Flow Cytometry-Based Quantification and Analysis of Myocardial B-Cells
Summary
Here we report a protocol for the quantification and differentiation of myocardial B-lymphocytes based on their location in the intravascular or endothelial space using flow cytometry.
Abstract
A growing body of evidence shows that B-lymphocytes play an important role in the context of myocardial physiology and myocardial adaptation to injury. However, the literature reports contrasting data on the prevalence of myocardial B-cells. B-cells have been reported to be both among the most prevalent immune cells in the rodent heart or to be present, but at a markedly lower prevalence than myeloid cells, or to be quite rare. Similarly, several groups have described that the number of myocardial B-cells increases after acute ischemic myocardial injury, but one group reported no changes in the number of B-cells of the injured myocardium. Implementation of a shared, reproducible method to assess the prevalence of myocardial B-cells is critical to harmonize observations from different research groups and thus promote the advancement of the study of B-cell myocardial interactions. Based on our experience, the seemingly contrasting observations reported in the literature likely stem from the fact that murine myocardial B-cells are mostly intravascular and connected to the microvascular endothelium. Therefore, the number of B-cells recovered from a murine heart is exquisitely sensitive to the perfusion conditions used to clean the organ and to the method of digestion used. Here we report an optimized protocol that accounts for these two critical variables in a specific way. This protocol empowers reproducible, flow cytometry-based analysis of the number of murine myocardial B-cells and allows researchers to distinguish extravascular vs. intravascular myocardial B-cells.
Introduction
B-lymphocytes are highly specialized immune cells that play an important role in both adaptive and innate immune responses1. There are two main populations of B-cells: a smaller population of B1 cells that are mostly produced during embryonic life, and a preponderant population of B2 cells that are produced in adult life in the bone marrow1. After maturing in the bone marrow, B-cells migrate to primary and secondary lymphoid organs. From there they continuously recirculate between lymphoid organs traveling through blood vessels and lymphatic vessels2. B-cells express specific antibodies on their surface, which function as receptors. When B-cells encounter an antigen that binds to their receptor, an activating signal can be triggered. Activated B-cells either migrate to the tissue where the antigen was found or go back to the bone marrow where they can mature into antibody-producing plasma cells3,4.
Recently, it has been appreciated that the heart harbors a sizeable population of B-cells. Studies in rodents have shown that B-cells colonize the heart early during embryonic development5, and that myocardial-associated B-cells are mostly intravascular, naive B2 cells adhered to the endothelium6,7, with a small percentage of B1 cells7. There are still many areas of uncertainty, but the available data indicates that B-cells play an important role both in the naive heart and in the context of myocardial adaptation to injury.
Studies in the naive murine heart have shown that at baseline myocardial B-cells are mostly located in the intravascular space, adhered to the endothelium (>95% of murine cardiac B-cells were found to be located in the intravascular space). These B cells were found to have gene expression patterns different from those of circulating B cells isolated from the peripheral blood. Analysis of naive hearts from B-cell-deficient animals and syngeneic controls found that animals lacking B-cells had smaller hearts and higher ejection fraction6. All this evidence suggests that B-cells might modulate myocardial growth and/or myocardial function, and that not only interstitial but also intravascular B-cells could be responsible for such observations. B-cells were also found to modulate the phenotype of myocardial resident macrophages8.
Several studies have shown that B-cells play an important role in the context of myocardial adaptation to injury8,9,10,11,12,13. B-cells accumulate transiently in the injured heart, likely through a CXCL13-CXCR5 dependent mechanism11,13. From there, B-cells promote adverse cardiac remodeling through several mechanisms that include cytokine-mediated monocyte recruiting9,12. In addition, B-cells can produce antibodies against cardiac proteins that can promote the extension of cardiac damage and adverse cardiac remodeling through several mechanisms14,15,16,17,18,19,20,21,22,23,24,25. B-cells can also exert protective effects on the injured heart through the secretion of IL-1010.
As the number of groups investigating the role of B-cells in the naive and injured heart grows, it is becoming more and more important to define shared protocols to properly quantify and assess myocardial B-cells and thus avoid inconsistencies that have already started to appear in the literature. So far B-cells have in fact both been reported to be one of the most prevalent immune cells in the rodent heart7 and to be present at a markedly lower prevalence than myeloid cells26,27, or to be quite rare28. Similarly, several groups have described that the number of myocardial B-cells increases after acute ischemic myocardial injury7,9,13, but one group reported no changes in the number of B-cells of the injured myocardium29. Studies on cardiac immune cells rarely give details on perfusion conditions and there is no consensus on the digestion conditions. Since in the rodent heart a large proportion of B-cells are intravascular and extraction of immune cells from the myocardium is highly dependent on the digestion method used, the differences reported in the literature might be the result of differences in organ perfusion and tissue digestion.
Presented here is a detailed method for flow cytometry-based quantification of murine myocardial B-cells that maximizes the yield of B-cell recovery by optimizing perfusion and digestion conditions and allows the discrimination of intravascular vs. extravascular myocardial B-cells6. This protocol is an adaptation and optimization of other similar protocols that distinguish between intravascular and interstitial immune cells28,30,31.
