Diffusion Tensor Magnetic Resonance Imaging in Chronic Spinal Cord Compression
Summary
Here, we present a protocol for the application of diffusion tensor imaging parameters to evaluate spinal cord compression.
Abstract
Chronic spinal cord compression is the most common cause of spinal cord impairment in patients with nontraumatic spinal cord damage. Conventional magnetic resonance imaging (MRI) plays an important role in both confirming the diagnosis and evaluating the degree of compression. However, the anatomical detail provided by conventional MRI is not sufficient to accurately estimate neuronal damage and/or assess the possibility of neuronal recovery in chronic spinal cord compression patients. In contrast, diffusion tensor imaging (DTI) can provide quantitative results according to the detection of water molecule diffusion in tissues. In the present study, we develop a methodological framework to illustrate the application of DTI in chronic spinal cord compression disease. DTI fractional anisotropy (FA), apparent diffusion coefficients (ADCs), and eigenvector values are useful for visualizing microstructural pathological changes in the spinal cord. Decreased FA and increases in ADCs and eigenvector values were observed in chronic spinal cord compression patients compared to healthy controls. DTI could help surgeons understand spinal cord injury severity and provide important information regarding prognosis and neural functional recovery. In conclusion, this protocol provides a sensitive, detailed, and noninvasive tool to evaluate spinal cord compression.
Introduction
Chronic spinal cord compression is the most common cause of spinal cord impairment1. This condition can be due to posterior longitudinal ligament ossification, hematoma, cervical disc herniation, vertebral degeneration, or intraspinal tumors2,3. Chronic spinal cord compression can lead to various degrees of functional deficits; however, there are clinical cases with serious spinal cord compression without any neurological symptoms and signs, as well as patients with mild spinal cord compression but serious neurological deficits4. Under these circumstances, sensitive imaging is essential to evaluate compression severity and identify the range of damage.
Conventional MRI plays a significant role in elucidating spinal cord anatomy. This technique is usually utilized to evaluate the compression degree because of its sensitivity to soft tissues5. Many parameters can be measured from MRI, such as MR signal intensity, cord morphology, and spinal canal area. However, MRI has some limitations and only provides qualitative information rather than quantitative results6. Patients with chronic spinal cord compression often have abnormal signal changes of MRI intensity. However, discrepancies between clinical symptoms and MRI intensity changes make it hard to diagnose a functional condition based solely on MRI characteristics7. Previous studies highlight this controversy in terms of the prognostic value of MRI T2 hyperintensity in the spinal cord8. Two groups reported that T2 hyperintensity of the spinal cord is a poor prognostic parameter after surgery for chronic spinal cord compression8,9. In contrast, some authors found no significant association between T2 signal changes and prognosis8,9. Chen et al. and Vedantam et al. divided MRI T2 hyperintensities into two categories corresponding to different prognostic outcomes10,11. Type 1 showed faint, fuzzy, indistinct borders, and this category demonstrated reversible histologic changes. Type 2 images presented intense, well-defined borders, which corresponded to irreversible pathologic damage. Conventional T1/T2 MRI techniques do not provide adequate information to identify these two categories and evaluate patient prognosis. By contrast, DTI, a more sophisticated imaging technique, may help obtain more specific prognostic information by quantitatively detecting microstructural changes in tissues via water molecule diffusion.
In recent years, DTI has garnered increasing attention due to its ability to describe spinal cord microarchitecture. DTI can measure the direction and magnitude of water molecule diffusion in tissues. DTI parameters can quantitatively evaluate neural damage in patients with chronic spinal cord compression. FA and the ADC are the most commonly applied parameters during spinal cord evaluation. The FA value reveals the degree of anisotropy to orientate surrounding axonal fibers and describe anatomic boundaries12,13. The ADC value provides information on the characteristics of molecular motion in many directions in a three-dimensional space and reveals the mean of diffusivities along the three principal axes6,12. Changes in these parameters are associated with microstructural alterations that influence water molecule diffusion. Therefore, surgeons can utilize/measure DTI parameters to identify spinal cord pathology. The present study provides DTI methods and processes that provide more detailed prognostic information to treat patients with chronic spinal cord compression.
