Labeling and Imaging of Amyloid Plaques in Brain Tissue Using the Natural Polyphenol Curcumin
Summary
Curcumin is an ideal fluorophore for labeling and imaging of amyloid beta protein plaques in brain tissue due to its preferential binding to amyloid beta protein as well as its structural similarities with other traditional amyloid binding dyes. It can be used to label and image amyloid beta protein plaques more efficiently and inexpensively than traditional methods.
Abstract
Deposition of amyloid beta protein (Aβ) in extra- and intracellular spaces is one of the hallmark pathologies of Alzheimer's disease (AD). Therefore, detection of the presence of Aβ in AD brain tissue is a valuable tool for developing new treatments to prevent the progression of AD. Several classical amyloid binding dyes, fluorochrome, imaging probes, and Aβ-specific antibodies have been used to detect Aβ histochemically in AD brain tissue. Use of these compounds for Aβ detection is costly and time consuming. However, because of its intense fluorescent activity, high-affinity, and specificity for Aβ, as well as structural similarities with traditional amyloid binding dyes, curcumin (Cur) is a promising candidate for labeling and imaging of Aβ plaques in postmortem brain tissue. It is a natural polyphenol from the herb Curcuma longa. In the present study, Cur was used to histochemically label Aβ plaques from both a genetic mouse model of 5x familial Alzheimer's disease (5xFAD) and from human AD tissue within a minute. The labeling capability of Cur was compared to conventional amyloid binding dyes, such as thioflavin-S (Thio-S), Congo red (CR), and Fluoro-jade C (FJC), as well as Aβ-specific antibodies (6E10 and A11). We observed that Cur is the most inexpensive and quickest way to label and image Aβ plaques when compared to these conventional dyes and is comparable to Aβ-specific antibodies. In addition, Cur binds with most Aβ species, such as oligomers and fibrils. Therefore, Cur could be used as the most cost-effective, simple, and quick fluorochrome detection agent for Aβ plaques.
Introduction
Alzheimer's disease (AD) is one of the most common, age-related, progressive neurological disorders and one of the leading causes of death worldwide1,2. Learning, memory, and cognition impairment, along with neuropsychiatric disorders, are the common symptoms manifested in AD3. Although the etiology of AD has not been fully elucidated, the available genetic, biochemical, and experimental evidence indicates that gradual deposition of Aβ is a definitive biomarker for AD4. This misfolded protein accumulates in intracellular and extracellular spaces and is thought to be involved in synaptic loss, increased neuroinflammation, and neurodegeneration in the cortical and hippocampal regions in brain affected by AD5. Therefore, histochemical detection of Aβ in AD tissue is a crucial first step in developing non-toxic, anti-amyloid drugs to prevent AD progression.
During the past few decades, several dyes and antibodies have been used by many research laboratories to label and image Aβ plaques in brain tissue, but some of these methods are time consuming and the dyes or antibodies used are expensive, requiring several accessory chemicals. Therefore, the development of an inexpensive means of detection of Aβ plaques in the AD brain would be a welcome new tool. Many laboratories started using Cur, a promising anti-amyloid natural polyphenol, for labeling and imaging Aβ, as well as a therapeutic agent for AD6,7,8,9. Its hydrophobicity and lypophilic nature, structural similarities with classical amyloid binding dyes, strong fluorescent activity, as well as strong affinity to bind with Aβ makes it an ideal fluorophore for labeling and imaging of Aβ plaques in AD tissue10. Cur binds with Aβ-plaques and oligomers and its presence is also detected in intracellular spaces7,11,12,13. In addition, it has been shown that minimal amounts (1−10 nM) of Cur can label Aβ plaques in 5x familial Alzheimer's disease (5xFAD) brain tissue7. Although the 1 nM concentration does not provide the optimal fluorescence intensity for counting of Aβ plaques, a 10 nM or higher concentration of Cur does. Ran and colleagues14 reported that doses as low as 0.2 nM of difluoroboron-derivatized Cur can detect in vivo Aβ deposits nearly as well as an infrared probe. Whether this dose is sufficient to label Aβ plaques in tissue is still not clear. Most previous studies have used 20−30 min for staining Aβ plaques using Cur, but optimal staining may require much less time.
