Visualization and Quantification of Endogenous Intra-Organelle Protein Interactions at ER-Mitochondria Contact Sites by Proximity Ligation Assays
Summary
The need for new approaches to study membrane contact sites (MCSs) has grown due to increasing interest in studying these cellular structures and their components. Here, we present a protocol that integrates previously available microscopy technologies to identify and quantify intra-organelle and inter-organelle protein complexes that reside at MCSs.
Abstract
Membrane contact sites (MCSs) are areas of close membrane proximity that allow and regulate the dynamic exchange of diverse biomolecules (i.e., calcium and lipids) between the juxtaposed organelles without involving membrane fusion. MCSs are essential for cellular homeostasis, and their functions are ensured by the resident components, which often exist as multimeric protein complexes. MCSs often involve the endoplasmic reticulum (ER), a major site of lipid synthesis and cellular calcium storage, and are particularly important for organelles, such as the mitochondria, which are excluded from the classical vesicular transport pathways. In the last years, MCSs between the ER and mitochondria have been extensively studied, as their functions strongly impact cellular metabolism/bioenergetics. Several proteins have started to be identified at these contact sites, including membrane tethers, calcium channels, and lipid transfer proteins, thus raising the need for new methodologies and technical approaches to study these MCS components. Here, we describe a protocol consisting of combined technical approaches, that include proximity ligation assay (PLA), mitochondria staining, and 3D imaging segmentation, that allows the detection of proteins that are physically close (>40 nm) to each other and that reside on the same membrane at ER-mitochondria MCSs. For instance, we used two ER-anchored lipid transfer proteins, ORP5 and ORP8, which have previously been shown to interact and localize at ER-mitochondria and ER-plasma membrane MCSs. By associating the ORP5-ORP8 PLA with cell imaging software analysis, it was possible to estimate the distance of the ORP5-ORP8 complex from the mitochondrial surface and determine that about 50% of ORP5-ORP8 PLA interaction occurs at ER subdomains in close proximity to mitochondria.
Introduction
Inter-organelle communication is a defining characteristic of eukaryotic cells. One way in which organelles communicate is by forming membrane contact sites (MCSs), which are close membrane oppositions between two organelles that are maintained by structural and functional proteins, such as tethers, lipid transfer proteins, and calcium channels1. MCSs can be established between similar or different organelles, and they mediate the exchange of cellular components, which is important for maintaining cellular homeostasis. To date, several MCSs have been identified, including endoplasmic reticulum (ER)-mitochondria, ER-plasma membrane (PM), and ER-lipid droplet (LD) contacts1. Among them, those formed between the ER and the mitochondria (MERCSs) are among the most studied as they are involved in the regulation of several cellular functions, including lipid and calcium homeostasis2. As mitochondria are largely excluded from the classical vesicular transport pathways, they rely on MERCS and on their molecular constituents to import key lipids or lipid precursors from the ER. The non-vesicular transport of these lipids across MERCSs ensures the maintenance of proper mitochondrial lipid composition, as well as their functional and structural integrity3.
Given the crucial involvement of MCSs in various cellular functions, the interest in providing a deeper understanding of their molecular components has greatly increased in the last years. Several types of imaging-based approaches have been used to advance the knowledge on MCSs. Among them, the fluorescence probe-based proximity ligation assay (PLA) has been widely used as an indicator of the abundance of MCSs by detecting inter-organelle protein-protein interactions (in a detection range of 40 nm) at endogenous levels4. For instance, MERCSs have been visualized and quantified by using PLA between several mitochondria-ER proteins pairs, including VDAC1-IP3R, GRP75-IP3R, CypD-IP3, and PTPIP51-VAPB5,6,7,8. Although this technology has been used to detect and quantify inter-organelle protein-protein interactions that are present at the MCS5,7,9,10,11, most of the studies did not combine PLA with organelle staining. Consequently, a quantitative method that allows the measurement of the proximity between PLA interactions and associated organelles has not been developed yet. Thus, so far, in the case of ER proteins, their interaction within membrane subdomains in contact with other organelles has not been distinguished from their interaction within the widely distributed ER network.
