of DNA polymerase. Mix well by vortexing and spin to collect the contents. Keep on ice.
- Add 74 µL of the above mix to each tube from step 3.2.2. Mix well by pipetting 2–3 times and flicking 3-4 times, then spin to collect the contents. Keep on ice and hold.
- Pre-cool the thermal cycler to 16 °C by turning off the lid heat. Place the tubes in the thermal cycler for 2 h at 16 °C.
- After the second-strand synthesis step, place the tubes on ice to proceed to the cDNA purification step, or freeze at -20 °C.
NOTE: cDNA purification is recommended before freezing.
- Perform cDNA purification (second round).
NOTE: All centrifugations are performed at ~8,000 x g at room temperature. Never exceed 16,000 x g to avoid damage to the filter cartridge.- Start heating nuclease-free water to 50–55 °C for later use in a dry heating block. Never exceed 58 °C to prevent partial denaturation of cDNA.
- Transfer the cDNA to a 1.5 mL nuclease-free tube. Add 250 µL of cDNA binding buffer to each tube. Check for precipitates in the buffer and warm the buffer solution to 37 °C for 10 min to re-dissolve. Mix thoroughly by pipetting 2–3 times and flicking 3-4 times. Spin to collect the contents.
- Firmly put the cDNA filter cartridge in the wash tubes. Add the above mix to the center of the filter. Centrifuge at 8,000 x g for ~1 min or until it is through the filter. Discard the flow-through and replace the wash tube.
- Add 500 µL of wash buffer. Make sure ethanol has been added to the wash buffer previously. Centrifuge at 8,000 x g for ~1 min or until it is through the filter.
- Discard the flow-through, and centrifuge again at 8,000 x g for ~1 min to empty the cartridge. Transfer the cDNA filter to a cDNA elution tube.
- Apply 8.5 µL of pre-warmed nuclease-free water to the center of the filter. Wait for 2 min and centrifuge at 8,000 x g for ~1.5 min. Elute again with additional 8.5 µL of pre-warmed nuclease-free water.
NOTE: Double-stranded cDNA recovery volume will be ~16 µL. - Proceed immediately to second round IVT or freeze the cDNA at -20 °C overnight.
- Perform a second round of aRNA production by IVT.
- Set hybridization air oven to 37 °C (36 °C setpoint).
- Prepare the master mix for IVT on ice in the following order: to 16 µL of the eluted double-stranded cDNA from step 3.3.7, add 4 µL of T7 ATP solution (75 mM); 4 µL of T7 CTP solution (75 mM); 4 µL of T7 GTP solution (75 mM); 4 µL of T7 UTP solution (75 mM); 4 µL of T7 10x reaction buffer; and 4 µL of T7 enzyme mix. Mix well, spin briefly to collect the contents, and hold on ice.
- Add 24 µL of the IVT master mix to each tube containing 16 µL of cDNA. Mix the contents by pipetting gently and thoroughly. Incubate tube at 37 °C for 14 h.
NOTE: Incubation for < 12 h severely affects yield. - Add 60 µL of non-DEPC treated RNase-free water to increase the volume to 100 µL and stop the IVT reaction.
- Perform aRNA purification (second round).
NOTE: All centrifugations are performed at ~8,000 x g at room temperature. Never exceed 16,000 x g to avoid damage to the filter cartridge.- Start heating ~200 µL of nuclease-free water to 50–55 °C for later use in a dry heating block or PCR machine. Never exceed 58 °C to prevent partial denaturation of the aRNA.
- Transfer the aRNA to a 1.5 mL nuclease-free tube. Add 350 µL of aRNA binding buffer to each aRNA sample. Add 250 µL of ACS grade 100% ethanol to each tube and mix 3 times by pipetting (do not vortex to mix and spin).
NOTE: RNA will start precipitating upon the addition of ethanol. - Transfer the mix immediately to the RNA purification column by adding it gently to the center of the filter cartridge. Centrifuge at 8,000 x g for ~1 min or until the mix has passed entirely through the filter. Discard the flow-through and reuse the waste collection tube.
- Add 650 µL of wash buffer to each filter cartridge. Centrifuge for ~1 min at 8,000 x g or until the entire buffer has passed. Discard the flow-through and spin the filters for an additional ~1 min to remove traces of the wash buffer.
- Transfer the filters to a fresh aRNA collection tube. Add 100 µL of pre-heated nuclease-free water to the center of the filter. Wait for 2 min, then centrifuge for ~1.5 min at 8,000 x g or until it has passed.