Protocol
The study was approved by the local Medical Ethics Committee in Guangzhou First People’s Hospital in China. Signed informed consent forms were received from healthy volunteers and participants prior to participation. All of the studies were conducted in accordance with the World Medical Association Declaration of Helsinki.
1. Subject Preparation
- Ensure that each participant meets the following criteria for chronic spinal cord compression: a) a history of loss of significant neurological function, b) a positive myelopathy physical examination, and c) MRI evidence of cervical cord compression.
NOTE: The exclusion criteria are a) incapability of providing written consent and b) inability to obtain DTI parameters of artifacts. For controls, inclusion criteria are a) no history of significant back or neck injuries, neurological disorders, or spine surgeries; b) no MRI evidence of cervical cord compression. - sk each participant to complete and sign a consent form that lists MRI safety guidelines and the imaging protocol. Specifically, patients with chronic spinal cord compression are examined by MRI preoperatively and 1 year postoperatively.
- rovide earplugs for each participant. Place them in a supine position with a head/neck coil enclosing the cervical region, and a landmark at the thyroid cartilage level. Ensure that each participant is in a comfortable position that effectively reduces movement.
2. Structural MRI Parameters
NOTE: Anatomical T1-weighted (T1 W) images, T2-weighted (T2 W) images, and DTI acquired on a 3 Tesla MRI scanner with a 16-channel head coil.
- Use fast perturbation gradient echo (FPGR) for localization scanning to obtain axial, sagittal, and coronal position maps.
- Position the sagittal positioning line with the coronal position maps to ensure that the positioning baseline is parallel to the spinal canal (spinal cord); first locate the sagittal plane T2 W, then copy and paste the sagittal T1 W positioning line to the T2 W positioning line.
- Use the following imaging parameters for T1 W and T2 W sagittal imaging: field of view (FOV) = 240 mm x 240 mm, voxel size = 1.0 mm x 0.8 mm x 3.0 mm, slice gap = 0.3 mm, slice thickness = 3 mm, number of excitation (NEX) = 2, fold-over direction = feet/head (FH), and time of echo (TE)/time of repetition (TR) = 10/700 ms (T1 W) and 101/2,500 ms (T2 W). Obtain nine sagittal images covering the entire cervical spinal cord.
- Position the axial positioning line on the sagittal T2 W image and cover the intervertebral disc from C2/3 to C6/7, centering on the anteroposterior diameter of the intervertebral space. Use the following imaging parameters: FOV = 180 mm x 180 mm, voxel size = 0.7 mm x 0.6 mm x 3.0 mm, slice thickness = 3 mm, fold-over direction = anterior/posterior (AP), NEX = 2, and TE/TR = 120/3,000 ms.
- Position the axial positioning line on the sagittal T2 W image, centering on the anteroposterior diameter of the intervertebral space, with 45 slices covering the cervical spinal cord from C1 to C7.
- Obtain DTI via the following sequence: single-shot spin-echo echo-planar imaging (SE-EPI) with 20 orthogonal directions. Non-coplanar diffusion directions with b-value = 800 s/mm2.
- Use the following imaging parameters: FOV = 230 mm x 230 mm, acquisition matrix = 98 x 98, reconstructed resolution = 1.17 x 1.17, slice thickness = 3 mm, fold-over direction = AP, NEX = 2, EPI factor = 98, and TE/TR = 74/8,300 ms. Provide a time course summarizing the steps in the MRI protocol, as shown in Figure 1.
NOTE: The time course summarizing the MRI and DTI protocol is shown in Figure 1.
3. Image Postprocessing and Data Measurement Indexes
- Automatically convey all scanning images to the Syngo MR B17. Load the T2 W sagittal and axial imaging of the intervertebral space in the filming interface and find the most compressed portion of the cervical spinal cord.
- In the 2:1 viewing interface, load the FA image and click the Position Display: Series tab. Count and record the level of highest compression from the top to the bottom of the location map.
- Click the File tab to select the tensor image, then use the applications toolbar at the top left of the screen to select Neuro 3D(MR) to automatically create ADC and FA colormaps.