Here, we describe a protocol to detect PLA interactions between proteins that reside in the membrane of the same organelle and to analyze their proximity to the membrane of the partner organelle at the MCS. This protocol was developed based on two premises: 1) previous studies showing that, in overexpression conditions, the ER lipid transfer proteins ORP5 and ORP8 co-localize and interact at ER-mitochondria and ER-PM MCSs12,13,14,15 and that ORP5 localizes at ER-LD contacts16,17; 2) existing technologies, including PLA, confocal microscopy, organelle labeling, and 3D imaging analysis.
Protocol
1. Mitochondrial staining and proximity ligation assay (PLA)
- Plate 0.5 x 105-2 x 105 HeLa cells, maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% non-essential amino acids at 37 °C with 5% CO2, in 13 mm glass coverslips in 1.5 in 24-well plates at a dilution that allows 75%-90% cell confluence on the day of the procedure.
NOTE: Of note, HeLa cells can be submitted to additional treatments, such as siRNA treatment and/or DNA plasmid transfection, before mitochondrial staining and PLA. - Mitochondrial staining
- Wash the cells once in serum-free medium pre-warmed at 37 °C. Incubate the cells with pre-warmed serum-free medium for 10 min at 37 °C with 5% CO2.
- Prepare 1 µM red mitochondrial marker in pre-warmed serum-free medium.
- Incubate the cells with 500 µL of mitochondrial marker solution for 30 min at 37 °C with 5% CO2. During the mitochondrial marker treatment and the following steps of the protocol, keep the cells on coverslips protected from light as much as possible.
- Following the incubation, wash the cells 1x in pre-warmed serum free medium and 2x in 1x phosphate buffered saline (PBS). Perform this step as quickly as possible since long washing steps before fixation may affect the mitochondrial morphology.
- Remove the 1x PBS from the coverslips, and fix the cells by incubating them in freshly prepared 4% PFA (in 1x PBS) for 30 min at room temperature (in the dark) under the chemical hood. Wash 3x for 2 min in 500 µL of 1x PBS (using disposable pipets or a vacuum system connected to a pump).
- Remove the 1x PBS, and incubate the coverslips with 500 µL of 50mM NH4Cl for 15 min at room temperature (in the dark). Wash 1x for 2 min in 500 µL of 1x PSB using a vacuum system.
- Wash 3x for 2 min in 500 µL of blocking buffer 1 (BB1, 1%BSA, 0.1% saponin in 1x PBS) for the ORP5-ORP8 PLA or blocking buffer 2 (BB2, 2% BSA, 0.1% normal goat serum, 0.1 saponin in 1x PBS) for the ORP8-PTPIP51 PLA.
- Transfer the coverslips from the 24-well plate into a humidity chamber. After transferring the coverslips into the chamber, add 100 µL of BB (BB1 or BB2, according to the pairs of antibodies used) to avoid dryness.
NOTE: A humidity chamber protected from light can be easily and rapidly obtained by wrapping a Petri dish (plastic or glass) in aluminum foil and adding some pieces of wet paper towel in the periphery of the Petri dish.
- Proximity ligation assay (PLA)
NOTE: The PLA protocol follows the manufacturer's instructions with slight modifications.- Primary antibody incubation
- Prepare the primary antibody working solution. Dilute rabbit anti-ORP5 (1:150) plus mouse anti-ORP8 (1:200) in BB1, mouse anti-ORP8 (1:200) plus rabbit anti-PTPIP51 (1:200) in BB2, and rabbit anti-ORP5 (1:150) plus mouse anti-PTPIP51 (1:200) in BB1. Prepare (at least) 40 µL of primary antibodies solution per coverslip (e.g., 0.27 µL of rabbit anti-ORP5, 0.2 µL of mouse anti-ORP8, 39.53 µL of BB1).
- Remove the BB from the previous wash (step 1.2.8) using a vacuum system, and add 40 µL of primary antibody solution to the coverslips. Incubate for 1 h at room temperature in a humidity chamber protected from light.
- Wash coverslips 3x for 5 min with 100 µL of the corresponding BB using the vacuum system.