- Aliquot 2 µL in a tube for spectrophotometer (at 260 nm wavelength) and bioanalyzer spread analyses to check for aRNA yield and quality.
NOTE: Concentration must be at least 5 ng/µL; otherwise, reject the sample. The sample can be stored for ~1 year at -80 °C. Avoid freeze-thawing the samples. - Check the samples using a bioanalyzer to visualize proper spreading of aRNA products following the manufacturer's protocol (Figure 2).
4. Amplified RNA Fragmentation and Cleanup
- Mix the following on ice (total volume 40 µL, combine 2 tubes of aRNA non-overlapping sample bar codes): 36 µL of aRNA (200 ng total) and 4 µL of RNA fragmentation buffer. Incubate the mixture at 94 °C for 90 s.
- Immediately move the mixture to ice and add 4 µL of RNA fragmentation stop buffer. Adjust the volume to 100 µL by adding 56 µL of nuclease-free water.
- Add 350 µL of RLT buffer from the RNA purification kit (see Table of Materials) and mix well by pipetting. Add 250 µL of EtOH, mix well by pipetting, and transfer the sample to the RNA purification spin column. Spin for 15 s at 8,000 x g.
- Transfer the column to new collection tube and add 500 µL of RPE buffer. Spin for 15 s at 8,000 x g. Discard the flow-through. Add 500 µL of 80% EtOH and spin for 2 min at 8,000 x g.
- Transfer the column to a new collection tube, open lid of column, and spin for 5 min at full speed.
- Transfer the column to a new collection tube, and elute with 16 µL of nuclease-free water, spinning at full speed for 1 min.
5. Library Preparation
NOTE: IVTs can be pooled at this point, if there is no overlap in barcodes used. The phosphatase treatment time is 40 min. Poly-nucleotide kinase treatment time is 1 h.
- To 16 µL of fragmented aRNA in a 0.7 mL PCR tube, add 4 µL of the following mix: 2 µL of 10x phosphatase buffer, 1 µL of Antarctic phosphatase, and 1 µL of recombinant ribonuclease inhibitor (e.g., RNaseOUT). Incubate in a thermal cycler with the following protocol: 37 °C for 30 min, 65 °C for 5 min, and hold at 4 °C.
- To the 0.7 mL PCR tube from step 5.1, add 30 µL of the following mix: 17 µL of nuclease-free water, 5 µL of 10x phosphatase buffer, 5 µL of ATP (10 mM), 1 µL of recombinant ribonuclease inhibitor, and 2 µL of PNK. Incubate in the thermal cycler at 37 °C for 60 min, then hold at 4 °C.
- Perform a column cleanup of phosphatase and PNK-treated aRNA.
- Adjust volume to 100 µL by adding 50 µL of nuclease-free water. Add 350 µL of RLT buffer and mix well. Add 250 µL of EtOH, mix well by pipetting, and transfer the sample to the RNA purification spin column.
- Spin for 15 s at 8,000 x g. Transfer the column to a new collection tube and add 500 µL of RPE buffer.
- Spin for 15 s at 8,000 x g. Discard the flow-through and add 500 µL of 80% EtOH.
- Spin for 2 min at 8,000 x g. Transfer the column to a new collection tube, open the lid of column, and spin for 5 min at full speed.
- Transfer the column to a new collection tube and elute with 14 µL of nuclease-free water, spinning at full speed for 1 min to recover a ~10 µL volume of material. Discard the column. Reduce the volume to 5 µL using a vacuum concentrator for ~10 min.
- Ligate a 3' adapter using a commercial kit (see Table of Materials).
- Dilute the 3' adapter (RA3) from TrueSeq kit 3 folds with nuclease-free water and store the aliquots at -20 °C.
- To 5 µL of phosphatase and PNK-treated RNA, add 1 µL of diluted 3' adaptor. Incubate at 70 °C for 2 min, and then immediately place the tube on ice to prevent secondary structure formation.
- Add 4 µL of the following mix: 2 µL of 5x HM ligation buffer (HML), 1 µL of RNase inhibitor, and 1 µL of T4 RNA ligase 2 (truncated). Incubate the tube on the pre-heated thermal cycler at 28 °C for 1 h (unheated or with the lid open).