- Turn to the level of the highest compression site, and create spherical regions of interest (ROIs) of identical volumes (with a size of 6 mm3) using the Start Evaluation Mode tab. The ROIs must be selected, including the inner spinal cord to exclude the partial volume effects of cerebrospinal fluid (CSF).
- Calculate and display the FA and ADC values at the bottom right of the screen automatically. Display the E1, E2, and E3 values by clicking the Diffusion toolbar and choosing them.
NOTE: All measurements were performed by two radiologists blinded to the patients’ clinical details. The final results were determined as the average of the two. - Perform image processing of the DTI datasets using a Syngo MR B17 Advantage Workstation, following the steps in Figure 2.
Representative Results
This is a summary of results obtained from healthy volunteers and patients with cervical spondylotic myelopathy. The protocol enabled the physician to view DTI maps. This technology could serve as an objective measure to measure functional status in myelopathic conditions. DTI maps of healthy volunteers are shown in Figure 3. The DTI parameters of healthy volunteers were as follows: FA = 0.661; ADC = 1.006 x 10-3 mm2/s; E1 = 1.893 x 10-3 mm2/s; E2 = 0.746 x 10-3 mm2/s; E3 = 0.377 x 10-3 mm2/s (Figure 3). DTI maps of chronic spinal cord compression patients are displayed in Figure 4 and have the following parameters: FA = 0.605; ADC = 1.522 x 10-3 mm2/s; E1 = 2.731 x 10-3 mm2/s; E2 = 1.058 x 10-3 mm2/s; E3 = 0.776 x 10-3 mm2/s (Figure 4). Postoperative imaging was also performed. Figure 5 shows DTI maps of patients with chronic spinal cord compression who underwent surgery. The DTI parameters are as follows: FA = 0.616; ADC = 1.210 x 10-3 mm2/s; E1 = 2.190 x 10-3 mm2/s; E2 = 0.858 x 10-3 mm2/s; E3 = 0.582 x 10-3 mm2/s (Figure 5).
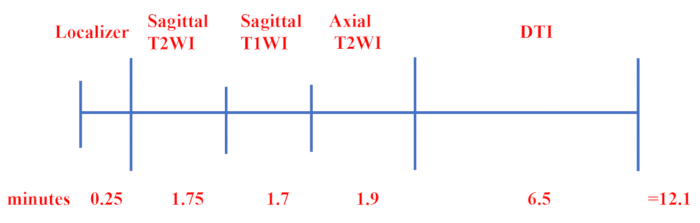
Figure 1: Time course of the clinical MRI protocol. First, the FSPGR sequence was selected for localization scanning, and then the rapid recovery of fast spin echo was performed to acquire the sagittal T2 W and T1 W images and axial T2 W images. Finally, DTI was performed using single-shot SE-EPI with 20 orthogonal directions. Please click here to view a larger version of this figure.
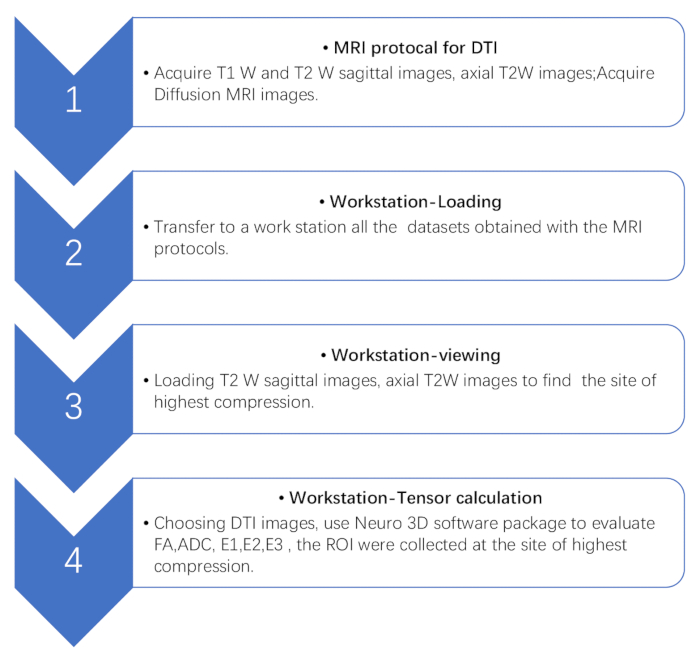
Figure 2: Flowchart of the steps involved in DTI processing. Flowchart showing four DTI postprocessing steps with a workstation. First, acquire conventional MRI and DTI in the workstation. Then, find the site of the highest compression based on conventional MRI images. Finally, perform the tensor calculation. Please click here to view a larger version of this figure.
