High Throughput In Vitro Assessment of Latency Reversing Agents on HIV Transcription and Splicing
Summary
A high throughput protocol for functional assessment of HIV efficient reactivation and clearance of latent proviruses is described and applied by testing the impact of interventions on HIV transcription and splicing. Representative results of the effect of latency reversing agents on LTR-driven transcription and splicing are provided.
Abstract
HIV remains incurable due to the existence of a reservoir of cells that harbors stable and latent form of the virus, which stays invisible to the immune system and is not targeted by the current antiretroviral therapy (cART). Transcription and splicing have been shown to reinforce HIV-1 latency in resting CD4+ T cells. Reversal of latency by the use of latency reversal agents (LRAs) in the "shock and kill" approach has been studied extensively in an attempt to purge this reservoir but has thus far not shown any success in clinical trials due to the lack of development of adequate small molecules that can efficiently perturb this reservoir. The protocol presented here provides a method for reliably and efficiently assessing latency reversal agents (LRAs) on HIV transcription and splicing. This approach is based on the use of an LTR-driven dual color reporter that can simultaneously measure the effect of an LRA on transcription and splicing by flow cytometry. The protocol described here is adequate for adherent cells as well as the cells in suspension. It is useful for testing a large number of drugs in a high throughput system. The method is technically simple to implement and cost-effective. In addition, the use of flow cytometry allows the assessment of cell viability and thus drug toxicity at the same time.
Introduction
Despite effective long-term antiretroviral therapy, HIV persists in a latent state as an integrated provirus in memory CD4+ T cells1. The chromatin structure of the HIV-1 5' long terminal repeat (LTR) promoter and epigenetic modifications such as histone methylation and deacetylation by DNA methyltransferases (DNMT) and histone deacetylases (HDAC) are important mechanisms leading to transcriptional repression and thus post-integration latency2,3. A large variety of latency reversing agents (LRAs) has been investigated for their efficacy to induce virus production in vitro and in vivo from latently infected resting CD4+ T cells4,5,6,7,8. Among the LRAs tested, HDACi (HDAC inhibitors) and BET bromodomain inhibitors (BETis) induce chromatin decondensation and release of the positive transcription elongation factor b (P-TEFb) respectively, leading to subsequent relieve of the transcriptional repression at the 5'LTR and activation of HIV expression9,10,11,12,13. However, the magnitude of reactivation achieved by these LRAs was limited as only a modest increase in cell-associated unspliced HIV mRNA (US RNA), indicative of viral transcription, was observed ex vivo14,15. Importantly, these LRAs also failed to induce a reduction in the frequency of latently infected cells.
HIV expression may be further restricted by inefficient splicing16 as well as defects in nuclear export of multiply spliced HIV RNA (MS RNA)17. Thus, identifying new classes of LRAs that are more potent and can affect distinct aspects of virus production post-integration are needed. In addition, the development of novel assays that help defining the optimal compounds to efficiently reverse latency is required.
Here, a protocol is presented, which utilizes a high-throughput approach for functional assessment of the impact of interventions on HIV LTR-driven transcription and splicing. In brief, a new LTR-driven dual color reporter system pLTR.gp140/EGFP.RevΔ38/DsRed (Figure 1) is used to assess HIV reactivation by flow cytometry. In this fluorescent reporter, the expression of unspliced HIV mRNA (4 kb) leads to enhanced green fluorescent protein (EGFP) expression, while the expression of spliced mRNA (2 kb) would lead to Discosoma sp. red (DsRed) fluorescent protein expression. Briefly, we used a fluorescent Env-EGFP fusion protein, gp140unc.EGFP, where the coding sequence of EGFP was placed in frame with an un-cleaved and truncated form of the envelope (Env). Changes were introduced to ablate the cleavage site preventing the dissociation of Env into gp120 and gp41-EGFP, and to truncate the gp160 protein prior to the transmembrane domain creating a soluble Env analogue, which facilitates the correct folding and expression of EGFP. Upon the expression within a cell, Rev localizes to the nucleus where it mediates the nuclear-cytoplasmic export of the 4 kb env mRNA via the interaction with the rev responsive element (RRE). The truncation of Env does not compromise the RRE, which lies between gp120 and gp41, and the A7 3' splice site. In this system, splicing at HIV-1 splice donor 4 (SD4) and splice acceptor 7 (SA7) results in the production of a 2 kb mRNA encoding a non-functional Rev protein truncated at amino acid 38 fused to DsRed fluorescent protein, RevΔ38-DsRed. Briefly, DsRed was inserted into the 2nd exon of Rev at amino acid 38 by overlap extension18. To facilitate the nuclear export of unspliced mRNA, a mammalian expression vector encoding Rev (pCMV-RevNL4.3) was co-transfected with the fluorescent reporter construct (Figure 2). This unique reporter construct described here is useful in high-throughput assessment of HIV transcription and splicing, without the need to use viral vectors.
Protocol
NOTE: Procedures for cloning, transformation and sequencing are discussed elsewhere18,19. The protocols herein begin from the transfection of the mammalian expression vectors (Figure 3).
1. Transfection of HEK293T Cells with Dual Color Reporter Construct
- Cultivate HEK293T cells in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), penicillin (100 U/mL) and streptomycin (100 μg/mL) in a 5% CO2 incubator at 37 °C. After thawing, passage HEK293T cells 2-3 times before using them in transfection experiments. This would give the cells time to recover from the thawing procedure.
CAUTION: Mycoplasma contamination of cell cultures remains a serious problem. Good laboratory practice and routine testing of cell cultures are essential to decrease the risk of mycoplasma contamination and avoid diffusion20. - Split the cells 1:2 one day before seeding and transfer them into fresh DMEM medium. Cultivate the cells for 24 h in a 5% CO2 incubator at 37 °C.