The present study was designed to test the minimum time required by Cur to label Aβ plaques in AD brain tissue and to compare the sensitivity for labeling and imaging of Aβ plaques in brain tissue from the 5xFAD mice after staining with Cur with other conventional Aβ-binding dyes, such as Thioflavin-S (Thio-S), Congo red (CR), and Fluoro-jade C (FJC). The Aβ labeling capability of these classical amyloid binding dyes was compared with Cur staining in paraffin-embedded and cryostat coronal brain sections from 5xFAD mice and from aged-matched human AD and control brain tissue. The findings suggest that Cur labels Aβ plaques in a manner similar to Aβ-specific antibodies (6E10) and moderately better than Thio-S, CR, or FJC. In addition, when intraperitoneal injections of Cur to 5xFAD mice were administered for 2−5 days, it crossed the blood-brain barrier and bound with Aβ plaques7. Interestingly, nanomolar concentrations of Cur have been used to label and image Aβ plaques in 5xFAD brain tissue7,14. Moreover, morphologically distinct Aβ plaques, such as core, neuritic, diffuse, and burned-out plaques can be labeled by Cur more efficiently than with any of the other conventional amyloid binding dyes7. Overall, Cur can be applied to label and image Aβ plaques in postmortem brain tissue from AD animal models and/or human AD tissue in an easy and inexpensive way, as a reliable alternative to Aβ-specific antibodies.
Protocol
All methods described here have been approved by the Animal Care and Use Committee (ACUC) of Saginaw Valley State University. The human tissue was obtained from an established brain bank at the Banner Sun Health Institute in Arizona15,16.
1. Perfusion of the animals
- Prepare the fixative and perfusion buffers.
- Prepare 0.1 M sodium phosphate buffer by adding 80 g of sodium chloride (NaCl), 2 g of potassium chloride (KCl), 21.7 g of disodium hydrogen phosphate (Na2HPO4·7H2O), 2.59 g of potassium dihydrogen phosphate (KH2PO4), and double distilled water to make a total of 1 L.
- Prepare 4% paraformaldehyde (PFA).
- Add 40 g of paraformaldehyde to 1 L of PBS (0.1 M, pH 7.4).
- Heat the PFA solution to 60−65 °C and mix using a magnetic stirrer.
NOTE: The temperature should not exceed 65 °C. - Add few drops of NaOH (1 N) with a dropper to dissolve the PFA completely.
- Filter the PFA solution with medium to fine filter paper and store at 4 °C.
NOTE: The solution is good for a month.
- Perform animal anesthesia and perfusion.
NOTE: Twelve-month-old B6SJL-Tg APP SwFlLon, PSEN1*M146L*L286V, 1136799Vas/J (5×FAD) age-matched control mice (n = 6 per group) were purchased from vendors and bred in the animal house of Saginaw Valley State University. Genotyping was confirmed by polymerase chain reaction (PCR) as described previously7. Human AD brain tissue includes postmortem AD brain tissue and age-matched control tissue.- Anesthetize the animal with an appropriate anesthetic agent, such as sodium pentobarbital (390 mg/kg body weight), or a ketamine/xylazine mixture (up to 80 mg/kg body weight ketamine and 10 mg/kg body weight xylazine) by intraperitoneal injection (27 G needle and 1 mL syringe). Check the level of anesthesia by pinching a toe. If the animal is unresponsive, then it is ready for perfusion surgery.
- Place anesthetized animal in the supine position on the perfusion surgery tray and using small iris scissors make an incision to the posterior end of the left ventricle.
- Insert a 22 G perfusion needle to the left ventricle and make a small incision at the right auricle to remove perfusion fluid from the body. Use a gravity-fed perfusion system to allow the ice-cold perfusion fluid (0.1 M PBS, pH 7.4) to flow for 5−6 min (flow rate 20−25 ml/min).
NOTE: A clear liver is the indicator of optimum perfusion. - Switch the buffer valve to an ice-cold 4% paraformaldehyde solution for fixing and allow it to flow for 8−10 min.
NOTE: Tremor followed by hardened or stiff limbs are indicators of good fixation. - Remove the brain from the skull using scissors. Using a spatula, collect the brain and place it in a vial of 4% PFA (at least 10x the volume of the brain volume) and store at 4 °C until further use.
2. Tissue processing
- Cut cryostat sections.
- Transfer the brain to graded sucrose solutions (10%, 20%, and 30%) and store at 4 °C for 24 h each, until use.
- Using a cryostat at -22 °C, cut 40 µm-thick sections. Collect 10−20 sections per well in a 6 well plate filled with PBS and sodium azide (0.02%).
- Paraffin embed the sections for mouse and human brain tissue.
- For paraffin sections, dehydrate the perfused and 24 h post-fixed brain tissue with graded alcohols (50%, 70%, 90%) for 2 h each, followed by 100% alcohol 2x for 1 h each), and then with xylene 2x for 1 h each) at room temperature.
- Penetrate the tissue with xylene-paraffin (1:1) 2x for 1 h at 56 °C in a glass conical flask covered with aluminum foil.
- Immerse the tissue in melted paraffin (56 °C) for 4−6 h.
- Cut 5 µm-thick sections using a rotary microtome at room temperature and place them in a tissue water bath at 45 °C.