- With the reaction tube remaining on the thermal cycler, add 1 µL of stop solution (STP) and gently pipette the entire volume up and down 6–8 times to mix thoroughly. Continue to incubate the reaction tube on the thermal cycler at 28 °C for 15 min, then place the tube on ice.
- Add 3 µL of nuclease-free water to obtain a total volume of 12 µL. Use 6 µL and store the remaining 6 µL at -80 °C.
- Perform a reverse transcription reaction.
- Combine the following in a PCR tube: 6 µL of adapter-ligated RNA and 1 µL of RNA RT primer (RTP). Incubate the tube at 70 °C for 2 min, then immediately place the tube on ice.
- Add 5.5 µL of the following mix: 2 µL of 5x first strand buffer, 0.5 µL of 12.5 mM dNTP mix, 1 µL of 100 mM DTT, 1 µL of RNase inhibitor, and 1 µL of reverse transcriptase. Incubate the tube in the pre-heated thermal cycler at 50 °C for 1 h, then place the tube on ice (samples can be kept at -20 °C).
- Perform PCR amplification with 11-15 cycles to enrich 5', 3'-primer ligated product.
- To each reverse transcription reaction, add 35.5 µL of the following mix: 8.5 µL of ultra-pure water, 25 µL of PCR mix (PML), and 2 µL of RNA PCR primer (RP1). To each reaction, add 2 µL of a uniquely indexed RNA PCR primer (RPIX, X = 1 through 24).
- Amplify the tube in the thermal cycler using the following PCR cycling conditions: 98 °C for 30 s; 12-15 cycles of 98 °C for 10 s; 60 °C at 30 s; 72 °C for 30 s; 72 °C for 10 min; then hold at 4 °C.
6. PCR Product Cleanup and Size Selection
- Prewarm AmpureXP magnetic beads at room temperature. Vortex the beads until they are well-dispersed, then add 50 µL to the 50 µL PCR reaction (1:1 PCR product:beads). Mix the contents up to ten times to mix thoroughly.
- Incubate at room temperature for 15 min. Place on a magnetic stand for at least 5 min, until the liquid appears clear. Remove and discard 95 µL of the supernatant.
- Add 200 µL of freshly prepared 80% EtOH. Incubate for at least 30 s, then remove and discard the supernatant without disturbing the beads. Repeat this once more.
- Air-dry the beads for 15 min or until completely dry. Resuspend with 32.5 µL of resuspension buffer. Pipette the entire volume up and down ten times to mix thoroughly.
- Incubate at room temperature for 2 min. Place on a magnetic stand for 5 min, until the liquid appears clear. Transfer 30 µL of the supernatant to a new tube. Add 20 µL of nuclease-free water to obtain a 50 µL total volume.
- Perform size selection of the PCR products with solid phase reversible immobilization (SPRI) magnetic beads.
- Add 0.7x volume (35 µL) of SPRI beads to the tube prepared in step 6.5 and mix thoroughly by pipetting. Incubate for 5 min at room temperature.
- Place the tube on a magnetic stand and wait for 5 min or until the beads separate. Remove the supernatant carefully without disturbing the beads.
- Add 200 µL of freshly prepared 85% EtOH. Wait for 30 s, then remove the EtOH. Air-dry the beads for 10 min.
- Add 20 µL of nuclease-free water. Place the tube back on the magnet, wait for 5 min, and pipette off the liquid portion without disturbing or touching the beads. Store the DNA at -20 °C.
7. Determination of Library Amount and Quality
- Check the concentration of DNA with a spectrophotometer at 260 nm wavelength (expected concentration is at least ~5–10 ng/µL).
- Run 1 µL of each sample on a bioanalyzer using a high-sensitivity DNA chip to examine size distribution (expected peak is at 300–400 bp (see Figure 3)).
8. Sample Submission
- Combine non-overlapping sequencing PCR barcodes (1:1 ratio). For example, combine PCR products of samples with RPI-1 and RPI-2, RPI-3 and RPI-4 together in equal nanogram amounts each, according to the RNA sequencing core's total input sample amount requirement recommendations.
- After combining, adjust the total concentration to 5 ng/µL and at least a 10 µL volume or as recommended by the sequencing facility core.