- The day before transfection, remove the medium from the flask by aspiration and wash the cells with Dulbecco’s phosphate buffered saline (DPBS) solution free of calcium (Ca2+) and magnesium (Mg2+) to remove traces of serum.
- Dispense 5 mL of trypsin-EDTA solution (0.05% – 10 mM in PBS) into the culture vessel and place it at 37 °C in a 5% CO2 incubator for 2 to 5 min.
- When the cells are detached, add 5 mL of DMEM supplemented with 10% (v/v) FBS to further inhibit the trypsin activity which may damage the cells.
- Resuspend gently by pipetting the cell suspension up and down to break up the clumps.
- Dilute the cells 1:10 in trypan blue stain (0.4%).
- Count the live cells that do not take up trypan blue using a microscope (10x objective) and a haemocytometer.
- Calculate the number of viable cells per milliliter by taking the average of the cell count and multiplying it by 10,000 and by the dilution factor (1:10) from the trypan blue stain.
- Plate 2 x 104 cells in 100 μL of DMEM medium supplemented with 10% FBS without antibiotics in a 96-well flat-bottom plate for 24 h.
- For each well, dilute 400 ng of pLTR.gp140/EGFP.RevD38/DsRed, 20 ng of pCMV-RevNL4.3 and 100 ng of pCMV-Tat101AD8-Flag DNA in 50 μL of serum free medium. Mix gently. For experiments without Tat, use a matched empty Tat vector pcDNA3.1 (-).
NOTE: The use of endotoxin-free DNA is highly recommended. For that, prepare endotoxin-free DNA using a midi or maxiprep nucleic acid purification kit. Determine the DNA purity by measuring the 260/280 OD ratio, which should be between 1.7 and 1.9. - Mix the lipid reagent gently before use, then dilute 0.65 μL in 50 μL of serum free medium. Mix gently and incubate for 5 min at room temperature.
- Combine the diluted DNA with the diluted lipid reagent.
- Mix gently and incubate for 20 min at room temperature. The solution may appear cloudy.
NOTE: Proceed to step 1.15. within 30 min. - Dispense 100 μL per well of the lipid reagent-DNA complexes drop-wise on top of the cells.
- Mix gently by rocking the plate back and forth.
- Incubate the plate for 5 h in a 5% CO2 humidified incubator at 37 °C.
- Each reaction mix is sufficient for a single well (96-well) transfection. Adjust the amount and volume of components according to the number of drug and combination that would be tested, and accounts for pipetting variations. All conditions are usually tested in triplicates.
- Transfect additional wells with 100 ng of CMV-driven EGFP and DsRed-Express DNA plasmid for compensation purposes.
2. Treatment of Transfected HEK293T Cells with Latency Reversing Agents
NOTE: Prior to each assay, determine the physiological condition of each LRA by measuring the viability of the cells after exposure to high and low dose of the drug with cell proliferation assay.
- Dilute each LRA to the appropriate working concentration with growth medium (e.g., 1 μM for JQ1(+)).
CAUTION: Reconstitute the lyophilized drugs with the appropriate solvent to a concentration of 10 mM. Store all stock LRAs at -80 °C in a single use aliquot (5 μL each) in order to avoid repeated freezing-thawing cycles. - Carefully aspirate transfection medium with a multichannel and replace with 100 μL medium containing appropriate LRA (Table 1). Add medium very gently alongside the wall of the well to avoid detaching the transfected HEK293T cells, which are known to easily detach from the culture plate surface.
- Incubate the plate for 48 h in a 5% CO2 humidified incubator at 37 °C.
3. Staining of Transfected Cells with Fixable Viability Dye for Flow Cytometry Analysis
CAUTION: Removing dead cells and debris is essential to eliminate false positives and to obtain results of the highest quality.
- Detach the cells into the media using 100 μL of phosphate-buffered saline (PBS) per well and by gently pipetting up-and-down (~5 times) with a multichannel, while avoiding frothing of the media. If needed, detach the cells from the plate using 35 µL per well of trypsin-EDTA solution (0.05% – 10 mM in PBS) and incubating 5 min at 37 °C, before neutralizing with medium containing serum.
- Transfer the cells to a 96-well V-bottom plate.
- Spin the cells for 5 min at 500 x g at 4 °C, then carefully aspirate the medium/PBS without touching the cells.
- Wash the cells at least 1 time with 200 μL of protein/serum free PBS.
- Centrifuge at 500 x g for 5 min at 4 °C, then discard the supernatant by tilting the plate and removing the wash buffer without touching the cells.
- Prepare a working solution of fixable viability dye by diluting the viability dye 1:1000 in protein/serum free PBS. Prepare 50 µL of the diluted stain per well.
NOTE: Viability dyes are available in a range of colors suitable for use with blue, red and violet lasers. Prepare a stock solution of fixable viability dye by resuspending one vial of the lyophilized dye (component A) with 50 μL anhydrous DMSO (component B). Store at -20 °C in a single use aliquot (1 μL each), protected from the light. - Add 50 μL of the diluted viability dye to each well and mix the cells by pipetting up and down with a multichannel.
- Stain for 10-15 min at 4 °C, protected from the light.
- Wash 1-2 times with 150 μL of wash buffer (PBS with 1% bovine serum albumin and 2 mM EDTA).
- Centrifuge at 500 x g for 5 min at 4 °C. Discard the supernatant.
- Fix the cells with 100 μL of freshly prepared 1% formaldehyde in wash buffer for 10-15 min at 4 °C in the dark.
CAUTION: Formaldehyde is very toxic. Prepare 1% formaldehyde solution in a fume hood to avoid inhalation while wearing gloves and safety glasses for protection. - Wash the cells 1-2 times with 100 μL of wash buffer.