- Histochemically label the Aβ plaques in the cryostat sections with Cur.
- Rinse the sections from step 2.1.2 with PBS (pH 7.4) 3x for 5 min each.
- Immerse the sections in 70% ethanol for 2 min at room temperature.
- Dissolve stock Cur (1 mM) in methanol and dilute with 70% ethanol to obtain a final working concentration of 10 µM.
- Immerse the sections with working Cur solution for 1−5 min at room temperature on a shaker at 150 rpm.
- Discard Cur solution and wash with 70% ethanol 3x for 2 min each.
- Put the sections on poly-L-lysine coated glass slides and mount with a coverslip using organic mounting media, such as distyrene plasticizer xylene (DPX).
- View under a fluorescence microscope using 480/550 nm excitation/emission filters.
- Histochemically label the Aβ plaques in the paraffin-embedded mouse and human brain sections with Cur.
- Deparaffinize the tissue sections from step 2.2.4 with xylene 2x for 5 min each at room temperature.
- Rehydrate with graded alcohol solutions (100%, 80%, 70%, 50% for 1 min each) and with distilled water 2x for 5 min each at room temperature.
- Stain sections with Cur (10 µM) for 10 min at room temperature in the dark, shaking at 150 rpm. Wash with 70%, 90%, and 100% alcohol for 2 min each.
- Clear with xylene 2x for 5 min each and cover slip with DPX.
- Visualize under a fluorescence microscope as mentioned in step 2.3.7.
- Colocalize Cur with the Aβ antibody in Aβ plaques and oligomers.
- Wash cryostat sections from step 2.1.2 with PBS 3x in a 12 well plate.
- Block the sections with 10% normal goat serum (NGS) dissolved in PBS with 0.5% Triton-X-100 at room temperature for 1 h.
- Discard the blocking solution. Incubate the sections with Aβ-specific antibodies (6E10 or A11, diluted 1:200) dissolved in fresh blocking solution containing 10% NGS and 0.5% Triton-X100 overnight at 4 °C in a shaker at 150 rpm.
- Discard the antibody solution and wash the sections with PBS 3x for 10 min each.
- Incubate with the secondary antibody tag with red fluorophore (e.g., Alexa 594) for 1 h at room temperature in the dark.
- Wash with PBS 3x for 10 min each.
- Wash with 70% alcohol 1x.
- Incubate the sections with Cur (10 µM) for 5 min at room temperature.
- Wash with 70% alcohol 3x for 1 min each.
- Dehydrate with 90% and 100% alcohol for 1 min each, clear with xylene 2x for 5 min each, and mount on slides using DPX.
- Visualize using a fluorescence microscope with appropriate excitation/emission filters for the red and green signals.
- For intracellular Aβ colocalization, stain the sections using Aβ-antibody (6E10), restain with Cur at room temperature in the dark with shaking at 150 rpm, and counterstain with either Hoechst-33342 (1 mg/ml) and/or DAPI (1ug/ml) for 10 min at room temperature in the dark with shaking at 150 rpm. Wash with PBS 3x.
- Take images with the red, green, and blue filters with a 100x objective (total magnification 1,000x).
- Label Aβ plaques with Thio-S, CR, and FJC.
NOTE: Detailed protocols for Thio-S and CR labeling were previously reported7.- For FJC staining, wash the free-floating sections obtained from step 2.1.2 with PBS 3x for 5 min each.
- Place the sections in a 12 well plate and stain with FJC (0.001%) for 10 min in the dark at room temperature.
- Discard FJC solution and wash with PBS 3x for 5 min each.
- Incubate with ammonium chloride (NH4Cl, 50 mM dissolved in PBS) for 10 min at room temperature.
- Discard the NH4Cl solution and wash with PBS 3x for 5 min each.
- Following the steps in section 2.4, dehydrate with graded alcohol solutions, clear, mount, and view under a fluorescence microscope using 450/520 nm excitation/emission filters.
Representative Results
Curcumin labels Aβ plaques within a minute. When we stained 5xFAD tissue with Cur, we found that Cur label Aβ plaques within 1 min. Although increased incubation time with Cur slightly increased the fluorescence intensity of Aβ plaques, the number of observed Aβ plaques was not significantly different between 1 min and 5 min staining time (Figure 1).
Cur can label Aβ plaques in cryostat-prepared, paraffin-embedded mouse and human AD tissue that colocalizes with Aβ-specific antibodies in mouse AD brain tissue. When we stained cryosection, paraffin-embedded sections (Figure 2A) and human AD tissue (Figure 2B), we observed Cur-labeled Aβ plaques in all types of tissue sections. Additionally, to confirm that Cur is binding to Aβ plaques, we first labeled the plaques with 6E10, followed by Cur staining. We observed that the Cur was completely co-localized with Aβ at the same plaques that bound the 6E10 (Figure 2C).