In this protocol, we standardize myocardial perfusion to eliminate B-cells floating in the intravascular space without removing biologically relevant B-cells adhered to the microvascular endothelium. Moreover, building on prior protocols that have described the use of intravenous injection of antibodies to distinguish intravascular from interstitial immune cells32, and taking advantage of the fact that B-cells express the surface marker B22033, we demonstrate how to distinguish intravascular vs. extravascular myocardial B-cells through intravascular injection of a B220-specific antibody immediately before the animal sacrifice and cardiac perfusion. This protocol is relevant to the research of any scientist interested in including the analysis of myocardial B-cells in the naive and injured heart. The widespread implementation of this protocol will reduce inconsistencies between research groups, will allow the analysis of changes in the intravascular and extravascular myocardial B-cell pools, and thus will bolster the advancement of discoveries in the field of cardiac immunology.
In summary, the protocol represents an optimized workflow to quantify and analyze myocardial B-cells via flow cytometry, and at the same time distinguish between cells located in the extravascular space and the intravascular space.
Protocol
All experiments described in this manuscript were performed with the approval of the IACUC at Johns Hopkins University School of Medicine.
1. Preparations
- Prepare the FACS Buffer, as described in Table 1.
- Ensure there is enough CO2 to euthanize the animals.
- Prepare the dissection space (place the bench pad and place the tape and dissection tools nearby).
- Label the 15 mL tubes, put 3 mL of HBSS with calcium and magnesium in each tube (see Table of Materials) and place them on ice (one tube per heart and an extra one for the Reference group/flow cytometry controls). Also, fill the small (35 mm) Petri dishes with HBSS.
- Turn on the temperature-controlled shaker, set it to 37 °C, and pre-chill the centrifuge at a temperature of 4 °C.
- Prepare the syringe pump and set the flow rate to 3 mL/min. Fill a 20 mL syringe with HBSS, prime the tube line with buffer, and remove any bubbles.
2. Intravascular B-cell staining (in vivo)
- Weigh a 12-week-old male mouse of the C57BL/6J strain. Load a 3/10 cc insulin syringe with 100 µL of the B220 antibody dilution (1:10, in PBS). Cover the syringe with aluminum foil to protect from light.
- Anesthetize one mouse.
- Apply an intraperitoneal injection of 2% 2,2,2-tribromoethanol with a dose of 400 mg/kg and wait 10-20 min.
NOTE: Monitor the mouse's breathing and movement. Once the mouse stops moving, stimulate pain by pinching the toe; the absence of a withdrawal reflex indicates adequate anesthesia. - If adequate anesthesia is not achieved within the course of 20 min, an additional dose of anesthetic at 100 mg/kg can be administered, and an assessment of mouse kinetics, withdrawal reflex, and respiration should be made every 5 min.
- Apply an intraperitoneal injection of 2% 2,2,2-tribromoethanol with a dose of 400 mg/kg and wait 10-20 min.
- Place the mouse on the lab bench on a bench pad inclined toward one side, gently pull down the skin of the back, and slightly press the body against the bench to make the eye protrude.
- Inject the diluted B220 antibody from step 2.1 by retroorbital injection.
- Introduce the syringe into the eye at 45° from the sagittal plane of the mouse's head. Slowly inject the solution. If resistance is felt, remove the syringe and enter again. Wait for 2 min.
NOTE: Prolonged incubation time would jeopardize the ability to discriminate intra-vascular vs extra-vascular B cells as it would give time to the injected antibody to diffuse outside of the vasculature.
- Introduce the syringe into the eye at 45° from the sagittal plane of the mouse's head. Slowly inject the solution. If resistance is felt, remove the syringe and enter again. Wait for 2 min.
- Euthanize the mouse as per the IACUC regulations.
- Open the CO2 flow to the euthanasia chamber at a rate of 0.5 L/min, or the recommended flow rate based on the size of your chamber.
- Place the mouse in the chamber for the recommended time, ensuring it is no longer breathing. Remove it and perform cervical dislocation as a secondary method of euthanasia.
- Once the mouse is euthanized, promptly proceed to organ harvesting and perfusion.
3. Heart collection
- Chest opening.
- Hold the skin of the mouse with forceps at the epigastric area. Make a 2 mm opening in the skin using scissors and use the forceps to peel the skin away to reveal the fascia layer, a shiny translucent layer, of the chest and abdomen. Cut at the epigastrium through the fascia and peritoneum to open the abdominal wall and reveal the xiphoid process. Clamp the xiphoid process using hemostatic forceps.
- With the scissors, make a vertical cut through the chest wall at the level of the mid-clavicular line on each side and lift the anterior chest wall to reveal the mediastinum content, using hemostatic forceps as a weight to maintain the opening.
- Heart perfusion and extraction.
- Using forceps, remove any fat or thymic tissue surrounding the heart.
- Hold the aorta securely from behind using forceps. Introduce the syringe needle (25 G) into the apex of the heart. Perfuse with 3 mL of HBSS at a rate of 3 mL/min.
NOTE: Monitor the perfusion. The liver should fill, and blood in the coronary vessels should be entirely removed. Use of a syringe pump calibrated to 1 mL/ minute is recommended to maintain a steady flow. - Cut the aorta using scissors and take out the heart. Wash the heart in the Petri dish with HBSS to remove any remaining blood. Using scissors and forceps, remove the fat, connective tissue and thymus, and dry the heart. Weigh the isolated heart and note the weight in milligrams. Mince the heart at room temperature into small pieces (1-2 mm) with a blade.