- Centrifuge at 500 x g for 5 min at 4 °C. Discard the supernatant.
- Resuspend the cell pellet in 70 μL of wash buffer.
NOTE: The protocol can be paused here. Fixed cells could be stored at 4 °C in the dark for analysis the next day on the flow cytometer.
4. EGFP and DsRed Measurements by Flow Cytometry and Data Analysis
NOTE: Analyze HIV transcription (% EGFP) and splicing (% DsRed) on a flow cytometer. Filter the sample before the run with a 70 μm cell-strainer or 100 μm nylon mesh to avoid clogging up the nozzle.
- Start up the cytometer and computer at least 10 min prior to use to ensure lasers warm up.
- Prior to running samples and commencing data acquisition, fill the sheath tank with 0.9% saline solution and ensure that a sodium hypochlorite tablet is added to the waste tank.
- Check the flow cell for air bubbles.
- Verify that the detectors and filters are appropriate for the experiment.
NOTE: For EGFP, use of blue laser (488 nm) and 530/30 bandpass, while DsRed Express is optimally detected using the yellow laser (561 nm) and 610/20 bandpass. A blue laser (488 nm) and 610/20 bandpass can also be used to detect DsRed expression. - Check the cytometer performance and sensitivity across detectors by running calibration beads.
- Adjust the forward (FSC-A) and side (SSC-A) scatter voltages with unstained sample so that the main population is on-screen and clearly discernable.
NOTE: FSC (Forward-scattered light) is a measurement of light diffraction in a flat angle, which depends on the volume of the cell (cell surface area or size), while SSC (side-scattered light) is a measurement of light diffraction in a right angle, which is proportional to cell granularity and internal complexity. - Perform manual or automatic compensation by using the single-stained samples ensuring minimal spillover of EGFP+ population into the DsRed detector and vice-versa.
CAUTION: For compensation purposes, use a sample of cells expressing each of the single color fluorescent protein as well as cells singly stained with the fixable viability dye. Do not use FITC and PE compensation beads for EGFP and DsRed fluorescent proteins compensations due to different spectral characteristics. Compensation controls should be bright enough to resolve positive and negative population. - Create plots and set the gates using fluorescence minus one (FMO) controls.
- Acquire and record a minimum of 10,000 viable cell events per sample. Run samples at medium or low speed to avoid doublets passing through the laser intercept. Use pulse geometry gating such as SSC-A versus SSC-H to eliminate doublets. Check the stability of the run by plotting time versus SSC-A to see how even the flow is during the run.
- At the end of the run, clean the flow cytometry fluidics with concentrated cleaning agents then water for 5 min each.
- Shutdown the system, discard the waste and re-fill the sheath tank after acquisition.
- Analyze the data using a flow cytometry data analysis software. Exclude cell debris and clump (doublets) based on forward and side scatter, then eliminate the dead cells using the viability dye stain (negative population = live cells). Identify the cells expressing EGFP and DsRed (Figure 4), as well as the percentage of spliced product DsRed/(DsRed + EGFP) (Figure 5).
- Determine the effect of the LRA on HIV transcription and splicing by comparing untreated cells and the cells exposed to individual or a combination of LRAs (Figure 5).
Representative Results
Representative results are shown in Figure 5 for the expression of HIV-1 unspliced (EGFP) and spliced (DsRed) products following treatment with bromodomain inhibitor JQ1. Both JQ1(+) and Tat significantly increased the percentage of cells expressing EGFP (2.18 and 4.13 FC over DMSO respectively; n = 3) indicative of unspliced transcripts. Moreover, JQ1(+) significantly increased the percentage of cells expressing DsRed (46.6 FC over DMSO) as well as the proportion of spliced product (7.37 FC over DMSO) to a similar level as Tat (59.6 and 5.83 FC over DMSO, respectively) confirming the ability of JQ1(+) to turn on HIV transcription and splicing. On the other hand, treatment with the stereoisomer control JQ1(-) abolishes JQ1(+) effect on HIV transcription and splicing confirming the success of the protocol.
In a recent publication, we have shown by RNA analysis an accumulation of HIV spliced transcripts following JQ1(+) treatment21. In fact, our data revealed consistent changes in the levels of unspliced and spliced transcripts that were mirrored by the EGFP and DsRed protein expression in this model following treatment with JQ1(+). These results indicate that EGFP and DsRed expressing cells reflect the ability of the bromodomain inhibitor JQ1(+) to induce HIV transcription and splicing.
In summary, we validated the use of the pLTR.gp140/EGFP.RevΔ38/DsRed reporter in a high-throughput set-up for the assessment of the effect of LRAs on HIV-1 transcription and splicing. Sub-optimal amount of LRA or increased toxicity could lead to scud results. Optimizing the amount of drug used while preserving cell viability can improve the accuracy of the results.
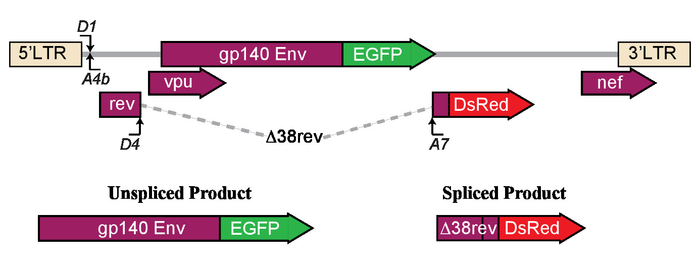
Figure 1: Schematic of the model used to determine the effects of latency reversing agents on LTR-driven transcription and splicing. The LTR construct expresses either unspliced envelope (Env) protein fused to enhanced green fluorescent protein (EGFP) or spliced Δ38rev protein with DsRed. This figure has been reprinted from Khoury et al.21. Please click here to view a larger version of this figure.