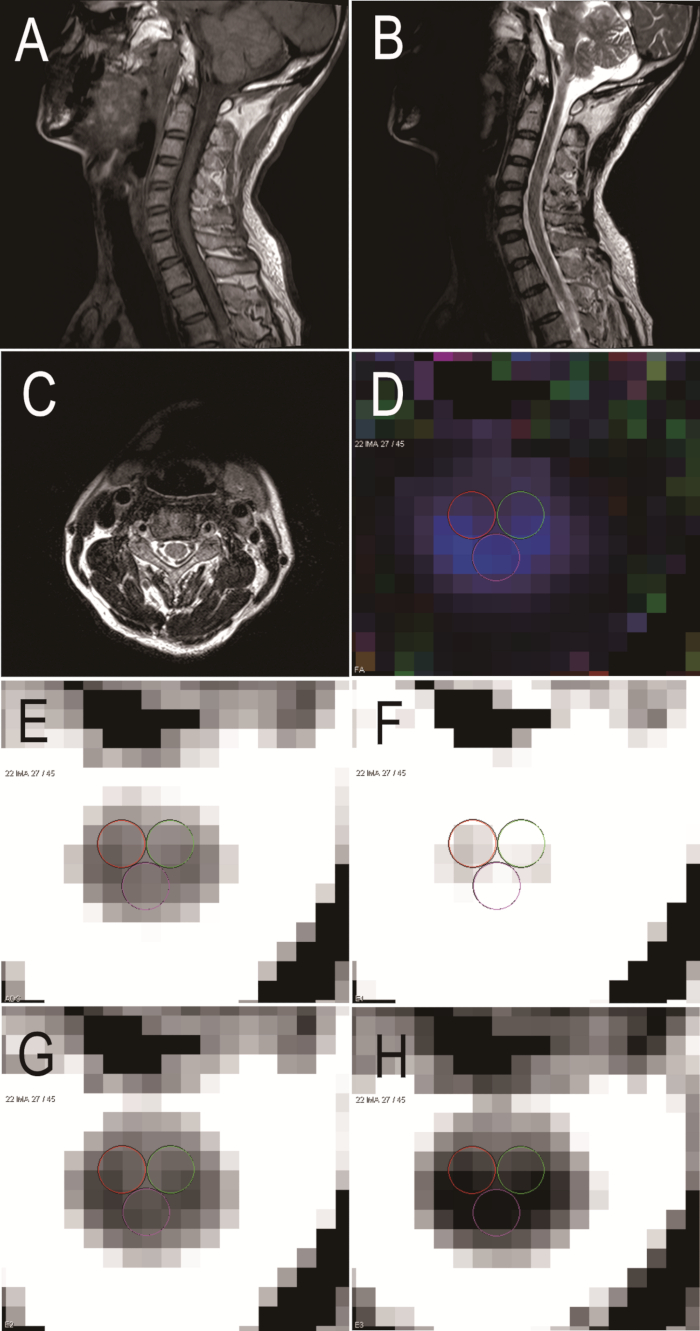
Figure 3: Sagittal and axial MRI and DTI in a healthy volunteer. (A) Sagittal MRI T1 W. (B) Sagittal MRI T2 W. (C) Axial MRI T2 W. (D) FA. (E) ADC. (F) E1. (G) E2. (H) E3. Please click here to view a larger version of this figure.
