A Screening Method for Identification of Heterochromatin-Promoting Drugs Using Drosophila
Summary
Drosophila is a widely used experimental model suitable for screening drugs with potential applications for cancer therapy. Here, we describe the use of Drosophila variegated eye color phenotypes as a method for screening small-molecule compounds that promote heterochromatin formation.
Abstract
Drosophila is an excellent model organism that can be used to screen compounds that might be useful for cancer therapy. The method described here is a cost-effective in vivo method to identify heterochromatin-promoting compounds by using Drosophila. The Drosophila's DX1 strain, having a variegated eye color phenotype that reflects the extents of heterochromatin formation, thereby providing a tool for a heterochromatin-promoting drug screen. In this screening method, eye variegation is quantified based on the surface area of red pigmentation occupying parts of the eye and is scored on a scale from 1 to 5. The screening method is straightforward and sensitive and allows for testing compounds in vivo. Drug screening using this method provides a fast and inexpensive way for identifying heterochromatin-promoting drugs that could have beneficial effects in cancer therapeutics. Identifying compounds that promote the formation of heterochromatin could also lead to the discovery of epigenetic mechanisms of cancer development.
Introduction
Heterochromatin is a condensed form of DNA that plays a central role in gene expression, in regulating chromosome segregation during cell division, and in protecting against genome instability1. Heterochromatin has been considered to be a gene repression regulator and to protect chromosome integrity during cell mitosis2,3. It is associated with the di- and tri-methylation of histone H3 lysine 9 (H3K9me) during lineage commitment4,5. Moreover, recruitment of Heterochromatin Protein 1 (HP1) chromodomain proteins is also considered to be associated with heterochromatin and epigenetic repression of gene expression6. These proteins are essential components and markers of heterochromatin formation.
Since genomic instability enables cells to acquire genetic alterations that promote carcinogenesis, heterochromatin is becoming more recognized in cancer development and may be targeted for cancer treatment7,8. Currently, there are no drugs that are well-established in assisting heterochromatin formation. Here, we present a simple and quick yet efficient method for screening small-molecule compounds that promote heterochromatin formation. The screening is done by treating Drosophila with a library of small-molecule drugs. This method takes advantage of a variegated eye color phenotype in the DX1Drosophila strain that is influenced by heterochromatin levels. DX1 flies contain a tandem array of seven P[lac-w] transgenes, which have the variegated expression/depression depending on heterochromatinization, therefore, the extent of variegation in the eye color reflect the heterochromatin level. Specifically, increasing heterochromatin could be detected by the rising proportion of variegated eye color (white eye). On the contrary, decreasing heterochromatin would be detected by the rising proportion of the P[lac-w] transgene expression (red eye)9,10,11,12.
Therefore, we take advantage of this Drosophila transgene system that produces a variegated eye color phenotype since its expression is directly correlated to the amount of heterochromatin present. Upon discovery of compounds that are suspected to promote heterochromatin formation, we may confirm this suspicion using other methods such as western blot. These heterochromatin-promoting substances may be further developed for clinical trials in patients in the future.
Protocol
1. Drug library preparation
- Identify and prepare a drug library to be screened using appropriate solvent at a desired concentration (e.g., 10 mM in DMSO).
NOTE: A specific example for a drug library is the Oncology Set III from National Cancer Institute (NCI) Developmental Therapeutics Program (DTP). Compounds in this set are provided as 20 µL at 10 mM in 100% DMSO in two 96-well PP U-bottom plates and stored at -20 °C. The NCI Plate maps, and the basic chemical data of each drug could be found at the following websites:
NCI plate number 4740
NCI plate number 4741
2. Screen for heterochromatin-promoting drugs using Drosophila
- Drosophila Breeding strategy (Figure 1A)
- Day 1: Cross three w1118/Y; DX1/CyO male flies and three or more virgin w1118 female flies in a 25 mm x 95 mm food vial with 9 mL of standard Drosophila food media (Bloomington recipe). Set up three replicate vials for each drug and one control for the screen.
NOTE: DX1 flies were kindly provided by James Birchler (University of Missouri)9,13. - Day 2 and Day 3: Allow the flies to lay eggs for 2 days at room temperature (22 °C).
- Day 4: Use a short burst of CO2 gas to anesthetize the parent flies and dispose of the parent flies in a "fly morgue" (a bottle containing 70% alcohol).
- Day 1: Cross three w1118/Y; DX1/CyO male flies and three or more virgin w1118 female flies in a 25 mm x 95 mm food vial with 9 mL of standard Drosophila food media (Bloomington recipe). Set up three replicate vials for each drug and one control for the screen.
- Feed drugs to Drosophila for screening.