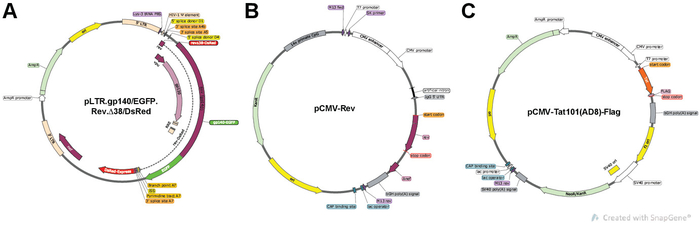
Figure 2: Constructs design. (A) pLTR.gp140/EGFP.RevΔ38/DsRed splicing reporter allows the expression of envelope fused to EGFP (gp140-EGFP) and non-functional Rev protein truncated at amino acid 38 fused to DsRed fluorescent protein (RevΔ38-DsRed). (B) pCMV-RevNL4.3 allows the expression of Rev protein. (C) pCMV-Tat101AD8-Flag allows the expression of Tat101(AD8) protein with a C-terminal flag-tag. The expression plasmids are constructed using PCR overlaps and restriction digest. All vector maps were created using SnapGene software, version 4.1.9. Please click here to view a larger version of this figure.
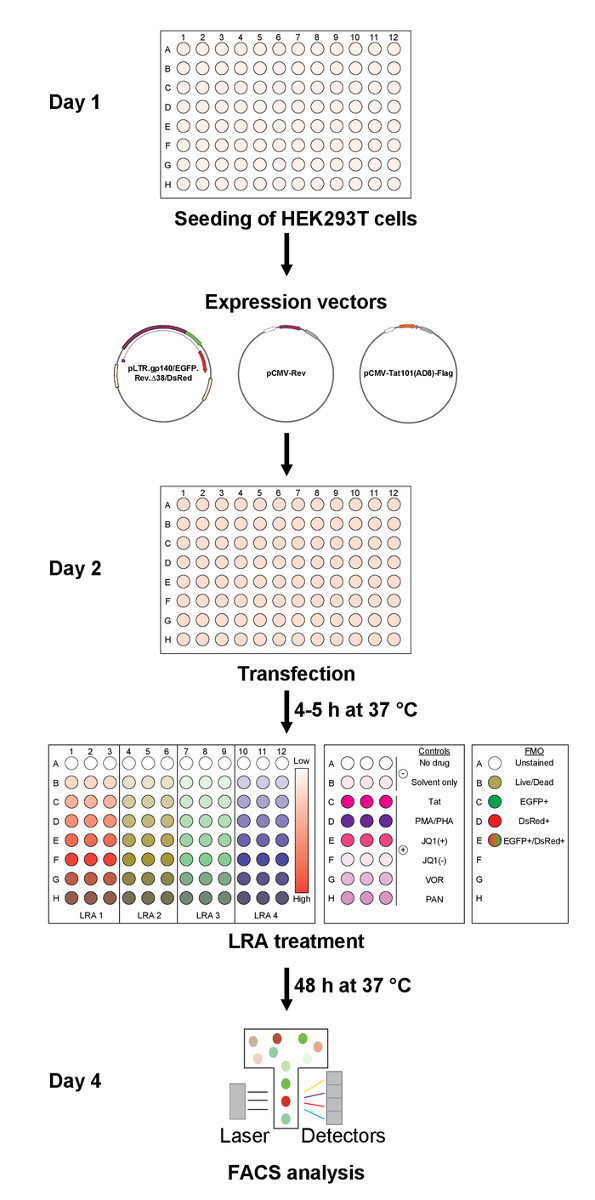
Figure 3: Assay design for rapid assessment of HIV latency reversing agents. The current method for the assessment of the effect of LRAs on HIV-1 transcription and splicing includes i) seeding of HEK293T cells one day prior to ii) transfection with expressions vectors followed by iii) LRA treatment. iv) Cells are analyzed by flow cytometry 48 h post-treatment. Using this protocol, 32 (in triplicate) to 96 conditions could be tested per plate. Please click here to view a larger version of this figure.
Table 1: Representative example of the plate set-up and controls needed. Columns 1 to 3 correspond to negative (-) controls with no drug and solvent only (DMSO 1:5000) as well as positive (+) controls such as Tat (100 ng), PMA/PHA = phorbol myristate acetate/phytohaemagglutinin (10 nM PMA, 10 μg/mL PHA), JQ1 (+/-) (1 μM), VOR = vorinostat (0.5 μM), and PAN = panobinostat (30 nM). Unknown LRAs are tested in triplicate over a range of concentrations (15.625 to 1000 nM). For compensation purposes, cells expressing each of the single-color fluorescent protein (EGFP+ and DsRed+) as well as cells stained with the viability dye and unstained cells are included in each run. Please click here to download this file.