Cur labeled Aβ oligomers and intracellular Aβ aggregates. To check whether Cur could label Aβ oligomers, 5xFAD mouse sections were stained with an Aβ oligomer-specific antibody (A11), followed by Cur staining. We observed that Cur colocalized with A11 in Aβ plaques (Figure 3A). Similarly, Cur also colocalized with the 6E10 antibody in intracellular spaces (Figure 3B), indicating that it can label the intracellular Aβ.
Cur labeled Aβ more prominently than classical amyloid binding dyes. Aβ labeling by Cur was compared with commercially available amyloid binding dyes Thio-S, CR, FJC. The Aβ-specific antibody 6E10 was used as standard control. We observed that Cur labeled Aβ more prominently than the conventional amyloid binding dyes (Figure 4).
Cur derivatives bis-demethoxycurcumin (BDMC) and demethoxycurcumin (DMC), also present in turmeric extract, label Aβ plaques comparatively to Cur in 5xFAD brain tissue. Two other major components, such as BDMC and DMC, are present in turmeric extract. We tested whether these two compounds also label Aβ plaques similar to Cur. When 5xFAD mouse brain sections were stained with these derivatives, both BDMC and DMC also labeled Aβ plaques, parallelling Cur (Figure 5A). Different mounting media were investigated to check for interference with Aβ-imaging after staining with Cur. The fluorescent signal was intact in both aqueous and organic mounting media, such as DPX (Figure 5B). The staining of Aβ plaques with Cur was appropriate after immunofluorescent labeling and followed by counterstaining with Hoechst 33342 solution (1 mg/ml) or 4′,6-diamidino-2-phenylindole (DAPI, 1µg/ml). The immunofluorescent signals and counterstaining intensity were maintained after Cur staining (Figure 5C).
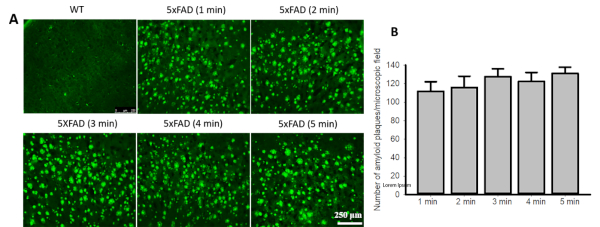
Figure 1: Curcumin labels Aβ plaques within a minute. (A). Brain sections from 5xFAD mice or human control and AD cortical tissue were stained with Cur (10 µM) for 1−5 min and the number of visible plaques were counted. (B). No difference was observed in the number of Aβ-plaque counts between the 1 min and 5 min staining times. Data are represented as mean ± standard error of mean (SEM). Please click here to view a larger version of this figure.
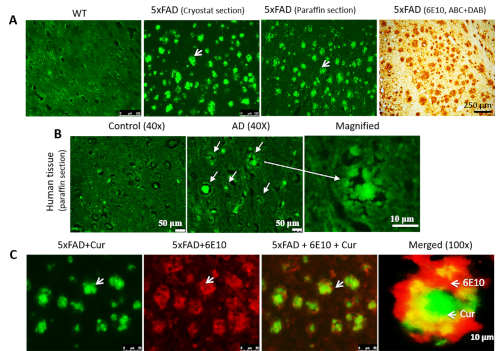
Figure 2: Colocalization of Cur with Aβ antibody in Aβ plaques. Cur can label Aβ plaques in cryostat-prepared, paraffin-embedded sections (A) and human AD tissue (B). The Aβ labeling paralleled the labeling with Aβ-specific antibody (6E10, DAB staining). (C). The 5xFAD sections were first labeled with Aβ antibody (6E10), followed by staining with Cur. Red = 6E10 bound by a secondary antibody tagged with Alexa fluorophore 594. Green = Cur. Cur completely colocalized with Aβ at the same plaques that bound to 6E10. Arrows indicate Aβ plaques. Scale bar indicates 50 µm (total magnification = 400x) in all three images on the left, and 10 µm (total magnification = 400x). Please click here to view a larger version of this figure.