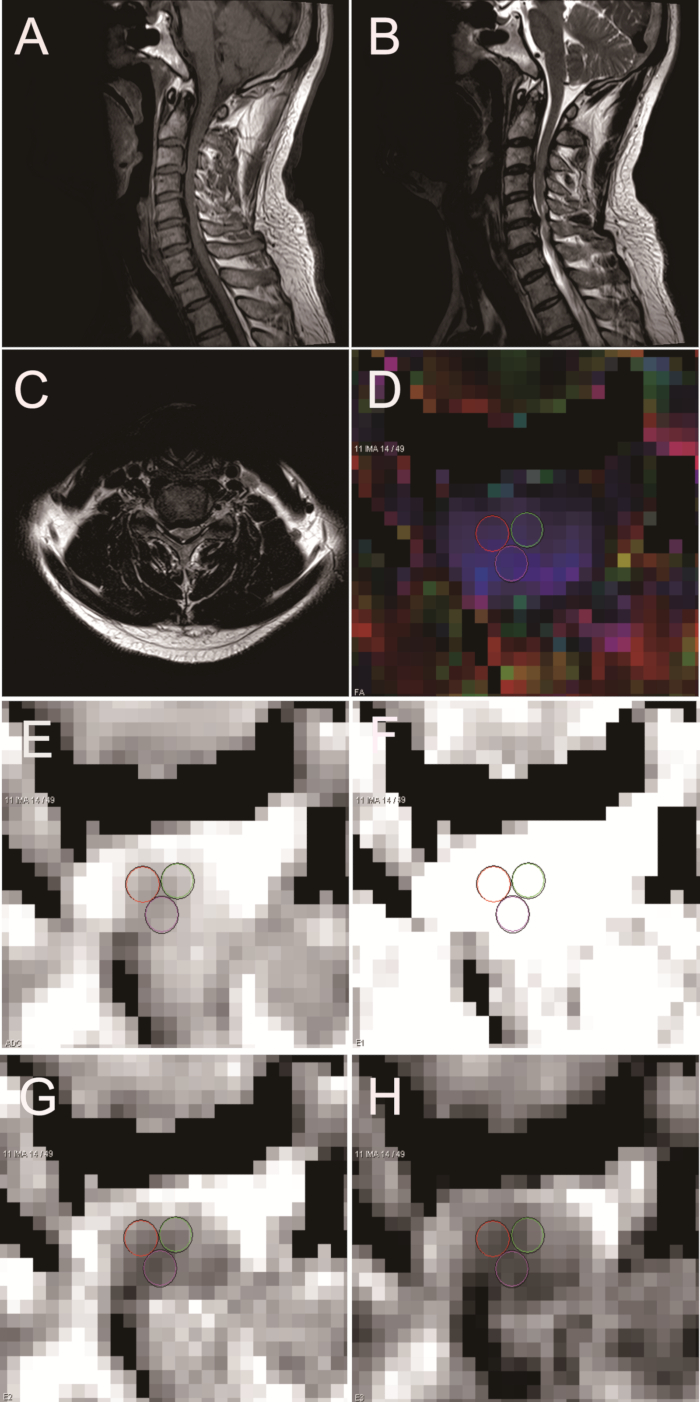
Figure 4: Sagittal and axial MRI and DTI in a patient with chronic spinal cord compression. (A) Sagittal MRI T1 W. (B) Sagittal MRI T2 W. (C) Axial MRI T2 W. (D) FA. (E) ADC. (F) E1. (G) E2. (H) E3. Please click here to view a larger version of this figure.