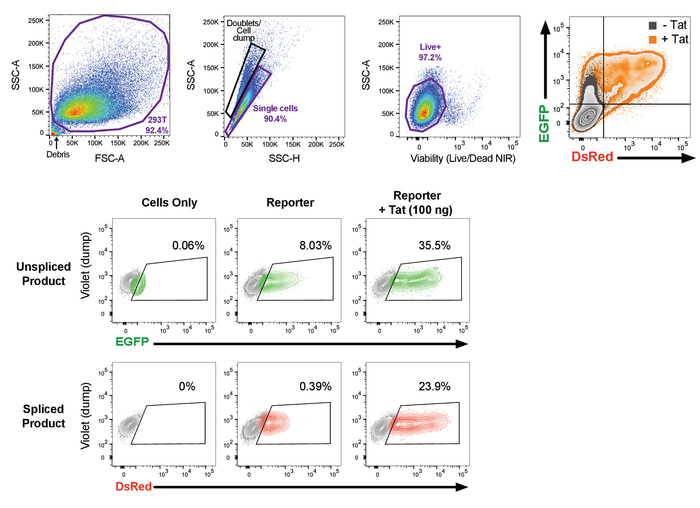
Figure 4: Gating strategy used in the flow cytometry analysis. The first step in the gating strategy is based on the forward and side scatter, which allows the distinction of the cells of interest based on the size and granularity properties of these cells. It is recommended that this gating be as generous as possible to include all events, while removing cellular debris and dead cells, which are found at the bottom left corner of the density plot. Then remove doublets from the dataset using the pulse geometry gating, SSC-A versus SSC-H, and further narrow on the live cells using the viability dye. Finally, a dump channel (violet) and EGFP or DsRed are used to define to cells of interest. The use of fluorescence minus one (FMO) controls are critical in defining the population of interest and addressing the issues of spillover from another fluorochrome in the channel of interest. After acquisition, data are analyzed using flow cytometry data analysis software. This figure has been reprinted in adapted form Khoury et al.21. Please click here to view a larger version of this figure.
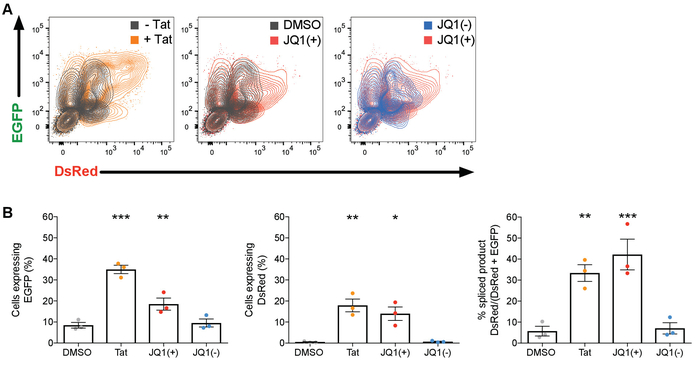
Figure 5: Representative results of the effect of bromodomain inhibitors on HIV transcription and splicing. (A) Examples of two-parameter dual color fluorescence EGFP versus DsRed density plots from untransfected cells (- Tat), cells transfected with 100 ng of pCMV-Tat101AD8-Flag (+ Tat) and cells treated with DMSO, JQ1 (+) or JQ1 (-). (B) The mean percentage (%) of cells expressing EGFP, DsRed or spliced product (DsRed/DsRed+EGFP) from 3 independent experiments is shown. Comparisons of each condition to DMSO were made using the 2-way ANOVA test. Only statistically significant comparisons are shown. *p < 0.05; **p < 0.01; ***p < 0.001. The black lines represent the mean±SEM. DMSO (1:5000), JQ1 (+) and JQ1(-) (1 μM). Please click here to view a larger version of this figure.
Discussion
Given the difficulty in measuring virus reactivation ex vivo, a wide range of in vitro models were developed over the time in order to study HIV latency including latently infected T cell-lines (J-Lats, ACH2, U1), primary models of latent infection of resting (O'Doherty, Lewin, Greene and Spina models) or pre-activated CD4+ T cells (Sahu, Marini, Planelles, Siliciano, Karn models) with single round or replication competent reporter viruses22. To model the physiological conditions of HIV latency in resting CD4+ T cells, dual fluorescent reporter systems ensued such as the RGH (Red-Green-HIV) reporter developed by Ivan Sadowski's group with an LTR-driven Gag-eGFP marker and a CMV-driven mCherry in place of Nef23. A similar dual color reporter virus, Duo-Fluo, was developed by E. Verdin laboratory with an LTR-driven EGFP expression and an mCherry fluorescent marker driven by an EF1α promoter24. In this system, the EGFP was inserted at the 3' end of the provirus in place of Nef. The deletion in the envelope in both these systems prevents multiple rounds of infections. Moreover, the combination of the two fluorescent proteins allows the detection of productively (eGFP+ mCherry+) and latently (eGFP- mCherry+) infected cells. However, several shortcomings of the dual color reporter viruses were noticeable in CD4+ T cells as the direct infection of latently infected cells is largely dependent on the cellular activation state of the T-cells23,24. In fact, very low rate of infection was observed using these two reporter viruses in resting CD4+ T cells: 0.1% for RGH23 and 0.2% for DuoFluo25. In addition, as the CMV promoter has been shown to be expressed at lower levels in non-activated cells26,27,28, an inactive CMV or EF1α promoter would lead to underestimation of the latently infected populations.
We have generated and characterized a new HIV reporter vector to study the establishment of latency and virus reactivation in a high throughput assay. Several advantages to our screening method include i) cost effectiveness with the ability to assess transcription and splicing simultaneously, ii) the ability to test a large number of drugs or combinations in a single experiment, iii) quick assessment of the effect of LRAs by flow cytometry (30-45 min typical read time for 96 well plate independent of the number of fluorescent parameters), iv) high dynamic range, v) suitable for automated high throughput transfection and flow cytometry using robot arms and HTS platform, vi) fast curing of data using a flow cytometry workspace template, vii) optimal for suspension and adherent cells, viii) simplicity of the model and adaptability to luminescence and plate reader measurements (dual luciferase Firefly and Renilla), and ix) scalability (96- and 384-well pate formats).