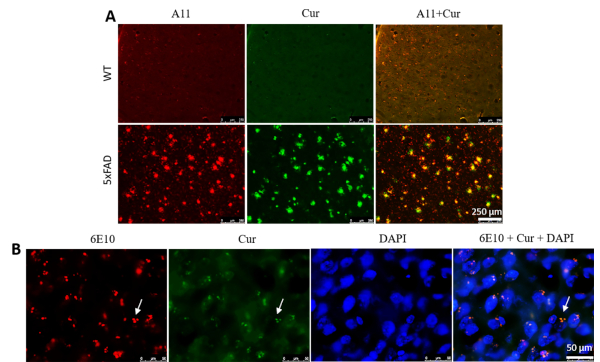
Figure 3: Curcumin labels Aβ oligomers and intracellular Aβ. (A) Aβ-oligomer was detected immunohistochemically using an Aβ-specific antibody (A11), followed by staining with Cur. Cur completely colocalized with Aβ, at the same oligomer where A11 binds. Scale bar = 250 µm and total magnification = 100x. (B) Similarly, intracellular Aβ was detected immunohistochemically using an Aβ-specific antibody (6E10), followed by staining with Cur. Note that Cur completely colocalized with Aβ in the same areas bound to 6E10. Scale bar = 50 µm and total magnification = 1,000x. Please click here to view a larger version of this figure.
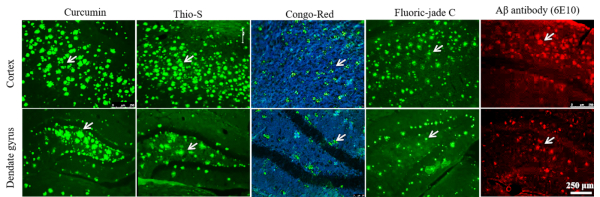
Figure 4: Comparison of different amyloid binding dyes with Cur to label Aβ plaques. Cryostat sections (40 µm) from the cortex of 12-month-old 5xFAD mice were stained with Cur, Thioflavin-S, Congo red, Fluoro-jade C, and with 6E10 antibody. Cur labeled Aβ plaques more prominently than Thio-S, CR, and FJC. Arrows indicate Aβ plaques. Please click here to view a larger version of this figure.
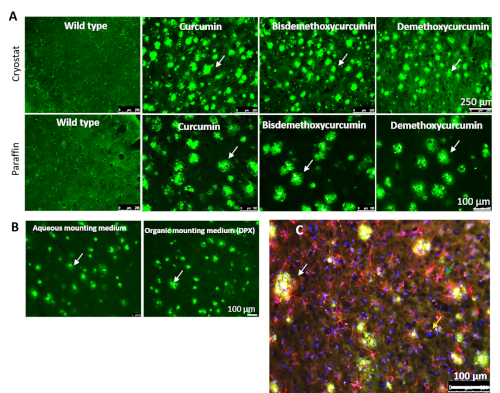
Figure 5: Cur-derivatives bis-demethoxy curcumin and demethoxy curcumin also label Aβ similarly to Cur. (A) Both cryostat and paraffin-embedded 5xFAD sections were stained with Cur-derivatives bis-demethoxy curcumin and demethoxy curcumin. Both these derivatives label Aβ plaques in a manner similar to Cur. (B) Both aqueous and organic mounting media (DPX) were used to mount tissue sections after labeling Aβ plaques with Cur. (C) Immunolabeled sections were used for Aβ labeling with Cur. The white arrows indicate Aβ plaques, the green arrow indicates activated astrocyte (GFAP), and the yellow arrow indicates nuclear stain (DAPI). Scale bars = 100 µm. Please click here to view a larger version of this figure.
| Features | Aβ-antibody | Curcumin | Thio-S | Congo red | Fluoro-Jade C |
| Duration of staining | ~24-48 h | ~1-5 min | ~10 min | ~60 min | ~30 min |
| Accessory chemicals | Secondary antibodies, several chemicals to make buffer, normal goat serum | Methanol | Ethanol | NaOH and ethanol | NaOH and ethanol, potassium permanganate |
| Cost | Costly: one Aβ-specific antibody vial requires ~$200-300 | Cost effective: ~$5-10/1 g Cur and can be applied for many tissues | Cost effective: ~$5/1 g, can be applied for few tissues | Cost effective: >$5/1 g Congo red, and can be applied for several tissues | Costly: ~$15.500/1 g FJC powder |
| Specificity | Different antibodies are required for Aβ oligomers and fibrils | Curcumin binds with Aβ oligomers and fibrils | Can bind only fibrils, not monomers, or oligomers | Can only bind with Aβ-protofibrils and fibrils16,17 | Can only bind with Aβ-fibrils and degenerated neurons |
| Stability | Depending on the dye attached to the secondary antibody | Very stable, even at room temperature when bound with Aβ | Stable in methanol | Stable in ethanol | Not stable |
| Care after staining | Needs extra care after staining, such as being kept in the dark and frozen all the time | Not as light sensitive and more stable at room temperature | Light sensitive | Not light sensitive | Light sensitive |
| Microscope required | Compound light or fluorescent (depending on use of secondary antibody) | Fluorescent | Fluorescent | Light microscope or polarized microscope or polarize filter | Fluorescent |
| Background staining | Generally, no background | Very low background | High background due to binding with lipid membrane or lipid compounds in cell | Low background | High background |
| In vivo Aβ-imaging | May not be applicable | Highly applicable | May not be applicable | May not be applicable | May not be applicable |
Table 1: Comparison of Aβ labeling with different amyloid binding dyes and Cur7.