Representative Results
Using the protocol described above, GABAergic neurons were manually sorted (Figure 1) and RNA was amplified, then made into a cDNA library (Figure 2) and sequenced at high depth8. The amplified RNA (aRNA) products ranged between 200–4,000 bp in size, with a peak distribution slightly above 500 bp (Figure 3A). The bead-purified cDNA library was further size-restricted by a second round of purification using beads that eliminated smaller fragments less than 200 bp (Figure 3B and 3C) and with a peak at ~350 bp. Having shorter fragments will lead to empty reads (no mRNA inserts, only adapter and primer sequences), whereas longer fragments will occupy more space on the flow cells, reducing total read output. Upon sequencing, we routinely obtained a 4.8 x 105 median, or 6.9 x 105 average mapped reads per cell (Figure 4A). After duplicate RNA removal using UMIs, each single cell had a 1.0 x 105 median, or 1.4 x 105 average unique reads per cell (Figure 4B). In each single cell external RNA controls consortium (ERCC), spike-in RNA was used as an internal control for which the absolute number of molecules that are added to the sample can be calculated. There was a linear relationship of input to observed counts, with a slope of 0.92 and adjusted R2 = 0.94 (Figure 4C). We detected on average ~10,000 genes per single neuron (ranging from ~7,500 to 12,000 genes/cell), with > 95% of the single cells detecting > 6,000 genes (Figure 4D-4F). This number compares favorably against published data from similar mouse brain-derived single neurons (e.g., 1,865-4,760 genes in Zeisel et al.9, 7,250 genes in Tasic et al.10, and 8,000 genes in Okaty et al.11). Readers are directed to Poulin et al.12 for a detailed comparison.
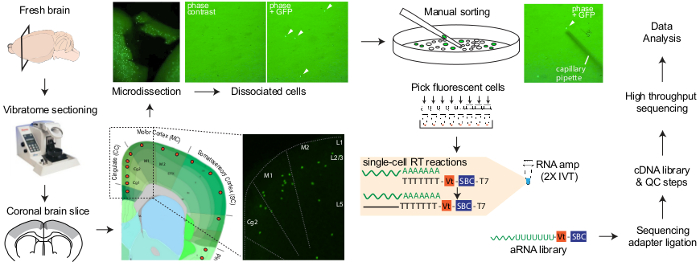
Figure 1: Workflow of manual sorting of neurons followed by DIVA-Seq. Fresh mouse brains were collected and sliced, and the region of interest was microdissected. Single neurons expressing fluorescent proteins were collected manually and amplified using two rounds of linear amplification by in vitro transcription. Please click here to view a larger version of this figure.
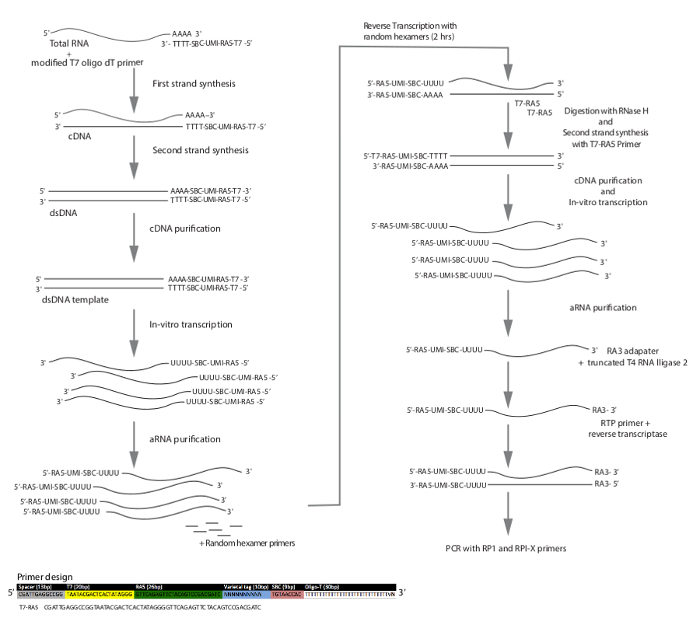
Figure 2: Schematic workflow of DIVA-Seq with two rounds of amplification while incorporating unique molecular identifiers (UMIs) or varietal-tags. Sample bar code (SBC) allows each single cell to be identified by its 9-nucleotide code (teal). UMI is a stretch of random nucleotides 10 bp in length that is different for each primer used. During the bioinformatics step, two mapped transcripts having the same UMI sequence will be counted only once, thus eliminating amplification duplicates and allowing for absolute transcript counting. RA5 and RA3 are sequencing primers, and T7-RA5 primer is needed to add the T7 sequences back to the first-round aRNA products so that the T7 RNA polymerase can rebind and perform a second round of linear amplification by in vitro transcription. Please click here to view a larger version of this figure.