The propensity of an LRA or a combination of LRAs to activate HIV LTR-driven transcription and splicing thus EGFP and DsRed fluorescent proteins expression could be examined by flow cytometry using our HIV splicing reporter. However, before testing the synergy of novel LRAs, it is highly recommended to determine the physiological conditions of each LRA by measuring the viability of the cells after exposure to a range of concentrations of the single drug. Therefore, including a marker of live and dead cells is essential to eliminate false positives and to obtain results of the highest quality. Another important aspect of flow cytometry analysis is the compensation controls or FMO. It is highly recommended to use singly stained samples ensuring minimal spillover of EGFP+ population into the DsRed and vice-versa. Moreover, it is essential to account for autofluorescence of the cells by including unstained cells. Finally, a regular control of the cytometer performance and sensitivity across the detectors is critical mostly when measuring samples for a study spanning a long period of time.
Although not discussed in the protocol section, there are several limitations of our splicing reporter system. For a more thorough discussion, please see our previous publication (Khoury et al., 2018). Export of the partially or unspliced variants of HIV mRNA is facilitated by the viral protein Rev and its association with the Rev Responsive Element (RRE) in these mRNA species29,30,31,32. The overexpression of Rev in our system allowed us to circumvent the major block of nuclear retention of multiply spliced and unspliced mRNAs observed in latently infected T cells17 and focus on understanding how the LRAs induce transcription and splicing thus virus reactivation. In addition, given that our system requires the transfection of 3 plasmids, we chose HEK293T cells because the high transfection efficiency of these cells. Owing the different temporal and spatial availability of cellular factors in T cells compared to a cancer cell line, it is important to validate the results of the splicing reporter acquired in HEK293T cells in T-cell lines and primary cells, which might provide great technical challenges due to their limited transfectability. Therefore, the use of alternative DNA delivery reagents for transfection such as DMRIE-C, which has been shown to be particularly effective for the transfection of suspension cells (e.g., Jurkat), or nucleofection methods such as Amaxa nucleofector or Neon transfection system that have been proven to facilitate efficient and reliable delivery of single or multiple plasmids to difficult-to-transfect cells including primary CD4+ T cells. Alternatively, it would be interesting to test this reporter system in the context of full-length virus in a primary cell model of latency using the aforementioned transfection methods. In fact, the differences in the availability of host transcription, elongation and splicing factors in a rCD4+ T cell may affect the capacity of an LRA to reactivate the latent provirus. Finally, our model does not address whether the LRA can affect transcription and splicing of bystander cells, and whether it can induce replication competent virus. However, we would suspect that by measuring an increase in cell-associated HIV US and MS RNA, this would lead to the production of replication competent virus in the culture supernatant. This could be confirmed by conducting the gold standard quantitative virus outgrowth assay (QVOA)33.
Due to the complex nature of latency and to ensure efficient virus reactivation, a multifaceted combinatorial strategy may be required34. Potential treatment needs to stimulate multiple pathways including transcription and splicing, which has been previously shown to play essential roles in the establishment of latency16,35,36,37. Our LTR-driven splicing reporter would allow a high throughput screening of drugs that can optimally target both steps, transcription and splicing, increasing the chance of finding molecules that can synergize efficiently, which would lead to a lower dose levels and thus toxicity of each drug.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by project grant APP1129320 and program grant APP1052979 from the NHMRC of Australia. We thank Dr. Adam Wheatley, Dr. Marina Alexander, Dr. Jenny L. Anderson and Michelle Y. Lee for providing essential constructs and advice for the successful completion of this work. We also thank the DMI Flow Facility staff for their advice and generous assistance in maintaining the flow cytometer used in this study.
Materials
| Cell culture | |||
| HEK293T cells (Human Embryonic Kidney cells) | ATCC | CRL-3216 | Replicates vectors carrying the SV40 region of replication. |
| Dulbecco's Modified Eagle's Medium (DMEM 1x + GlutaMAX-I) | Gibco | 10569-010 | + 4.5 g/L D-Glucose + 110 mg/L Sodium Pyruvate |
| Fetal Bovine serum | Gibco | 10099-141 | Origin Australia |
| Penicillin-Streptomycin | Sigma | P4458 | |
| Dulbecco's phosphate buffered saline (DPBS), no calcium, no magnesium | Gibco | 14190-136 | |
| Trypan blue Stain, 0.4% | Gibco | 15250 | |
| Trypsin-EDTA (0.05%), phenol red | Gibco | 25300054 | |
| Lipofectamine 2000 | Invitrogen | 11668-019 | Lipid transfection reagent |
| Opti-MEM I (1x) reduced serum medium | Gibco | 31985-070 | Serum free medium |
| NucleoBond Xtra Maxi | Marcherey-Nagel | 740414.50 | |
| pEGFP-N1 plasmid | Clontech (TaKaRa) | 6085-1 | Expression of EGFP in mammalian cells, CMVIE promoter. |