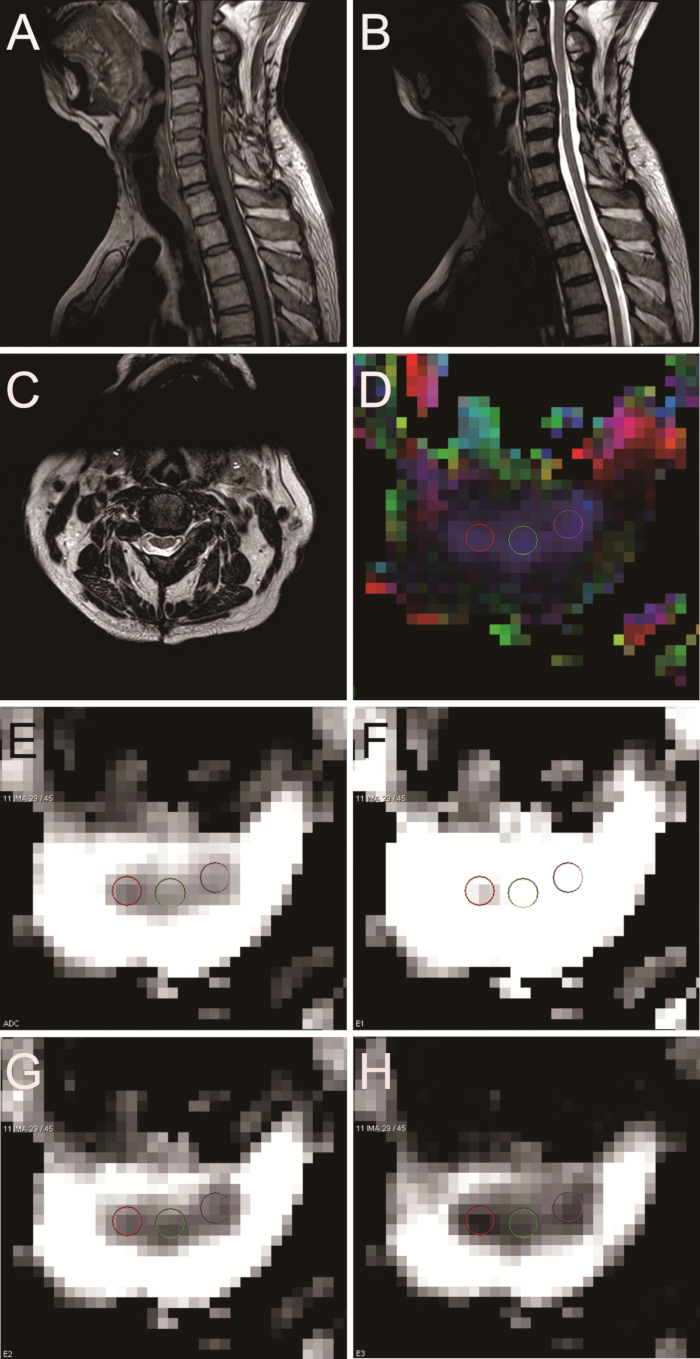
Figure 5: Sagittal and axial MRI and DTI in a patient with chronic spinal cord compression after surgery. (A) Sagittal MRI T1 W. (B) Sagittal MRI T2 W. (C) Axial MRI T2 W. (D) FA. (E) ADC. (F) E1. (G) E2. (H) E3. Please click here to view a larger version of this figure.
Discussion
Conventional MRI is usually utilized to assess the prognosis of patients with various spine conditions. However, this imaging modality provides macroscopic anatomic detail rather than microstructure evaluation14, which limits the prediction of neurological function. Furthermore, traditional MRI may underestimate the severity and extent of spinal cord damage. The emergence of DTI can help surgeons to evaluate spinal cord function more accurately by providing quantitative information on water molecule diffusion.