- Dilute each drug compound from the original stock (20 µL at 10 mM in 100% DMSO in 96-well PP U-bottom plates) to a 10 µM final concentration with 33% DMSO in water. Use 33% DMSO in water without any drug as the control.
NOTE: Some drugs are poorly soluble in water and were dissolved in DMSO. 100% DMSO is toxic to flies. 33% is tolerable. - Day 4: Pipette 60 µL of a 10 µM drug solution or control onto the top of fly food. At this point, ensure that the parent flies have been removed and there are fly eggs and crawling first-instar larvae on the food.
- Day 6: Repeat pipetting 60 µL of the same 10 µM drug solution to the food vial.
NOTE: Since drugs were added on to the fly food, the top surface food contains nearly 10 µM concentration and the bottom food could be not be fully penetrated. All the flies lay eggs on the food top and eat the top surface food.
- Dilute each drug compound from the original stock (20 µL at 10 mM in 100% DMSO in 96-well PP U-bottom plates) to a 10 µM final concentration with 33% DMSO in water. Use 33% DMSO in water without any drug as the control.
- Observe and score eye color changes.
- Two days after the F1 flies emerge (approximately on day 14), examine the flies. Use a short burst of CO2 to anesthetize the F1 flies and remove the flies from their vial onto a porous dissecting pad with CO2 sipping through from underneath a filter paper.
- Inspect the flies on this pad under a dissection microscope. Examine the whole fly and identify all w1118/Y; DX1/+ heterozygous males by their lacking the CyO dominant curly wing phenotype.
- Score the eye color of each of the w1118/Y; DX1/+ heterozygous male flies on a scale of 1 to 5 (Figure 1B). The percentage of white eye color was rated as follows:
1. <5% red scattered eye total surface area
2. 6% – 25% red spots eye total surface area
3. 26% – 50% red spots eye total surface area
4. 51% – 75% red spots eye total surface area
5. >75% red spots eye total surface area
NOTE: Alternatively, homogenize fly heads in 100% methanol and use a spectrometer to measure eye pigmentation at OD 450 nm. Select only males because PEV is more pronouced in DX1 males. Score more than 10 males in each vial. - Calculate the mean color index of all the males from each vial scored. Perform triplicates for each compound (Figure 1C).
NOTE: Since eye color varies with age, this is a time-sensitive step. The eye color should be calculated at the same age – 2 days old. Usually >10 flies should be scored in each vial. - To confirm that the compounds indeed promote heterochromatin formation, use a different technique such as western blotting to validate (Figure 1G).
Representative Results
This protocol was successfully used to screen compounds that promote heterochromatin formation in Drosophila, which is an efficient and low-cost in vivo system for drug development (Figure 1). We screened a small-molecule drug library, Oncology Set III, which is composed of 97 FDA authorized oncology drugs, using the DX1 strain of Drosophila melanogaster (Figure 1B). The results for a significant drug were represented in a bar graph (Figure 1C,D). According to the screening assay, methotrexate (4-aminopteroylglutamic acid), which is coded as E7 in the library, caused the most variegation in the DX1 strain, suggesting that methotrexate could be the most promising heterochromatin-promoting drug for future cancer targeted-therapy (Figure 1E,F). To further confirm that this is indeed testing the compounds promoting heterochromatin formation, western blot data shows that heterochromatin formation associated protein H3K9me3 is upregulated (Figure 1G), further verifying that methotrexate could be the most promising heterochromatin-promoting drug.