| pDsRed-Express-N1 | Clontech (TaKaRa) | 632429 | Expression of DsRed-Express in mammalian cells, CMVIE promoter. |
| pLTR.gp140/EGFP.RevD38/DsRed | Addgene | 115775 | |
| pCMV-RevNL4.3 | Addgene | 115776 | |
| pCMV-Tat101AD8-Flag | Addgene | 115777 | |
| Dimethyl sulfoxide (DMSO) | Millipore | 67-68-5 | |
| JQ1(+) | Cayman Chemical | 11187 | Stock at 10 mM in DMSO; working concentration 1 μM |
| JQ1(-) | Cayman Chemical | 11232 | Stock at 10 mM in DMSO; working concentration 1 μM |
| Phorbol Myristate Acetate (PMA) | Sigma-Aldrich | 16561-29-8 | Stock at 100 μg/mL in DMSO; working concentration 10 nM |
| Phytohaemagglutinin (PHA) | Remel | HA15/R30852701 | Stock at 1 μg/μL in PBS; working concentration 10 μg/mL |
| Vorinostat (VOR) | Cayman Chemical | 10009929 | Stock at 10 mM in DMSO; working concentration 0.5 μM |
| Panobinostat (PAN) | TRC | P180500 | Stock at 10 mM in DMSO; working concentration 30 nM |
| CellTiter 96 AQueous One Solution Cell Proliferation Assay | Promega | 63581 | |
| Venor GeM Classic | Minerva Biolabs | 11-1100 | Mycoplasma Detection Kit, PCR-based |
| Name | Company | Catalog Number | Comments |
| Flow cytometry reagents | |||
| LSR Fortessa | BD Biosciences | Flow cytometer (4 lasers-blue, red, violet and yellow) | |
| LSR II | BD Biosciences | Flow cytometer (3 lasers-blue, red and violet) | |
| LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit | Life Technologies | L34976 | Viability dye: for 633 or 635 nm excitation, 400 assays. Component A and B are both provided in the kit. |
| Bovine Serum Albumin | Sigma | A2153 | |
| EDTA 0.5M pH8 | Gibco | 15575-038 | |
| Formaldehyde Solution 37/10 (37%) | Chem-Supply | FA010 | |
| BD FACS Diva CS&T Research Beads | BD Biosciences | 655050 | Calibration beads |
| Sphero Rainbow Calibration Particles (8 peaks) | BD Biosciences | 559123 | 3.0 – 3.4 mm |
| Sheath solution | Chem-Supply | SA046 | 90 g NaCl in 10 L water |
| HAZ-Tabs | Guest Medical | H8801 | Chlorine release tablets for disinfection |
| Decon 90 | Decon Laboratories Limited | N/A | Concentrated cleaning agents of flow cytometer. Working solution Decon 90 5%. |
| Sodium Hypochlorite (12-13% Solution) | Labco | SODHYPO-5L | Concentrated cleaning agents of flow cytometer. Working solution bleach 1%. |
| 7x | MPBio | IM76670 | Concentrated cleaning agents of flow cytometer. Working solution 7x 1%. |
| Name | Company | Catalog Number | Comments |
| Materials | |||
| Tissue culture flasks (75 cm2, canted neck, cap vented) | Corning | 430641U | |
| Tissue culture plates (96 well flat bottom with lid) | Costar | 3599 | |
| Tissue culture plates (96 well V-bottom without lid) | Costar | 3896 | |
| Centrifuge tubes (10 mL) | SARSTEDT | 62.9924.284 | 100×16 mm |
| Centrifuge tubes (50 mL) | CellStar | 227261 | 30×115 mm |
| Microcentrifuge tubes (1.5 mL) | Corning Axygen | MCT-150-C | |
| Serological Pipette (25 mL), sterile | Corning | CLS4489-200EA | |
| Serological Pipette (10 mL), sterile | Corning | CLS4488-200EA | |
| Serological Pipette (5 mL), sterile | Corning | CLS4487-200EA | |
| Reagent reservoirs (50 mL), sterile | Corning | CLS4470-200EA | |
| 5 mL Round-Bottom polystyrene test tube, with cell-strainer cap | Corning | 352235 | 12 x 75 mm style, 70 mm |
| Nylon Mesh | SEFAR | 03-100/32 | 100 mm |
| Titertube Micro test tubes, bulk | BIO-RAD | 2239391 | microfacs tubes |
| 5 mL Round-Bottom polystyrene test tube, without cap | Corning | 352008 | 12×75 mm style |
| Snap Caps for 12×75 mm Test Tubes | Corning | 352032 | |
| Counting chamber, Neubauer improved double net ruling, bright-line (Haemocytometer, LO-Laboroptik) | ProSciTech | SVZ4NIOU | 3×3 large squares of 1 mm2; Depth 0.100 mm; volume 0.1 mL; area minimum 0.0025 mm2 |
| Coverslips (Menzel-Gläser) | Grale Scientific | HCS2026 | 20 x 26 mm |
| Microscope | Nikon TMS | 310528 | |
| Centrifuge 5810R refrigerated | Eppendorf | 5811000487 | with rotor A-4-81 including adapters for 15/50 mL conical tubes |
| FLUOstar Omega microplate reader | BMG Labtech | N/A | Plate reader for cell proliferation assay. Filter 490 nm. |
| Name | Company | Catalog Number | Comments |
| Softwares | |||
| FACS Diva | BD Biosciences | Flow cytometer data acquisition and analysis program, version 8.0.1 | |
| FlowJo | FlowJo | FlowJo 10.4.2 | Flow cytometer data analysis program, FlowJo Engine v3.05481 |
| Omega | BMG Labtech | FLUOstar multi-user reader control, version 5.11 | |
| Omega – Data Analysis | BMG Labtech | MARS | FLUOstar data analysis, version 3.20R2 |
| Microsoft Excel | Microsoft | Excel:mac 2011 | version 14.0.0 |
| Prism | GraphPad | Prism 7 | version 7.0c |
Referências
- Siliciano, J. D., et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nature Medicine. 9 (6), 727-728 (2003).
- Khoury, G., et al. Ch. 8. HIV vaccine and cure – The Path Towards Finding an Effective Cure and Vaccine. 1075, (2018).
- Van Lint, C., Bouchat, S., Marcello, A. HIV-1 transcription and latency: an update. Retrovirology. 10, 67 (2013).
- Archin, N. M., et al. HIV-1 Expression Within Resting CD4+ T Cells After Multiple Doses of Vorinostat. Journal of Infectious Diseases. 210, 728-735 (2014).
- Elliott, J. H., et al. Activation of HIV Transcription with Short-Course Vorinostat in HIV-Infected Patients on Suppressive Antiretroviral Therapy. PLoS Pathogens. 10, (2014).
- Leth, S., et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. The Lancet HIV. 3, e463-e472 (2016).