In the present study, a methodological framework was described to demonstrate the application of DTI parameters in patients with chronic spinal cord compression. DTI is a sensitive technique to measure the direction and diffusion magnitude of water molecules in tissues15. Surgeons can quantitatively assess neural damage in various pathologies of the spinal cord by evaluating DTI parameters. In this protocol, we manually drew ROIs on axial slices because existing dedicated software for the automatic segmentation of CSF and myelin is not adequate for the spinal cord. The small spinal cord cross-sectional area is a major limitation to effectively apply automatic segmentation. We selected ROIs at the most serious compression site. ROIs must include the inner spinal cord to eliminate the partial volume effects of CSF. In addition, DTI processing should reduce the effects of artifactual factors such as EPI-related geometric distortion artifacts and eddy current artifacts. The available options of the software package could help operators obtain useful information depending on the orientation of the diffusion-weighting gradient and separate eddy current correction. Conventional MRI scanning in the present study applied a fast spin-echo sequence to provide more image information. The longer echo chain and smaller echo interval were specifically designed to minimize artifacts created by spinal instrumentation. We selected a short echo time, wide readout frequency band, and small voxels to reduce artifacts. FA and ADC are commonly used DTI parameters in the measurements of the spinal cord. FA represents the degree of anisotropy in a range of 0 to 1. FA values closer to 1 indicate high tissue anisotropy13. The ADC is related to the average value of diffusivities in the three principal axes, and its change is consistent with the process of histopathological tissue injury6. The present work confirmed that chronic spinal cord compression might result in decreased FA and increased ADC values, as previously reported12. Chronic spinal cord compression can cause recurrent ischemic damage to the spinal cord and produce histopathological changes in downstream nerve fibers, such as angioedema, gliosis, neuron function loss, and eventually necrosis16. In the present work, these above-mentioned changes were clearly visualized on DTI.
DTI can serve as a tool to assess functional improvement and provide valuable prognostic information. Previous studies showed that high preoperative FA might be related to better neural functional recovery after surgery17. Kerkovsky et al. reported that patients with symptomatic cervical spondylotic myelopathy had higher ADC values and lower FA values compared with those who had no relevant symptoms but had radiological evidence of cord compression18. In a previous study of a chronic spinal cord compression rat model, DTI parameters were associated with pathological spinal cord conditions. Importantly, DTI can quantitatively assess the functional status of the spinal cord16. An analysis of 66 patients with chronic spinal cord compression also showed that DTI parameters were related to the Japanese Orthopedic Association recovery rate of patients with chronic spinal cord compression, and ADC, mean diffusivity, radial diffusivity, and axial diffusivity values might reflect neurologic impairment and be useful for evaluating postoperative prognosis19. Compared with conventional MRI, DTI is a useful quantitative tool to measure the recuperative potential of the spinal cord.
There were some limitations to this study. First, adequate spatial resolution is still difficult to achieve. Motion artifacts, arising from respiratory and cardiac motion and CSF pulsation, can produce bad effects on DTI, especially in the lower cervical cord and thoracic cord20. The longer echo chain and smaller echo interval were specifically designed to minimize artifacts created by spinal instrumentation. In this protocol, we selected a short echo time, wide readout frequency band, and small voxels to reduce artifacts. In addition, it was difficult to distinguish between white and gray matter on DTI with a 3 Tesla MR system21, which meant that both gray and white matter might be included in the ROIs. That could significantly influence DTI parameter measurements. ROI-based quantification might lead to a biased identification of the tract caused by user experience and anatomical knowledge. This manual delineation approach can be tedious and time-consuming, especially if there are several spinal cord slices, tracts, and subjects. ROIs should be selected at the inner spinal cord to exclude partial volume effects because of CSF. Useful methods to segment gray and white matter regions and discern available and effective ROIs are required in future studies.
In summary, this methodological framework demonstrates the application of DTI parameters in chronic spinal cord compression. DTI provides a measure of water molecular direction and diffusion magnitude in tissues. Surgeons can use this sensitive technique to quantitatively assess neural damage in various spinal cord pathologies.
Declarações
The authors have nothing to disclose.
Acknowledgements
This study was supported by Guangzhou Science and Technology Project of China (No. 201607010021) and the Nature Science Foundation of JiangXi (No. 20142BAB205065)
Materials
| 3-Tesla MRI scanner | Siemens | 40708 | Software: NUMARIS/4 |
| Syngo MR B17 | Siemens | 40708 | Software: NUMARIS/4 |
Referências
- Sun, G. D., et al. A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord. Neural Regeneration Research. 12 (8), 1365-1374 (2017).
- Watanabe, N., et al. Neurological Recovery after Posterior Spinal Surgery in Patients with Metastatic Epidural Spinal Cord Compression. Acta Medica Okayama. 70 (6), 449 (2016).