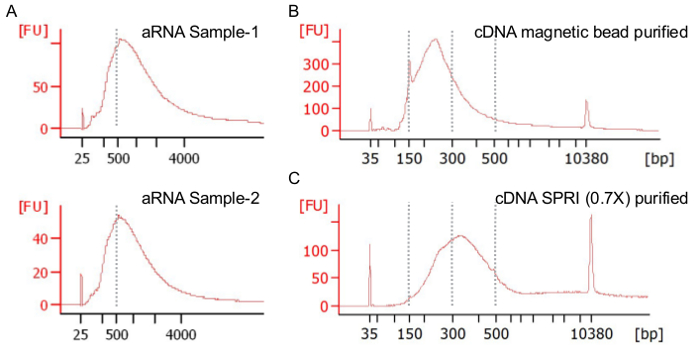
Figure 3: Example bioanalyzer plots. (A) aRNA size distributions should be between 200-4,000 bp with a peak at around 500 bp. X-axis has arbitrary fluorescence unit [FU]. (B) Size distribution of cDNA library products after bead cleanup. (C) Size distribution after 0.7x SPRI size selection (step 6.6) with a peak around 350 bp.
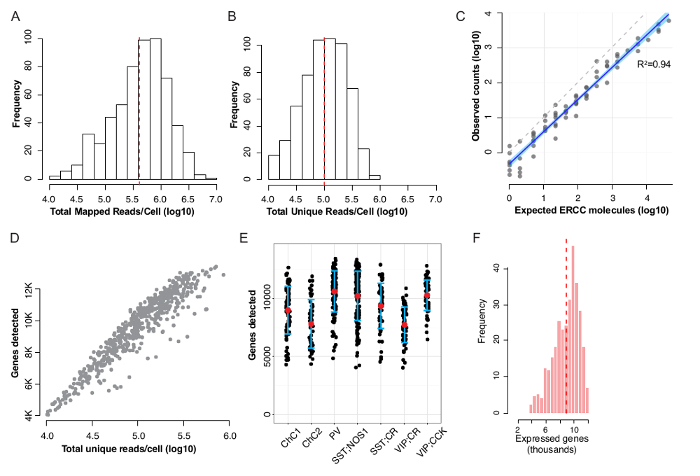
Figure 4: Example sequence read distributions and gene detection from manually-sorted neurons after DIVA-Seq8. (A) Total mapped read distribution. (B) Total unique reads distribution. (C) ERCC reads show linear relationship over 4 orders of magnitude. (D) Genes detected vs. read counts shows that >95% single cells have >6000 genes/cell. (E) Genes detected amongst 6 interneuron types are comparable. (F) Distribution of genes detected per cell, GEO accession #GSE92522. This figure has been adapted from Paul et al.8. Please click here to view a larger version of this figure.
| Buffer | Item | Concentration | Amount (µL) |
| Sample Collection buffer | Recombinant ribonuclease inhibitor | 55 | |
| ERCC | 1:50K diluted | 110 | |
| Nuclease free water | 605 | ||
| Aliquot 43.75 µL of above into 16 tubes (2 strips of 8; 200 µL PCR tubes); add following per tube | |||
| T7-UMI-primers (e.g. N10B1-N10B16) | 1 ng/µL | 6.25 µL/tube | |
| Final volume in each tube 50 µL (each tube can be split in 25 µL aliquots and frozen at -80 °C) | |||
| Solutions: | Item | Concentration | Amount |
| ACSF: to make 5 L dissolve | NaCl | 126 mM | 36.8 g |
| KCl | 3 mM | 1.15 g | |
| NaH2PO4 | 1.25 mM | 0.75 g | |
| NaHCO3 | 20 mM | 8.4 g | |
| To 500 mL of ACSF, bubble oxygen for 10-15 min then add following fresh each time: | |||
| D-glucose | 20 mM | 1.8 g | |
| MgSO4 | 2 mM | 0.5 mL from 4 M stock | |
| CaCl2 | 2 mM | 0.5 mL from 4 M stock | |
| Keep ACSF oxygenated through out | |||
| Solutions: | Item | Concentration | |
| Activity blocker cocktail: make a 100x stock | |||
| To 100 mL ACSF add | |||
| APV | 0.05 mM | ||
| CNQX | 0.02 mM | ||
| TTX | 0.0001 mM (0.1 µM) | ||
| Solutions: | Item | Amount | |
| Protease soltion (100 mL) | Protease from Streptomyces griseus | 100 mg | |
| Fetal bovine serum: commercial source, aliquot in 500 µL for each use. | |||
Table 1: List of solutions and buffers.