Discussion
Our hypothesis was that Cur could be used as the quickest, easiest, and least expensive way to label and image Aβ plaques in postmortem AD brain tissue when compared to other classical amyloid binding dyes, as well as Aβ-specific antibodies. The aims of this study were to determine the minimum time required to label and image Aβ plaques by Cur in postmortem AD brain tissue and determine whether Cur can be used as an alternative to Aβ antibody for labeling Aβ plaques. To this end, the Aβ-labeling capability of Cur was observed at different time points. Cur was able to label Aβ within one minute. In addition, labeling of Aβ by Cur was greater than other conventional amyloid binding dyes, such as Thio-S (0.1%), CR (1%), and FJC (0.001%).
Cur is considered a unique and ideal fluorophore for Aβ labeling, because it has most characteristics possessed by most of the conventional amyloid binding dyes, including structural, physical, chemical, and biological properties10. In addition, due to affordability, most researchers are interested in using this natural polyphenol. To show the specificity of Cur binding to Aβ plaques and oligomers, we used 6E10 and A11 (Aβ-oligomer-specific antibody). Cur showed nearly complete colocalization with all the different species of Aβ present in the tissue, which suggests that Cur is highly specific to Aβ7,17,18,19,20. In addition, Cur labeled Aβ oligomers (Figure 3A) and intracellular Aβ aggregates (Figure 3B) and colocalized with Aβ antibodies (Figure 2B), suggesting that Cur can label not only extracellular Aβ plaques, but also Aβ deposited in intracellular spaces7.
During the past few decades, several fluorophores and antibodies have been developed to label and image Aβ plaques histochemically. Undoubtedly, most of them are very specific to targeted Aβ species and for detecting Aβ plaques, but these are much more expensive and their use is more time consuming than using Cur. For example, we compared the Aβ plaque binding by Cur, with other amyloid binding dyes, such as Thio-S, CR, and FJC, where Aβ-specific antibody (6E10) was used as a reference control. These results suggest that Cur labels Aβ plaques more strongly than any of the other fluorophores. Most importantly, relative to Cur, some of the more commonly used fluorophores have distinct disadvantages in terms of labeling and imaging Aβ. For example, Thio-S can produce a distracting, high background because it binds with lipid membranes or lipid compounds in the cell21. Similarly, CR, which is commonly used to label Aβ, produces apple green birefringence (Figure 4) under a polarized microscope. CR does not label Aβ-plaques as readily as do Cur or Thio-S, labeling significantly fewer Aβ plaques than those detected by Cur7,22,23. Gutierrez et al. reported that FJC, which can bind with Aβ and with degenerated neurons, labels at a lower frequency than either Cur or Thio-S24. These results suggest that these commonly used classical markers have less affinity for binding to Aβ plaques than Cur.
In addition, labeling of different Aβ-plaque types (core, neuritic, diffuse, burned-out) may be optimized with Cur, rather than other amyloid binding fluorophores, because Cur can help to visualize and distinguish morphologically different Aβ plaques, whereas other techniques fail to distinguish these morphological subtypes7. Similarly, the use of Aβ-specific antibodies, which are very specific to different species of Aβ is very costly and time consuming, taking at least 24−48h via immunohistochemistry. Moreover, detecting different species of Aβ require different antibodies, as well as several accessory chemicals, which significantly adds to the total cost. Clearly, Cur is less costly, more readily available, and produces higher fluorescence intensity when it binds to Aβ plaques. Although CR is also a relatively cost-effective technique for labeling and imaging of Aβ plaques, Cur can bind and label more of the Aβ species, such as oligomers25 (Figure 3 and Figure 4), whereas CR only binds to protofibrils and fibrils23. Therefore, Aβ labeling with Cur can be achieved more efficiently and cost-effectively than by Thio-S, CR, or FJC (Table 1).
In summary, Cur can detect Aβ plaques and oligomers from AD brain tissue effectively, rapidly, and inexpensively. In addition, Cur binding to Aβ is very specific and its fluorescent activity is very stable. It requires minimal amounts (1-10 nM) to label Aβ. Moreover, Cur is also very specific to different Aβ species, such as fibrils or plaques, as well as oligomers7. Similarly, Cur derivatives demethoxycurcumin and bisdemethoxycurcumin also harbor amyloid binding properties and c label Aβ similarly to Cur10 (Figure 5A). Therefore, Cur is an ideal fluorophore for labeling and imaging of Aβ plaques in postmortem brain tissue. It can be used as a quick and easy alternative to detect Aβ plaque load after anti-amyloid therapy in experimental animal models of AD. Our findings confirm the reports of the high affinity of Cur to Aβ, reinforcing its potential use for monitoring Aβ-plaques in postmortem brain and in living tissue.