NOTE: Adult mouse hearts may weigh between 0.090-0.210 mg. - Measure the mass of heart tissue and place 60 mg of the minced heart to be digested in a 15 mL tube with 3 mL of HBSS. Retain the remaining heart tissue and place in a tube labelled "Reference control tissue"; this will be used for compensation control for live-dead signal and autofluorescence.
4. Heart digestion and cell staining
- Add enzymes to each tube containing minced tissue (see Table 1). Add 0.5 µL/mg of DNase 1 (300 Units for 60 mg of heart tissue), 0.5 µL/mg of collagenase II (625 Units for 60 mg of heart tissue), and 0.2 µL/mg of hyaluronidase (50 Units for 60 mg of heart tissue) to the tube.
- Place the digestion reaction in the shaker for 30 min at 300 rpm. Adjust the shaker rack to about 25° of incline.
- Add 7 mL of HBSS to each of the 15 mL reaction tubes and centrifuge at 250 x g for 5 min at 4 °C with acceleration and breaking set to moderate or slow. Decant the supernatant and resuspend each pellet in 5 mL of ACK lysis buffer to remove red blood cells. Incubate in the ACK lysis buffer for 5 min at room temperature.
- Add 10 mL of PBS to each tube, mix, and then filter into a 50 mL tube with a 40 µm strainer. Ensure that all the debris is inside the filter chamber. Smash any debris on the filter with a syringe plunger until it is completely suspended within the filter chamber.
- Add PBS through the filter until the volume reaches 25 mL per heart; this will allow the suspended cells to pass through the filter. Add an additional 25 mL of PBS to the Reference control tissue tube and divide the volume into different tubes (25 mL each). Label one of the tubes "Unstained" and the other one as "Live-dead". Centrifuge at 250 x g for 5 min at 4 °C.
NOTE: At this point, prepare the dilution for the live-dead stain (dilution: 1:500 in 1x PBS). - Decant the supernatant, resuspend the pellet in the Unstained tube in 100 µL of PBS and each of the other pellets in 100 µL of the live-dead stain dilution. Incubate on ice for 30 min in the dark, covering the ice bucket with aluminum foil.
- Add 500 µL of FACS buffer to each sample and transfer it into a labelled FACS tube through a 40 µm filter. Centrifuge the FACS tubes at 250 x g for 5 min at 4 °C. Discard the supernatant. Resuspend the pellet in 500 µL of FACS buffer.
- Add 50 µL of Fc blocking solution (see Table 1) to each tube, except for the Unstained and Live-dead tubes. Mix and incubate for 5 min.
- Add 50 µL of antibody mix solution (see Table 1) to each tube, except for the Unstained and Live-dead tubes, and incubate for 30 min in the dark, covering the ice bucket with aluminum foil.
- Add 2 mL of FACS buffer to each tube and centrifuge at 250 x g for 5 min at 4 °C. Remove the supernatant and resuspend in 300-500 µL of FACS buffer.
5. Flow cytometry
- Setup instrument control settings.
NOTE: In this protocol the sample is analyzed with a 4 lasers Cytek Aurora flow cytometer. Another appropriate flow cytometer could be used to detect the markers of interest.- Open the instrument control software (see Table of Materials). At the top of the window, select the Acquisition tab and click on New +. A window called Create New Experiment will display.
- In the Library section, in the field Type to filter, type PE, PerCP-Cy5.5, BV421, Alexa Fluor 700, and Zombie Aqua, clicking Add after typing each. The fluorophore names should be seen to the right. Then click Next to proceed to the Group tab.
- In the Group tab, click the symbol with a tube to add the heart for the analysis.
- Click on the + Reference group and a window will display. In the fluorescent tag column select Zombie Aqua, and in the control type column select Cells, then click Save.
- Click Next > Markers tab; a table will display with the names of the fluorophores. Type the corresponding cell marker in the first row of each fluorophore column and type Enter: CD45 for PerCP-Cy5.5; CD19 for BV421; CD11b for Alexa Fluor 700; B220 for PE; and live-dead for Zombie Aqua.
- Click Next twice to go to the Acquisition tab. In Events to Record of the experiment group type 10,000,000, and in the same field for the reference group line type 200,000. Click Save and Open. The other settings should remain as default, Stopping Time to 10,000, and Stopping Volume to 3,000.
- On the bottom left click on Instrument control. Set Voltage to FSC-50, SSC-175, SSC-B-175. Then, select the Acquisition Control. For the Flow rate option, select High from the drop-down menu.
- Acquire reference samples with a cytometer and unmix in software.
- Vortex the Unstained heart tube and place it securely on the sample injection port (SIP). Ensure the green indicator arrow is pointing to the correct sample, then click Start in the Acquisition Control panel of the cytometer software and wait until the events are recorded. Do the same for the Live-dead-stained heart tissue.
- Select the Unmix menu and set the following controls:
- Select the checkboxes for PE, PerCP-Cy5.5, BV421, and Alexa Fluor 700 in the column from library. Ensure Zombie Aqua is unchecked in the same column. At the bottom, check the option of Autofluorescence as a Fluorescent Tag.
- Click Next to proceed to the Identify Positive/Negative Populations tab. In this tab, a plot of FSC-A vs. SSC-B-A will be displayed. Click and drag the points of the polygon to select an area between 1.0 M and 4.0 M on the FSC-A axis, and between 0.2 M and 3.0 M on the SSC-B-A axis.