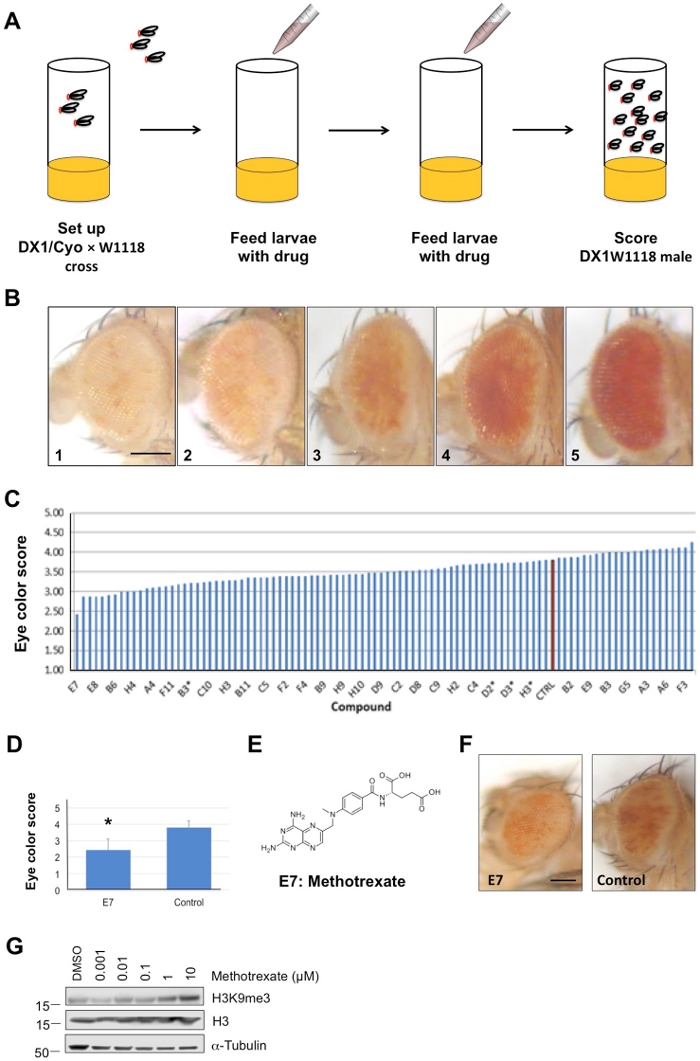
Figure 1: An illustration of the drug screening in Drosophila. (A) Drosophila screen methodology. An overview of scheme for heterochromatin-promoting compounds screening by using the Drosophila model. Three w1118/Y;DX1/CyO males and three virgin w1118 are crossed at room temperature. Then, remove the adult flies at day four and add 60 µL of the 10 µM drug dissolved in 33% DMSO solution to the top of the fly food at day four and day six, respectively. 33% DMSO solution was used as a control. The eye color (white to red) ratio of the F1 male generation were observed. (B) Eye color phenotype analysis and score. Representative images of eye color showing variegated phenotype. The percentage of variegation eye color was rated as following: 1, 1-5% red spots in total surface area; 2, 6-25% red spots in total surface area; 3, 26-50% red spots in total surface area; 4, 51-75% red spots in total surface area; and 5, >75% red spots in total surface area. (Scale Bar = 200 μm) (C) Each drug was assayed as the mean values from three independent experiments ± s.d. (standard deviation). Red bar shows the control. (D) Results of the scale performed in drug E7 and control. Differences between E7 and control groups were considered significant if P-values were <0.05 (*) by Student's t-Test. (E) The molecular structure of a chemical compound which termed E7 used in the example drug library. (F) Representative images of the fly eyes in E7 and control treatment group. (Scale Bar, 200 μm) (G) The western blot was performed with antibodies specific for H3K9me3, H3, or α-Tubulin by using the 3rd instar larvae total protein without or with methotrexate treatment at the indicated concentrations. This figure has been modified from Loyola et al14. Please click here to view a larger version of this figure.
Discussion
Heterochromatin is a condensed form of DNA that plays a central role in regulating gene expression. It is becoming increasingly more recognized in cancer and may serve as a potential target for cancer therapy15,16,17,18. Small-molecule compounds are commonly used in drug development due to advantages in manufacture, preservation, and metabolism in human bodies. To identify heterochromatin-promoting small-molecule compounds, an efficient method was designed and presented by using the DX1Drosophila strain, which has been proven to affect flies' eye color in a heterochromatin-dependent manner (Figure 1).
Our screening protocol is a simple, inexpensive and easy-to-follow in vivo system for drug development. However, one limitation of the screening strategy is determining an effective drug concentration. The female fruit fly lays her eggs on the surface of the semisolid fly food. For the drug treatment, we pipet the drug solution to the surface of the food, which is supposed to contain a predetermined concentration. However, since different drugs could have different efficiencies in diffusing to the bottom of the food, the drug concentration is not guaranteed to be consistent below the surface or towards the bottom of the food. At 10 µM concentration, lethality was seldom observed in larvae treated with any of the drugs in the library we screened. However, most of the drugs caused severe lethality to the larvae at 100 µM concentration, suggesting that drug concentration could also be considered as an important factor for the screen.
Here, we notice that tandem repeats such as those used here can trigger PEV by a mechanism that differs in some regards from that seen in pericentric heterochromatin. A drug that impacts PEV during embryonic and larval development (the test described here) will only identify drugs that impact the maintenance of heterochromatin in somatic cells and will not identify drugs that impact the initial formation of heterochromatin, which most likely occurs during blastoderm (nuclear cycles 10-14). Nonetheless, this could be a useful assay for a quick starting screen.
This protocol is reliable and cost-effective due to the sensitivity of this transgene cluster (DX1) to heterochromatin formation, reflected by the amount of red pigmentation present in the eye. Additionally, the short lifespan of Drosophila makes this protocol more efficient than other model organisms such as zebrafish or human cells. While there likely might be a reporter gene system present in zebrafish that is just as sensitive to heterochromatin levels, their lifespan is much longer than Drosophila and would take longer to obtain results. Additionally, using human cells would not be possible since this protocol specifically focuses on using phenotypic changes from epigenetic regulation to determine heterochromatin levels. Thus, this protocol provides an efficient in vivo method to determine which small drug molecule could potentially serve as a candidate for suppressing oncogenes in cancer therapeutics. While the Drosophila drug screening method is a slow approach to identifying compounds when compared with some in vitro screening methods, it is relatively sensitive and allows us to test compounds in vivo. In combination with the cell screening method, which is relatively inexpensive, easy to perform and is high throughput, the Drosophila screening method could also be used to confirm hits as a supplementary method.