- Tatsui, C. E., et al. Spinal Laser Interstitial Thermal Therapy: A Novel Alternative to Surgery for Metastatic Epidural Spinal Cord Compression. Neurosurgery. 79 Suppl 1 (suppl_1), S73 (2016).
- Zheng, W., et al. Application of Diffusion Tensor Imaging Cutoff Value to Evaluate the Severity and Postoperative Neurologic Recovery of Cervical Spondylotic Myelopathy. World Neurosurgery. 118, e849-e855 (2018).
- Ellingson, B. M., Salamon, N., Holly, L. T. Imaging techniques in spinal cord injury. World Neurosurgery. 82 (6), 1351-1358 (2014).
- Zhao, C., et al. Diffusion tensor imaging of spinal cord parenchyma lesion in rat with chronic spinal cord injury. Magnetic Resonance Imaging. 47, 25-32 (2018).
- Mohanty, C., Massicotte, E. M., Fehlings, M. G., Shamji, M. F. The Association of Preoperative Cervical Spine Alignment with Spinal Cord Magnetic Resonance Imaging Hyperintensity and Myelopathy Severity: Analysis of a Series of 124 Cases. Spine. 40 (1), 11-16 (2015).
- Tetreault, L. A., et al. Systematic review of magnetic resonance imaging characteristics that affect treatment decision making and predict clinical outcome in patients with cervical spondylotic myelopathy. Spine. 38 (22 Suppl 1), S89 (2013).
- Nouri, A. . The Role of Magnetic Resonance Imaging in Predicting Surgical Outcome in Patients with Degenerative Cervical Myelopathy. , (2015).
- Chen, C. J., Lyu, R. K., Lee, S. T., Wong, Y. C., Wang, L. J. Intramedullary high signal intensity on T2-weighted MR images in cervical spondylotic myelopathy: prediction of prognosis with type of intensity. Radiology. 221 (3), 789-794 (2001).
- Vedantam, A., Jonathan, A., Rajshekhar, V. Association of magnetic resonance imaging signal changes and outcome prediction after surgery for cervical spondylotic myelopathy. Journal of Neurosurgery Spine. 15 (6), 660 (2011).
- Vedantam, A., et al. Diffusion tensor imaging of the spinal cord: insights from animal and human studies. Neurosurgery. 74 (1), 1-8 (2014).
- Bazley, F. A., et al. DTI for assessing axonal integrity after contusive spinal cord injury and transplantation of oligodendrocyte progenitor cells. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2012 (4), 82-85 (2012).
- Lewis, M., Yap, P. T., Mccullough, S., Olby, N. The relationship between lesion severity characterized by diffusion tensor imaging and motor function in chronic canine spinal cord injury. Journal of Neurotrauma. 35 (3), (2018).
- Hagmann, P., et al. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 26 Suppl 1 (suppl_1), S205 (2006).
- Zheng, W., et al. Time course of diffusion tensor imaging metrics in the chronic spinal cord compression rat model. Acta Radiologica. , 284185118795335 (2018).
- Jones, J. G., Cen, S. Y., Lebel, R. M., Hsieh, P. C., Law, M. Diffusion Tensor Imaging Correlates with the Clinical Assessment of Disease Severity in Cervical Spondylotic Myelopathy and Predicts Outcome following Surgery. American Journal of Neuroradiology. 34 (2), 471-478 (2013).
- Kerkovský, M., et al. Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. Spine. 37 (1), 48-56 (2012).
- Zheng, W., et al. Application of Diffusion Tensor Imaging Cutoff Value to Evaluate the Severity and Postoperative Neurologic Recovery of Cervical Spondylotic Myelopathy. World Neurosurgery. 118, e849-e855 (2018).
- Thurnher, M. M., Law, M. Diffusion-weighted imaging, diffusion-tensor imaging, and fiber tractography of the spinal cord. Magnetic Resonance Imaging Clinics of North America. 17 (2), 225-244 (2009).
- Cadotte, A., et al. Spinal Cord Segmentation by One Dimensional Normalized Template Matching: A Novel, Quantitative Technique to Analyze Advanced Magnetic Resonance Imaging Data. PLOS ONE. 10 (10), e0139323 (2015).