Table 2: List of primers and sequences. N10B1-N10B96 are first strand primers and T7-RA5 is the second-round primer. Please click here to download this table.
Discussion
The manual sorting protocol is suitable for a supervised RNA sequencing of neuron populations that are either sparsely labeled in the mice brain or are representing a rare cell population that is otherwise not feasible to study using current high-throughput cell sorting and amplification methods. Cells subjected to FACS usually undergo sheath and sample line pressures in the range of ~9–14 psi, depending on nozzle size and desired event rates. In addition, upon being ejected from the nozzle, the cells can land hard on the surface of the collection tube or wells coated with sample buffer causing impact stress. During manual sorting, such high pressures are never applied, as the cells are sucked into the pipette by capillary action and expelled by gently blowing them out and simply breaking tips of the glass pipettes. The DIVA-Seq protocol is useful for RNA amplification from cells with small cellular volumes (<8 µL) and low starting material and consistently yields large numbers of detectable genes (8–10 K), which, when coupled with deep sequencing, allows for detailed reconstructions of a coherent molecular picture of cellular functions underlying cell identity8,13,14. Due to purity of cell collection steps, high sensitivity of gene detection, and the ability to perform absolute molecule counts, this method is useful for studying cellular states and disease pathophysiology with high depth and precision.
While the yield of amplified RNA and the degree of gene detection is relatively high in this protocol, certain procedural measures help maintain consistency. During second-strand synthesis, assembly must be done on ice, the thermal cycler must be pre-cooled before transfer to the unit, and the reaction must be done strictly at 16 °C (or slightly below) to avoid formation of hairpins that may reduce aRNA yield. It is also advised not to exceed 2 h at 16 °C during second-strand synthesis, and it is important to move to the cDNA purification step as soon as possible. During the IVT steps, incubation for less than 12 h might reduce aRNA yield, whereas exceeding 14 h of IVT time may result in some aRNA degradation.
We did not perform a comparative study with the same input sample from litter-mates subjected to FACS and manual sorting using DIVA-Seq; hence, we do not claim that any particular gene category is misregulated in FACS and not in manual sorting. Both FACS and manual sorting will likely introduce some degree of gene expression artifacts. For differential gene expression situations, any such effect should in theory cancel out one another, as it will be manifested in both the control and sample groups. Recently, a cocktail of transcription inhibitors have been used to prevent the activation of immediate early gene expression, and such steps can also be incorporated to this protocol15.
The manual sorting process is gentle and quicker (usually 90–160 min) compared to FACS (excluding the sample preparation times) that requires density gradient centrifugation, staining with viability, cytotracker dyes, and post-sorting visualization. Manual sorting does not subject the cells to high sheath pressure and impact stress upon sorting onto wells. It also allows near-constant access to oxygenated ACSF and overall provides a hospitable and less stressful sorting environment, which may be crucial for cells that are sensitive to stress such as fast spiking cells with high metabolic demands. In DIVA-Seq currently, up to 96 cells can be multiplexed to save reagent costs and provide absolute mRNA counting with high gene counts per cell.
However, there are drawbacks to this method; for example, manual sorting needs reliable fluorescently labeled cells as a starting population. It is inherently a low-throughput process, with each sorting session yielding 32–64 cells at its maximum, which is considerably lower than in FACS. Manual sorting also requires fine motor skills and some practice to manipulate glass pipettes under a dissection microscope and capture single cells in microcapillary pipettes. The DIVA-Seq amplification is 3'-biased; hence, it cannot be used for whole transcriptome amplification and is also not suitable for splice isoform detection.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the NIH (5R01MH094705-04 and R01MH109665-01 to Z.J.H.), by the CSHL Robertson Neuroscience Fund (to Z.J.H.), and by a NARSAD Post-Doctoral Fellowship (to A.P.).