In summary, with an interest in identifying compounds for further research and targeting heterochromatin for cancer therapy, although the mechanism in which this is occurring is not yet fully understood, a simple screen was performed in order to identify heterochromatin-promoting drugs. Discovering a low concentration at which a drug can promote heterochromatin formation could offer more unique treatments that may result in fewer side effects compared to modern chemotherapy treatments.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank J. Birchler, E. Bach and the Bloomington Drosophila Stock Center for various Drosophila strains; the National Cancer Institute (NCI) Developmental Therapeutics Program for the Oncology Set small-molecule drug library; UCSD undergraduate students including Amy Chang, Taesik You, Jessica Singh-Banga, Rachel Meza, and Alex Chavez. Research reported in this publication was supported by a research grant from American Thoracic Society to J.L. and funding from NIH: R01GM131044 to W.X.L.
Materials
| Drosophila | DX1 strain | DX1 flies were kindly provided by James Birchler (University of Missouri) | |
| Drosophila food media | UCSD fly kitchen | ||
| Methotrexate | NCI drug library | ||
| Dimethyl sulfoxide (DMSO) | Sigma | D2650 | |
| Ethyl alcohol | Sigma | E7023-500ML |
Referências
- Morgan, M. A., Shilatifard, A. Chromatin signatures of cancer. Genes Development. 29 (3), 238-249 (2015).
- Saksouk, N., Simboeck, E., Dejardin, J. Constitutive heterochromatin formation and transcription in mammals. Epigenetics Chromatin. 8, 3 (2015).
- Allshire, R. C., Madhani, H. D. Ten principles of heterochromatin formation and function. Nature Reviews Molecular Cell Biology. 19 (4), 229-244 (2018).
- Jenuwein, T., Allis, C. D. Translating the histone code. Science. 293 (5532), 1074-1080 (2001).
- Grewal, S. I., Moazed, D. Heterochromatin and epigenetic control of gene expression. Science. 301 (5634), 798-802 (2003).
- Heard, E. Delving into the diversity of facultative heterochromatin: the epigenetics of the inactive X chromosome. Current Opinion in Genetics & Development. 15 (5), 482-489 (2005).
- Ci, X., et al. Heterochromatin Protein 1alpha Mediates Development and Aggressiveness of Neuroendocrine Prostate Cancer. Pesquisa do Câncer. 78 (10), 2691-2704 (2018).
- Zhu, Q., et al. Heterochromatin-Encoded Satellite RNAs Induce Breast Cancer. Molecular Cell. 70 (5), 842-853 (2018).
- Dorer, D. R., Henikoff, S. Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell. 77 (7), 993-1002 (1994).
- Fanti, L., Dorer, D. R., Berloco, M., Henikoff, S., Pimpinelli, S. Heterochromatin protein 1 binds transgene arrays. Chromosoma. 107 (5), 286-292 (1998).
- Shi, S., et al. JAK signaling globally counteracts heterochromatic gene silencing. Nature Genetics. 38 (9), 1071-1076 (2006).
- Shi, S., et al. Drosophila STAT is required for directly maintaining HP1 localization and heterochromatin stability. Nature Cell Biology. 10 (4), 489-496 (2008).
- Ronsseray, S., Boivin, A., Anxolabehere, D. P-Element repression in Drosophila melanogaster by variegating clusters of P-lacZ-white transgenes. Genética. 159 (4), 1631-1642 (2001).
- Loyola, A. C., et al. Identification of methotrexate as a heterochromatin-promoting drug. Scientific Reports. 9 (1), 11673 (2019).
- Dialynas, G. K., Vitalini, M. W., Wallrath, L. L. Linking Heterochromatin Protein 1 (HP1) to cancer progression. Mutation Research. 647 (1-2), 13-20 (2008).
- Janssen, A., Colmenares, S. U., Karpen, G. H. Heterochromatin: Guardian of the Genome. Annual Review of Cell and Developmental Biology. 34, 265-288 (2018).
- Zhu, Q., et al. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature. 477 (7363), 179-184 (2011).
- Hu, X., et al. Unphosphorylated STAT5A stabilizes heterochromatin and suppresses tumor growth. Proceedings of the National Academy of Sciences of the United States of America. 110 (25), 10213-10218 (2013).