- In the next plot to the right, V5-A will be displayed on the X-axis. Move the gates to select half of the peak on the far right as positive and a region on the left side of the plot as negative. In the plot titled Reference Group – Unstained, select a population in a similar range. For this the unstained no positive-negative has to be set.
- Acquire experimental samples.
- For each tube containing a sample for an individual mouse, vortex the tube and place it securely on the SIP. Ensure the green indicator arrow is pointing to the correct sample, then click Start in the Acquisition Control panel of the cytometer software, and wait until the events are recorded.
- Gating strategy.
- Select the Default Unmixed Worksheet tab. Create an FSC-A vs. SSC-A plot using the Dot plot tool on top. Select events higher than 0.4 M on the FSC-A axis and higher than 0.8 M on the SSC-A axis using the Polygon selection tool. Double click in this selection and a new plot will display.
- From the newly created plot, right-click on the X axis label and select AF-A; right-click on the Y axis label and select Live-dead stain. Click on the Rectangle selection tool on top and select all the events below 105 on the live-dead axis that correspond to the lower live-dead signal. Double click in this selection and a new plot will display.
- In the new plot, right-click to select PerCP-Cy5.5-A for the X axis and SSC-A for the Y axis. Using the Rectangle selection tool, select the events higher than 104 on the PerCP-Cy5.5-A axis that correspond to the CD45 positive cells. Double click on the selection and a new plot will display.
- On the new plot, right-click to select FSC-A for the X axis and FSC-H for the Y axis. Using the Polygon selection tool select the events that follow a trend on which FSC-A value and SSC-A value are the same; with this the cell doublets will be removed and the population in the selection will be referred to as the CD45+ population. Double click on the selection to display a new plot with the CD45+ population.
- From the CD45+ population plot, right-click to select BV421 on the X axis vs. SSC-A on the Y axis. Using the Polygon selection tool, select the population to the right on the BV421 axis. This is the B-cell population. Double click twice in this population to create two new plots with the B-cell population.
- Right-click to setup the first B-cell plot with PE-A at the X axis and BV421-A on the Y axis. The population to the right corresponds to the intravascular B-cells, and the population to the left represents the interstitial B-cells. Use the Polygon selection tool to make a selection of both.
- Right-click to setup the second B-cell plot with Alexa Fluor 700-A on the X axis and SSC-A on the Y axis. The population at the left corresponds to the CD11b- cells, and the events at the right (if any) correspond to the CD11b+ cells.
- Right click on each selection and then click Gate Properties. Click on Count and % Parent to display the sizes and percentages. Record the number of events and percentages for further data analysis.
Representative Results
Once the acquisition is complete and all the events are collected, the data should be analyzed according to standard flow cytometry practice. The focus of the analysis will vary depending on the individual goal of each experiment. In this case, quantification of intravascular and extravascular B-cells was pursued, and it was expressed as the number of cells per mg of tissue.
When using a spectral cytometer, it is recommended to start the analysis with an initial gating to remove debris with sizes that are not in a range corresponding to immune cells, followed by removal of the live-dead stain+ signal. This allows the detection of a clean population of CD45 positive cells corresponding to leukocytes (Figure 1). After the removal of doublets, in a naive uninjured heart, it is expected that about 20% of CD45+ cells will be CD19+ B-cells (Figure 2A, 18.1% B-cells in this representative experiment). Of these cells, on average 5.5% are located in the interstitial space, while 94.5% are located in the intravascular space (Figure 2B). From the whole B-cell population found in the heart, only about 1.6% corresponds to B1 cells based on the surface marker CD11b (which is typically considered sufficient to classify a murine CD19+ cell as a B1 cell with a high degree of confidence1,34,35), and the other 98.4% corresponds to B2 cells (Figure 2C).
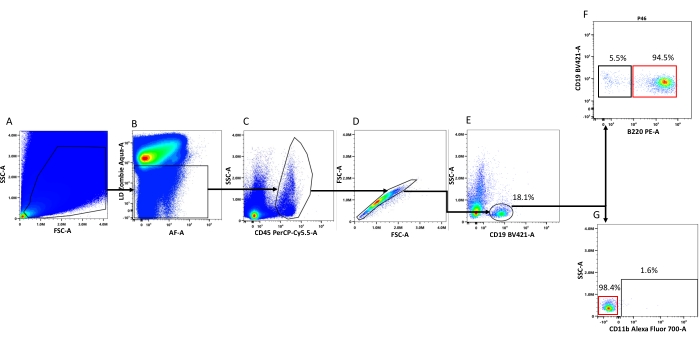
Figure 1: Flow cytometry gating strategy used to detect and characterize myocardial-associated B-cells. (A) FSC-A vs. SSC-A: The plot shows all the events collected, and the area selected is drawn to enrich the analysis with events that are in the range of size of leukocytes. (B) Autofluorescence vs. live-dead staining: The selected area corresponds to the lower live-dead signal. The gate drawn removes dead cells and some other cell debris that bind to the live-dead staining. (C) CD45 signal vs. SSC-A: The selected area corresponds to the CD45+ cells (i.e., myocardial leukocytes). (D) FSC-A vs. FSC-H: This gate is drawn to remove cell doublets (unselected events). (E) CD19 signal vs. SSC-A: The selected area corresponds to myocardial B-cells. (F) B220 vs. CD19: The area inside the black square corresponds to the intramyocardial B-cells (CD19+, B220-), and the area inside the red square corresponds to the intravascular B-cell population. (G) CD11b vs. SSC-A: The area inside the black circle corresponds to the CD11b+ (B1 cells), and the area inside the red circle corresponds to the CD11b- (B2 cells). The percentages displayed on each panel represent the average percentage that the population displayed represents from the parental population. Please click here to view a larger version of this figure.