For optimal Cur labeling it is recommended to not label Aβ with Cur in unperfused tissue and to avoid long-term tissue storage, as it can produce a greater amount of background, even when perfused. For colabeling, it is recommended to complete immunohistochemistry first with the specific antibody being used before staining with Cur and then follow this with counter-staining using DAPI or Hoechst.
Possible modifications to this method include increasing the incubation time of Cur with the tissue for up to 30 min, which will not interfere with signal intensity, although it may increase background while imaging. Decreasing concentration of Cur to less than 10 µM does not interfere in Aβ labeling to a significant level. To reduce background for human brain tissue, an alternative preparation method could be applied. For example, brain sections could be initially treated with 0.3% (w/v) Sudan Black B in 70% ethanol (v/v) for 10 min at room temperature. Then the section could be stained with Cur for 10 min at room temperature, and washed with PBS 3x for 15 min, counterstained, and mounted with antifading media13. Cryostat section thicknesses could also be reduced to 20−25 µm.
Potential caveats to this method include Cur binding with Aβ present in the blood vessels, which is different from the extracellular Aβ plaques. Thus, the investigator should be aware of the morphology of Aβ plaques and oligomers from Aβ in blood vessels. The investigator should be aware of auto-fluorescent signals. Excess green background can be seen occasionally after Cur staining, but this can be reduced by decreasing the staining time or by decreasing the concentration of Cur. Finally, the signal for colabeling with other markers may be reduced due to repeated treatments with the clearing agent (e.g., xylene).
Important limitations include the inability to colabel with any marker protein using secondary antibody tagged with green fluorescent dye, such as fluorescent isothiocyanate (FITC). These cannot be used because of the similar excitation/emission of Cur. Also, in the early stages of AD, only limited amounts of Aβ may be labeled by Cur. Finally, there is a need to do work in dark environment like any fluorescent dye.
Declarações
The authors have nothing to disclose.
Acknowledgements
Support for this study came from the Field Neurosciences Institute at Ascension of St. Mary's.
Materials
| 4′,6-diamidino-2-phenylindole (DAPI) | IHC world, Woodstock, MD | ||
| Aanimal model of Alzheimer's disease | Jackson's laboratory, Bar Harbor, ME | ||
| Absolute alcohol | VWR,Radnor, PA | ||
| Alexa 594 | Santacruz Biotech, Dallas, TX | ||
| Antibody 6E10 | Biolegend, San Diego, CA | ||
| Antibody A11 | Millipore, Burlington, MA | ||
| Compound light microscope | Olympus, Shinjuku, Japan | Olympus BX51 | |
| Congo red | Sigma, St. Louis, MO | ||
| Cryostat | GMI, Ramsey, MN | LeicaCM1800 | |
| Curcumin | Sigma, St. Louis, MO | ||
| Disodium hydrogen phosphate | Sigma, St. Louis, MO | ||
| Dystyrene plasticizer xylene | BDH, Dawsonville, GA | ||
| Filter papers | Fisher scientific, Pittsburgh, PA | ||
| Hoechst-33342 | Sigma, St. Louis, MO | ||
| Inverted fluorescent microscope | Leica, Buffalo Grove, IL | Leica DMI 6000B | |
| Inverted fluorescent microscope | Olympus, Shinjuku, Japan | Olympus 1×70 | |
| Normal goat serum | Sigma, St. Louis, MO | ||
| Paraffin | Sigma, St. Louis, MO | ||
| Paraformaldehyde | Sigma, St. Louis, MO | ||
| Ploy-lysine coated charged glass slide | Globe Scientific Inc, Mahwah, NJ | ||
| Potassium chloride | Sigma, St. Louis, MO | ||
| Potassium dihydrogen phosphate | Sigma, St. Louis, MO | ||
| Sodium azide | Sigma, St. Louis, MO | ||
| Sodium chloride | Sigma, St. Louis, MO | ||
| Sodium hydroxide | EMD Millipore, Burlington, MA | ||
| Sodium pentobarbital | Vortex Pharmaceuticals limited, Dearborn, MI | ||
| Thioflavin-S | Sigma, St. Louis, MO | ||
| Triton-X-100 | Sigma, St. Louis, MO | ||
| Xylene | VWR,Radnor, PA |
Referências
- Cummings, J. L. Alzheimer’s disease. New England Journal of Medicine. 351 (1), 56-67 (2004).