Materials
| ERCC RNA Spike-In Control Mixes | Thermo Fisher | Cat# 4456740 | |
| SuperScript III | Thermo Fisher | Cat# 18080093 | |
| RNaseOUT Recombinant Ribonuclease Inhibitor | Thermo Fisher | Cat# 10777019 | |
| RNA fragmentation buffer | New England Biolabs | Cat# E6105S | |
| RNA MinElute kit | Qiagen | Cat# 74204 | |
| Antarctic phosphatase | New England Biolabs | Cat# M0289 | |
| Poly nucleotide kinase | New England Biolabs | Cat# M0201 | |
| T4 RNA ligase2, truncated | New England Biolabs | Cat# M0242 | |
| Ampure Xp magnetic beads | Beckman Coulter | Cat# A63880 | |
| SPRIselect size selection magnetic beads | Thermo Fisher | Cat# B23317 | |
| DL-AP5 | Tocris | Cat# 0105 | |
| CNQX | Tocris | Cat# 1045 | |
| TTX | Tocris | Cat# 1078 | |
| Protease from Streptomyces griseus | Sigma-Aldrich | Cat# P5147 | |
| Message Amp II kit | Thermo Fisher | Cat# AM1751 | |
| Carbogen | Airgas | Cat# UN3156 | |
| Sylgard 184 | Sigma-Aldrich | Cat# 761036 | |
| Illumina TrueSeq smallRNA kit | Illumina | Cat# RS-200-0012 | |
| Bioanalyzer RNA Pico chip | Agilent | Cat# 5067-1513 | |
| Bioanalyzer High Sensitvity DNA chip | Agilent | Cat# 5067-4626 | |
| Bioanalyzer 2100 | Agilent | ||
| Dissection microscope with fluorescence and bright field illumination with DIC optics. (Leica model MZ-16F). | Leica | Model MZ-16F | |
| Glass microcapillary: Borosilicate capillary tubes 500/pk. OD= 1 mm, ID=0.58 mm, wall= 0.21 mm, Length= 150 mm. | Warner instruments | Model GC100-15, Order# 30-0017 | |
| Capillary pipette puller | Sutter Instruments Co | P-97 | |
| Vibratome | Thermo Microm | HM 650V | |
| Vibratome tissue cooling unit | Thermo Microm | CU 65 |
Referências
- Macosko, E. Z., et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell. 161 (5), 1202-1214 (2015).
- Klein, A. M., et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell. 161 (5), 1187-1201 (2015).
- Xin, Y., et al. Use of the Fluidigm C1 platform for RNA sequencing of single mouse pancreatic islet cells. Proceedings of the National Academy of Sciences of the United States of America. 113 (12), 3293-3298 (2016).
- Gao, R., et al. Nanogrid single-nucleus RNA sequencing reveals phenotypic diversity in breast cancer. Nature Communications. 8 (1), 228 (2017).
- Yuan, J., Sims, P. A. An Automated Microwell Platform for Large-Scale Single Cell RNA-Seq. Scientific Reports. 6, 33883 (2016).
- Eberwine, J., et al. Analysis of gene expression in single live neurons. Proceedings of the National Academy of Sciences of the United States of America. 89 (7), 3010-3014 (1992).
- Hashimshony, T., Wagner, F., Sher, N., Yanai, I. CEL-Seq: Single-Cell RNA-Seq by Multiplexed Linear Amplification. Cell Reports. 2 (3), 666-673 (2012).
- Paul, A., et al. Transcriptional Architecture of Synaptic Communication Delineates GABAergic Neuron Identity. Cell. 171 (3), 522-539 (2017).
- Zeisel, A., et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 1934, (2015).
- Tasic, B., et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nature Neuroscience. 19, 335-346 (2016).
- Okaty, B. W., et al. Multi-Scale Molecular Deconstruction of the Serotonin Neuron System. Neuron. 88 (4), 774-791 (2015).
- Poulin, J. F., Tasic, B., Hjerling-Leffler, J., Trimarchi, J. M., Awatramani, R. Disentangling neural cell diversity using single-cell transcriptomics. Nature Neuroscience. 19 (9), 1131-1141 (2016).
- Crow, M., Paul, A., Ballouz, S., Huang, Z. J., Gillis, J. Exploiting single-cell expression to characterize co-expression replicability. Genome Biology. 17, 101 (2016).
- Crow, M., Paul, A., Ballouz, S., Huang, Z. J., Gillis, J. Characterizing the replicability of cell types defined by single cell RNA-sequencing data using MetaNeighbor. Nature Communications. 9 (1), (2018).
- Hrvatin, S., et al. Single-cell analysis of experience-dependent transcriptomic states in the mouse visual cortex. Nature Neuroscience. 21 (1), 120-129 (2018).