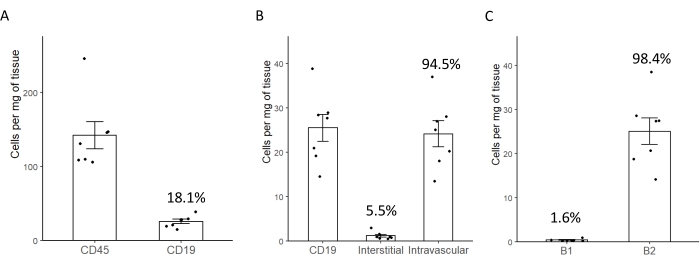
Figure 2: Representative plots reporting the average number of B-cells per milligram of myocardial tissue. (A) Average number of CD45+ and CD19+ cells/mg of murine myocardial tissue. The percentage displayed corresponds to the percentage of cells from the parental population (CD45+). (B) Average number of CD19+ cells and the percentage of the B-cells that are in the interstitial (extravascular) space of intravascular spaces. (C) Absolute number and percentage of myocardial B-cells that express markers of B1 or B2 cells. Error bars correspond to the standard error of the mean. Please click here to view a larger version of this figure.
Table 1: This table contains the solutions and reagents needed for the procedure. Please click here to download this Table.
Discussion
A growing body of evidence indicates that B-cells play an important role in the context of myocardial physiology and myocardial remodeling/adaptation to injury7,8,9,10,11,12,13,36. Flow cytometry is an excellent tool to study immune cell populations in any tissue and almost a mandatory tool for any study that focuses on B-cells in the heart.
The emerging literature on B-cells and the heart already reports contrasting findings on very basic aspects, such as the number of B-cells in the heart and changes in myocardial B-cell number with injury. B-cells have been reported as the most prevalent immune cell in rodent hearts7. However, other studies evaluated their relative prevalence compared to other myeloid cells and showed markedly lower numbers26,27. Pinto et al.26 found that B-cells comprised around 9% of the total leukocytes, while Yu et al.27 reported 10%. Adamo et al.7 reported that the average percentage of B-cells relative to leukocyte numbers was around 20%, which is very close to the 18.1% reported in this manuscript. Other studies have reported that B-cells have a very low prevalence in the myocardium28.
Several critical experimental steps could explain the variabilities in B-cell numbers reported in the literature. Namely, differences in the perfusion of the heart, tissue mincing, and digestion can significantly affect the number of B-cells recovered per mg of heart tissue. Perfusion with buffers containing calcium chelating agents and no calcium, or perfusion with a higher volume or perfusion rate, could detach the B-cells attached to the endothelium and reduce the recovery yield. Excessively fine tissue mincing could lead to over-digestion of the tissue and loss of cell viability. A reduction in cell viability could also be the result of digesting for a prolonged time or with higher enzyme concentrations.
The percentage of CD45+ cells that correspond to B-cells could be in a range between 15%-30% in a wild-type mouse. Depending on the mouse strain used, the age, and the genetic background, this value can vary. However, the percentage should remain similar in the studied group. Large variability in the number and percentage of B-cells between mice belonging to the same group suggests that an experimental error may be occurring. Low percentages could indicate an excess in perfusion strength and time. Global reduction in CD45+ cells could indicate that the tissue is being over-digested.
The protocol described here has been optimized to provide a consistent yield of B-cells from the digested murine heart and to allow the discrimination of intravascular vs. extravascular B-cells6. Discrimination of intravascular vs. extravascular B-cells is achieved via intravascular injection of an antibody immediately prior to sacrifice. The antibody injected intravascularly readily diffuses throughout the vasculature and encounters all the intravascular B-cells. If the animal is sacrificed soon enough and the heart is promptly harvested, the antibody does not have time to diffuse in the extravascular space and stain extravascular B-cells. Since the time between the intravascular injection of the antibody and organ harvest is critical, it is recommended to inoculate with the antibody and collect one individual mouse heart at a time. Intravascular injection of antibodies can be omitted if the discrimination of intravascular vs. extravascular B-cells is not of interest. The gating strategy displayed in Figure 1 would remain the same except for the last step, which would not be possible due to the lack of B220 labeling. Depending on the experimental design, any fluorophore from the panel shown can be modified to a color that fits with the panel used. Also, it is important to consider that the selection of the surface markers could be modified according to the needs of the group performing it; for instance, if in the experimental design it is believed that CD11b expression on B1 cells could be reduced, the addition of different markers is recommended, such as IgM, IgD, and CD537.
Please note that it might be necessary to process a heart just to run compensation controls for flow cytometry. This is recommended the first time the experiment is optimized. In the following experiments, saving some tissue at the moment of heart collection could be sufficient, as it would provide sufficient cells to run a negative "unstained" control and a positive "live-dead" stain control. Individual controls for the other colors could be either imported from prior experiments or generated using beads.
Some steps of the protocol can be modified without altering the outcomes of the experiment. For instance, the anesthesia method could be changed for a preferred one depending on the researcher's experience; the use of isoflurane instead of 2,2,2-tribromoethanol could reduce the time spent between anesthesia administration and heart collection. Also, the perfusion of the heart could be done manually without the use of a syringe pump as long as it is performed slowly, gently, and consistently.