- Rasmussen, T. A., et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. The Lancet HIV. 1, e13-e21 (2014).
- Søgaard, O. S., et al. The Depsipeptide Romidepsin Reverses HIV-1 Latency In Vivo. PLoS Pathogens. 11, (2015).
- Bartholomeeusen, K., Xiang, Y., Fujinaga, K., Peterlin, B. M. Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of Positive Transcription Elongation Factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. Journal of Biological Chemistry. 287, 36609-36616 (2012).
- Boehm, D., et al. BET bromodomain-targeting compounds reactivate HIV from latency via a Tat-independent mechanism. Cell Cycle. 12, 452-462 (2013).
- Contreras, X., et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. Journal of Biological Chemistry. 284 (11), 6782-6789 (2009).
- Rasmussen, T. A., et al. Comparison of HDAC inhibitors in clinical development: effect on HIV production in latently infected cells and T-cell activation. Human Vaccines & Immunotherapeutics. 9 (5), 993-1001 (2013).
- Wei, D. G., et al. Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing. PLoS Pathogens. 10 (4), e1004071 (2014).
- Blazkova, J., et al. Effect of histone deacetylase inhibitors on HIV production in latently infected, resting CD4(+) T cells from infected individuals receiving effective antiretroviral therapy. Journal of Infectious Diseases. 206 (5), 765-769 (2012).
- Bullen, C. K., Laird, G. M., Durand, C. M., Siliciano, J. D., Siliciano, R. F. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nature Medicine. 20 (4), 425-429 (2014).
- Yukl, S. A., et al. HIV latency in isolated patient CD4+T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Science Translational Medicine. 10, (2018).
- Lassen, K. G., Ramyar, K. X., Bailey, J. R., Zhou, Y., Siliciano, R. F. Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+T cells. PLoS Pathogens. 2, 0650-0661 (2006).
- Alexander, M. R., Wheatley, A. K., Center, R. J., Purcell, D. F. J. Efficient transcription through an intron requires the binding of an Sm-type U1 snRNP with intact stem loop II to the splice donor. Nucleic Acids Research. 38, 3041-3053 (2010).
- Anderson, J. L., Johnson, A. T., Howard, J. L., Purcell, D. F. J. Both Linear and Discontinuous Ribosome Scanning Are Used for Translation Initiation from Bicistronic Human Immunodeficiency Virus Type 1 env mRNAs. Journal of Virology. 81, 4664-4676 (2007).
- Nikfarjam, L., Farzaneh, P. Prevention and detection of Mycoplasma contamination in cell culture. Cell J. 13 (4), 203-212 (2012).
- Khoury, G., et al. HIV latency reversing agents act through Tat post translational modifications. Retrovirology. 15 (1), 36 (2018).
- Hakre, S., Chavez, L., Shirakawa, K., Verdin, E. HIV latency: experimental systems and molecular models. FEMS Microbiology Reviews. 36 (3), 706-716 (2012).
- Dahabieh, M. S., et al. Direct non-productive HIV-1 infection in a T-cell line is driven by cellular activation state and NFkappaB. Retrovirology. 11, 17 (2014).
- Calvanese, V., Chavez, L., Laurent, T., Ding, S., Verdin, E. Dual-color HIV reporters trace a population of latently infected cells and enable their purification. Virology. 446 (1-2), 283-292 (2013).
- Chavez, L., Calvanese, V., Verdin, E. HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells. PLoS Pathogens. 11 (6), e1004955 (2015).
- Hunninghake, G. W., Monick, M. M., Liu, B., Stinski, M. F. The promoter-regulatory region of the major immediate-early gene of human cytomegalovirus responds to T-lymphocyte stimulation and contains functional cyclic AMP-response elements. Journal of Virology. 63 (7), 3026-3033 (1989).
- Reeves, M., Sinclair, J. Aspects of human cytomegalovirus latency and reactivation. Current Topics in Microbiology and Immunology. 325, 297-313 (2008).
- Sambucetti, L. C., Cherrington, J. M., Wilkinson, G. W., Mocarski, E. S. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO Journal. 8 (13), 4251-4258 (1989).
- Kula, A., et al. Characterization of the HIV-1 RNA associated proteome identifies Matrin 3 as a nuclear cofactor of Rev function. Retrovirology. 8, 60 (2011).
- Kula, A., Marcello, A. Dynamic Post-Transcriptional Regulation of HIV-1 Gene Expression. Biology (Basel). 1 (2), 116-133 (2012).
- Yedavalli, V. S., Jeang, K. T. Rev-ing up post-transcriptional HIV-1 RNA expression. RNA Biology. 8 (2), 195-199 (2011).
- Zolotukhin, A. S., et al. PSF acts through the human immunodeficiency virus type 1 mRNA instability elements to regulate virus expression. Molecular and Cellular Biology. 23 (18), 6618-6630 (2003).
- Laird, G. M., Rosenbloom, D. I., Lai, J., Siliciano, R. F., Siliciano, J. D. Measuring the Frequency of Latent HIV-1 in Resting CD4(+) T Cells Using a Limiting Dilution Coculture Assay. Methods in Molecular Biology. 1354, 239-253 (2016).
- Cary, D. C., Peterlin, B. M. Targeting the latent reservoir to achieve functional HIV cure. F1000Res. 5, (2016).
- Han, Y., et al. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. Journal of Virology. 78 (12), 6122-6133 (2004).
- Laird, G. M., et al. Ex vivo analysis identifies effective HIV-1 latency – reversing drug combinations. Journal of Clinical Investigation. 125, 1901-1912 (2015).
- Lenasi, T., Contreras, X., Peterlin, B. M. Transcriptional interference antagonizes proviral gene expression to promote HIV latency. Cell Host Microbe. 4 (2), 123-133 (2008).