- Jack, C. R., Holtzman, D. M. Biomarker modeling of Alzheimer’s disease. Neuron. 80 (6), 1347-1358 (2013).
- Tarawneh, R., Holtzman, D. M. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harbor Perspectives in Medicine. 2 (5), (2012).
- Selkoe, D. J. Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nature Cell Biology. 6 (11), 1054-1061 (2004).
- Hardy, J., Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends in Pharmacological Sciences. 12 (10), 383-388 (1991).
- Chen, M., et al. Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease. Neural Regeneration Research. 13 (4), 742-752 (2018).
- Maiti, P., et al. A comparative study of dietary curcumin, nanocurcumin, and other classical amyloid-binding dyes for labeling and imaging of amyloid plaques in brain tissue of 5x-familial Alzheimer’s disease mice. Histochemistry and Cell Biology. 146 (5), 609-625 (2016).
- Maiti, P., Dunbar, G. L. Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases. International Journal of Molecular Sciences. 19 (6), (2017).
- Maiti, P., Dunbar, G. L. Comparative Neuroprotective Effects of Dietary Curcumin and Solid Lipid Curcumin Particles in Cultured Mouse Neuroblastoma Cells after Exposure to Abeta42. International Journal of Alzheimer’s Disease. , (2017).
- den Haan, J., Morrema, T. H. J., Rozemuller, A. J., Bouwman, F. H., Hoozemans, J. J. M. Different curcumin forms selectively bind fibrillar amyloid beta in post mortem Alzheimer’s disease brains: Implications for in-vivo diagnostics. Acta Neuropathologica Communications. 6 (1), 75 (2018).
- Koronyo, Y., et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight. 2 (16), (2017).
- Koronyo, Y., Salumbides, B. C., Black, K. L., Koronyo-Hamaoui, M. Alzheimer’s disease in the retina: imaging retinal abeta plaques for early diagnosis and therapy assessment. Neurodegenerative Diseases. 10 (1-4), 285-293 (2012).
- Koronyo-Hamaoui, M., et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. NeuroImage. 54 (Suppl 1), S204-S217 (2011).
- Ran, C., et al. Design, synthesis, and testing of difluoroboron-derivatized curcumins as near-infrared probes for in vivo detection of amyloid-beta deposits. Journal of the American Chemical Society. 131 (42), 15257-15261 (2009).
- Beach, T. G. The Sun Health Research Institute Brain Donation Program: Description and Experience, 1987-2007. Cell Tissue Bank. 9 (3), 229-245 (2008).
- Green, S. J., Killiany, R. J. Subregions of the inferior parietal lobule are affected in the progression to AD. Neurobiology of Aging. 31 (8), 1304-1311 (2010).
- Ono, K., Hasegawa, K., Naiki, H., Yamada, M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. Journal of Neuroscience Research. 75 (6), 742-750 (2004).
- Garcia-Alloza, M., Borrelli, L. A., Rozkalne, A., Hyman, B. T., Bacskai, B. J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. Journal of Neurochemistry. 102 (4), 1095-1104 (2007).
- Mutsuga, M., et al. Binding of curcumin to senile plaques and cerebral amyloid angiopathy in the aged brain of various animals and to neurofibrillary tangles in Alzheimer’s brain. Journal of Veterinary Medical Science. 74 (1), 51-57 (2012).
- Tei, M., Uchida, K., Mutsuga, M., Chambers, J. K., Nakayama, H. The binding of curcumin to various types of canine amyloid proteins. Journal of Veterinary Medical Science. 74 (4), 481-483 (2012).
- Liu, L., Komatsu, H., Murray, I. V., Axelsen, P. H. Promotion of amyloid beta protein misfolding and fibrillogenesis by a lipid oxidation product. Journal of Molecular Biology. 377 (4), 1236-1250 (2008).
- Wu, C., Scott, J., Shea, J. E. Binding of Congo red to amyloid protofibrils of the Alzheimer Abeta(9-40) peptide probed by molecular dynamics simulations. Biophysical Journal. 103 (3), 550-557 (2012).
- Wu, C., Wang, Z., Lei, H., Zhang, W., Duan, Y. Dual binding modes of Congo red to amyloid protofibril surface observed in molecular dynamics simulations. Journal of the American Chemical Society. 129 (5), 1225-1232 (2007).
- Gutierrez, I. L., et al. Alternative Method to Detect Neuronal Degeneration and Amyloid beta Accumulation in Free-Floating Brain Sections With Fluoro-Jade. ASN Neuro Methods. 10, 1-7 (2018).
- Yang, F., et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. Journal of Biological Chemistry. 280 (7), 5892-5901 (2005).