Despite the accurate execution of the protocol, inexperienced operators could encounter high variability between samples within the same experimental session or between different experimental sessions. Based on experience, this variability disappears once the scientist has had the opportunity to become fully familiar with the workflow. It is therefore recommended that anyone who wants to implement this protocol assess its reproducibility in two to three trial runs before collecting crucial data on their research projects.
In summary, existing methods for isolating immune cells from the heart and analyzing them via flow cytometry typically do not consider the ability of immune cells to attach to the endothelium, and either do not standardize perfusion or implement extensive perfusion, with the assumption that any immune cell in the intravascular space is a contaminant that must be purged. This ignores the notion that intravascular immune cells adhered to the endothelium have biological relevance. The protocol here presented has been optimized to maximize the recovery of myocardial immune cells with specific attention to myocardial B-cells, intravascular and extravascular. We expect that this protocol will be of interest to all those scientists who work in the field of cardiac immunology and are interested in exploring the function that B-cells might play in healthy and injured hearts.
Declarações
The authors have nothing to disclose.
Acknowledgements
This study was funded by NHLBI grants 5K08HLO145108-03 and 1R01HL160716-01 awarded to Luigi Adamo.
The Aurora Flow Cytometer used to develop this study was funded by NIH Grant S10OD026859. We acknowledge the support of the JHU Ross Flow Cytometry Core.
Materials
| Alexa Fluor 700 anti-mouse/human CD11b Antibody | 101222 | BioLegend | 100 µg 200 µL |
| (CellTreat 29481) Cell Strainer, 40 µm, Blue | QBIAP303 | Southern Labware | |
| 0.5 mL Natural Microcentrifuge Tube | 1605-0000 | SealRite, USA Scientific | |
| 0.9% Sodium Chloride Injection, USP | 114-055-101 | Quality Biological | 0.90% |
| 1.5 mL Natural Microcentrifuge Tube | 1615-5500 | SealRite, USA Scientific | |
| 10 µL Graduated TipOne Filter Tips | 11213810 | USA Scientific | |
| 1000 µL Graduated TipOne Filter Tips | 11267810 | USA Scientific | |
| 15 mL Centrifuge Tube, Plug Seal Cap, Polypropylene, RNase-/DNase-free | 430052 | Corning | |
| 1-Way Stop Valve, Polycarbonate | SVPT951 | ECT Manufacturing | |
| 2,2,2-Tribromoethanol | T48402 | Sigma-Aldrich | |
| 200 µL Graduated TipOne Filter Tips | 11208810 | USA Scientific | |
| 3-Way Stop Valve, Polycarbonate | SVPT953 | ECT Manufacturing | |
| 5 mL Polystyrene Round-Bottom Tube, 12 x 75 mm style | 352054 | Falcon, a Corning Brand | |
| 50 mL Centrifuge Tube, Plug Seal Cap, Polypropylene, RNase-/DNase-free | 430290 | Corning | |
| ACK (Ammonium-Chloride-Potassium) Lysing Buffer | 118-156-101 | Quality Biological | Osmolality: 290 + or -5% mOsm/Kg H20 |
| Adapter 4x50ml, for 250 mL rectangular bucket in Rotor A-4-63 | 5810759005 | Eppendorf | |
| Adapter for 15 mL Centrifuge Tubes, 9 Tubes per Adapter, Conical Bottom for use with Rotor Model A-4-62 | 22638289 | Eppendorf | |
| Adapter for 15 round-bottom tubes 2.6 – 7 mL, for 250 mL rectangular bucket in Rotor A-4-62 | 22638246 | Eppendorf | |
| Aluminum Foil 12 in x 75' Roll .0007 | UPC 109153 | Reynolds Wrap | |
| Anesthesia Induction Chamber – Mouse | RWD-AICMV-100 | Conduct Science | |
| BD Luer Slip Tip Syringe with attached needle 25 G x 5/8 in., sterile, single use, 1 mL | 309626 | BD Becton, Dickinson and Company | |
| Brandzig Ultra-Fine Insulin Syringes 29G 1cc 1/2" 100-Pack | CMD 2613 | Brandzig | |
| Brilliant Violet 421 anti-mouse CD19 Antibody | 115537 | BioLegend | 50 µg/mL |
| CAPS for Flow Tubes w/strainer mesh 35 µm, Dual position for 12 x 75 mm tubes, sterile | T9009 | Southern Labware | |
| Carbon Dioxide USP E CGA 940 | CD USPE | AirGas USA | |
| Cole-Parmer Essentials Low-Form Beaker, Glass, 500 mL | UX-34502-46 | Cole-Parmer | |
| Collagenase 2 | LS004176 | Sigma-Aldrich | |
| Connector brass chrome plated 1/4" female NPT x 1/4" barb | Y992611-AG | AirGas USA | |
| Cytek Aurora Flow Cytometer | Cytek Biosciences | ||
| Diss 1080 Nipple 1/4 BARB CP | M-08-12 | AirGas USA | |
| DNase I – 40,000 U | D4527 | Sigma-Aldrich | |
| Easypet 3 – Electronic Pipette Controller | 4430000018 | Eppendorf | |
| Electronic Balance, AX223/E | 30100606 | Ohaus Corp. | |
| Eppendorf 5810R centrifuge | 5810R | Eppendorf | |
| Eppendorf Research plus 1-channel variable pipettes | Eppendorf | ||
| FlowJo 10.8.1 | BD Becton, Dickinson and Company | ||
| GLACIERbrand, triple density Ice Pan (IPAN-3100) | Z740287 | Heathrow Scientific | |
| HBSS (1x) – Ca2+ [+] Mg2+ [+] | 14025076 | gibco | 1x |
| Hyaluronidase | H3506 | Sigma-Aldrich | |
| Kelly Hemostats, Straight | 13018-14 | Fine Science Tools | |
| Luer Slip Syringe sterile, single use, 20 mL | 302831 | BD Becton, Dickinson and Company | |
| M1 Adj. Reg 0-100 PSI/CGA940 | M1-940-PG | AirGas USA | |
| McKesson Underpads, Moderate | 4033-CS150 | McKesson | |
| Navigator Multi-Purpose Portable Balance | NV2201 | Ohaus Corp. | |
| PBS pH 7.4 (1X) Ca2+ [-] Mg2+ [-] | 10010023 | gibco | 1x |
| PE anti-mouse/human CD45R/B220 Antibody | 103208 | BioLegend | 200 µg/mL |
| PerCP/Cyanine5.5 anti-mouse CD45 Antibody | 103132 | BioLegend | 100 µg 500 uL |
| Petri dish, Stackable 35 mm x 10 mm Sterile Polystyrene | FB0875711YZ | Fisher Scientific | |
| Pkgd: Diss 1080 Nut/CO2/CO2-02 | M08-1 | AirGas USA | |
| Powerful 6 Watt LED Dual Goose-Neck Illuminator | LED-6W | AmScope | |
| PrecisionGlide Needle 25 G x 5/8 (0.5 mm x 16 mm) | 305122 | BD Becton, Dickinson and Company | |
| Purified Rat Anti-Mouse CD16/CD32 (Mouse BD Fc Block) Clone 2.4G2 (RUO) | 553141 | BD Becton, Dickinson and Company Biosciences | 0.5 mg/mL |
| R 4.1.1 | The R Foundation | ||
| Razor Blades | 9501250000 | Accutec Blades Inc | |
| Regulator analytical two stage 0-25 psi delivery CGA320 3500 psi inlet | Y12244A320-AG | AirGas USA | |
| Rotor A-4-62, incl. 4 x 250 mL rectangular buckets | Rotor A-4-62 | Eppendorf | |
| Serological pipette, plugged, 10 mL, sterile, non-pyrogenic/endotoxin-free, non-cytotoxic, 1 piece(s)/blister | 86.1254.001 | Sarstedt AG & Co KG | |
| Sigma label tape | L8394 | Sigma-Aldrich | |
| SpectroFlo 3.0.0 | Cytek Biosciences | ||
| Spex VapLock Luer Fitting, PP, Straight, Male Luer Lock x 1/8" Hose Barb; 1/EA | MTLL230-6005 | Spex | |
| Std Wall Lab Tubing, Size S2, Excelon, 1/8" ID x 3/16" OD x 1/32" Wall x 50' Long | CG-730-003 | Excelon Laboratory | |
| Syringe PP/PE without needle, 3 mL | Z683566 | Millipore Sigma | |
| Syringe pump | 55-1199 (95-240) | Harvard Apparatus | |
| Thomas 3-Channel Alarm Timer TM10500 | 9371W13 | Thomas Scientific | |
| Tube Rack, 12 positions, 6 for 5.0 mL and 15 mL tubes and 6 for 25 mL and 50 mL tubes, polypropylene, numbered positions, autoclavable | 30119835 | Eppendorf | |
| Tube Rack, 12 positions, for 5.0 mL and 15 mL tubes, polypropylene, numbered positions, autoclavable | 30119827 | Eppendorf | |
| TYGON R-3603 Laboratory Tubing, I.D. × O.D. 1/4 in. × 3/8 in. | T8913 (Millipore Sigma) | Tygon, Saint-Gobain | |
| Vortex-Genie 2 | SI-0236 | Scientific Industries, Inc. | |
| VWR Dissecting Forceps with Guide Pin with Curved Tips | 89259-946 | Avantor, by VWR | |
| VWR Dissecting Scissors, Sharp Tip, 4½" | 82027-578 | Avantor, by VWR | |
| VWR Incubating Orbital Shaker, Model 3500I | 12620-946 | Avantor, by VWR | |
| Zombie Aqua Fixable Viability Kit | 423102 | BioLegend |
Referências
- Adamo, L., Rocha-Resende, C., Mann, D. L. The emerging role of B lymphocytes in cardiovascular disease. Annual Review of Immunology. 38, 99-121 (2020).
- Gowans, J. L., Knight, E. J. The route of re-circulation of lymphocytes in the rat. Proceedings of the Royal Society of London. Series B: Biological Sciences. 159 (975), 257-282 (1964).
- Kunkel, E. J., Butcher, E. C. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 16 (1), 1-4 (2002).
- Tanaka, T., et al. Molecular determinants controlling homeostatic recirculation and tissue-specific trafficking of lymphocytes. International Archives of Allergy and Immunology. 134 (2), 120-134 (2004).
- Rocha-Resende, C., et al. Developmental changes in myocardial B cells mirror changes in B cells associated with different organs. JCI Insight. 5 (16), (2020).
- Adamo, L., et al. Myocardial B cells are a subset of circulating lymphocytes with delayed transit through the heart. JCI Insight. 5 (3), 139377 (2020).
- Adamo, L., et al. Modulation of subsets of cardiac B lymphocytes improves cardiac function after acute injury. JCI Insight. 3 (11), (2018).
- Rocha-Resende, C., Pani, F., Adamo, L. B cells modulate the expression of MHC-II on cardiac CCR2(-) macrophages. Journal of Molecular and Cellular Cardiology. 157, 98-103 (2021).
- Zouggari, Y., et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nature Medicine. 19 (10), 1273-1280 (2013).
- Wu, L., et al. IL-10-producing B cells are enriched in murine pericardial adipose tissues and ameliorate the outcome of acute myocardial infarction. Proceedings of the National Academy of Sciences. 116 (43), 21673-21684 (2019).
- Heinrichs, M., et al. The healing myocardium mobilizes a distinct B-cell subset through a CXCL13-CXCR5-dependent mechanism. Cardiovascular Research. 117 (13), 2664-2676 (2021).
- Sun, Y., et al. Splenic marginal zone B lymphocytes regulate cardiac remodeling after acute myocardial infarction in mice. Journal of the American College of Cardiology. 79 (7), 632-647 (2022).
- Yan, X., et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. Journal of Molecular and Cellular Cardiology. 62, 24-35 (2013).
- Iwata, M., et al. Autoimmunity against the second extracellular loop of beta(1)-adrenergic receptors induces beta-adrenergic receptor desensitization and myocardial hypertrophy in vivo. Circulation Research. 88 (1), 578-586 (2001).
- Jahns, R., et al. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. The Journal of Clinical Investigation. 113 (10), 1419-1429 (2004).
- Christ, T., et al. Autoantibodies against the beta1 adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. Journal of Molecular and Cellular Cardiology. 33 (8), 1515-1525 (2001).
- Jane-wit, D., et al. Adrenergic receptor autoantibodies mediate dilated cardiomyopathy by agonistically inducing cardiomyocyte apoptosis. Circulation. 116 (4), 399-410 (2007).
- Ludwig, R. J., et al. Mechanisms of autoantibody-induced pathology. Frontiers in Immunology. 8, 603 (2017).
- Haudek, S. B., et al. Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proceedings of the National Academy of Sciences. 105 (29), 10179-10184 (2008).
- Staudt, A., Eichler, P., Trimpert, C., Felix, S. B., Greinacher, A. Fc(gamma) receptors IIa on cardiomyocytes and their potential functional relevance in dilated cardiomyopathy. Journal of the American College of Cardiology. 49 (16), 1684-1692 (2007).
- Zhang, M., et al. The role of natural IgM in myocardial ischemia-reperfusion injury. Journal of Molecular and Cellular Cardiology. 41 (1), 62-67 (2006).
- Zhang, M., et al. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proceedings of the National Academy of Sciences. 101 (11), 3886-3891 (2004).
- Schulze, K., Becker, B. F., Schauer, R., Schultheiss, H. P. Antibodies to ADP-ATP carrier–an autoantigen in myocarditis and dilated cardiomyopathy–impair cardiac function. Circulation. 81 (3), 959-969 (1990).
- Matsumoto, Y., Park, I. K., Kohyama, K. B-cell epitope spreading is a critical step for the switch from C-protein-induced myocarditis to dilated cardiomyopathy. The American Journal of Pathology. 170 (1), 43-51 (2007).
- Caforio, A. L. P., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. European Heart Journal. 34 (33), 2636-2648 (2013).
- Pinto, A. R., et al. Revisiting cardiac cellular composition. Circulation Research. 118 (3), 400-409 (2016).
- Yu, Y. R., et al. A protocol for the comprehensive flow cytometric analysis of immune cells in normal and inflamed murine non-lymphoid tissues. PLoS One. 11 (3), 0150606 (2016).
- Epelman, S., et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 40 (1), 91-104 (2014).
- Horckmans, M., et al. Pericardial adipose tissue regulates granulopoiesis, fibrosis and cardiac function after myocardial infarction. Circulation. 137 (9), 948-960 (2017).
- Lavine, K. J., et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proceedings of the National Academy of Sciences. 111 (45), 16029-16034 (2014).
- Bajpai, G., Lavine, K. J. Isolation of macrophage subsets and stromal cells from human and mouse myocardial specimens. Journal of Visualized Experiments. (154), e60015 (2019).
- Anderson, K. G., et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nature Protocols. 9 (1), 209-222 (2014).
- Coffman, R. L., Weissman, I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 289 (5799), 681-683 (1981).
- Montecino-Rodriguez, E., Dorshkind, K. B-1 B cell development in the fetus and adult. Immunity. 36 (1), 13-21 (2012).
- Bermea, K., Bhalodia, A., Huff, A., Rousseau, S., Adamo, L. The role of B cells in cardiomyopathy and heart failure. Current Cardiology Reports. , 01722-01724 (2022).
- Zhao, T. X., et al. Rituximab in patients with acute ST-elevation myocardial infarction: an experimental medicine safety study. Cardiovascular Research. 118 (3), 872-882 (2022).
- Kushnir, N., et al. B2 but not B1 cells can contribute to CD4+ T-cell-mediated clearance of rotavirus in SCID mice. Journal of Virology. 75 (12), 5482-5490 (2001).