- PLA probe incubation
- Prepare the PLA probes working solution. Dilute rabbit PLUS (1:5) and mouse MINUS (1:5) PLA probes in BB, and mix. For each coverslip, prepare (at least) 40 µL of PLA probe solution (e.g., 8 µL of rabbit PLUS PLA probe, 8 µL of mouse MINUS PLA probe, 24 µL of BB). Allow the PLA probe solution to sit for 20 min at room temperature before use.
- Remove the BB from the coverslips (from step 1.3.2) using a vacuum system, and add 40 µL of PLA probe solution to the coverslips. Incubate for 1 h at 37°C in a humidity chamber protected from the light.
- Wash the coverslips 2x for 5 min in 100 µL of 1x wash buffer A at room temperature.
- Ligation
- Dilute 5x ligation buffer to 1:5 in ultra-pure water and mix. For each coverslip, prepare (at least) 40 µL of ligation solution (8 µL of 5x ligation buffer, 31 µL of ultra-pure water).
- Add the ligase (1 U/µL, supplied in the PLA kit) to 1x ligation buffer prepared in the previous step at a dilution of 1:40, and mix.
- Remove 1x wash buffer A from the coverslips (from step 1.3.4) using a vacuum system, add 40 µL of ligase solution to the coverslips, and incubate for 30 min at 37 °C in a humidity chamber protected from the light.
- Wash the coverslips 2x for 2 min in 100 µL of 1x wash buffer A at room temperature using the vacuum system.
- Polymerization
- Dilute 5x amplification buffer 1:5 in ultra-pure water, and mix. For each coverslip, prepare (at least) 40 µL of amplification solution (8 µL of 5x amplification buffer, 31.5 µL of ultra-pure water).
- Add the polymerase (10 U/µL, supplied in the PLA kit) to the 1x polymerization buffer prepared in the previous step at a dilution of 1:80, and mix.
- Remove 1x wash buffer A from the coverslips (from step 1.3.6) using a vacuum system, add 40 µL of polymerase solution to the coverslips, and incubate for 1 h 40 min at 37 °C in a humidity chamber protected from the light.
- Remove polymerase solution from coverslips, and wash 2x for 10 min in 100 µL of 1x wash buffer B at room temperature in a humidity chamber protected from the light.
- Wash the coverslips 1x for 1 min in 100 µL of 0.001x wash buffer B at room temperature in a humidity chamber protected from the light.
- Mount the coverslips on glass slides for microscopy using mounting medium with DAPI (concentration between 1.6-0.4 µg/mL). Seal with nail polish.
- Primary antibody incubation
2. Image acquisition
- Observe the PLA results, and acquire images using fluorescence confocal microscopy with a 63x oil immersion objective using the accompanying software.
- Set the fluorescence excitation using a 405 nm laser diode or a white light laser, and collect the spectral windows with GaAsP PMTs or hybrid detectors. At each focal plane (spanning 300 nm), acquire the fluorescence signals for PLA (λex = 488 nm, λem = 505-560 nm) and mitochondria (λex = 543 nm, λem = 606-670 nm).
3. Image processing and assessment of PLA spots associated with mitochondria
- Process the confocal images using a software for image analysis that generates distance maps between the cellular components. Follow the steps below to generate 3D reconstitutions of PLA spots and the mitochondrial network and to access the distance between them using cell imaging software (Figure 1).
- Installation of the 3D distance map extension
- Install both the cell analysis software package with the XT option and the MATLAB software or only a MATLAB compiler runtime (MRC), which can be freely downloaded from the website.
- Set up the cell analysis software to start MATLAB when an XTension is launched as follows: from the option Imaris (Mac OS X) or Edit menu (Windows), select Preferences, change to the Custom Tools panel, and then set the path:
C:Program FilesMATLABR201Xa_x64binwin64MATLAB.exe for Windows or/Applications/MATLAB_R201Xa.app/bin/matlab for Mac OS X.
- Import the images into the software
- Convert the confocal stack images into an .IMS file, either directly through the Arena section or using the standalone File Converter that allows batch conversion.
- After the importation, check that images remain properly calibrated. Click on Edit > Image Properties > Image Geometry, and check that the voxel size corresponds in X and Y to the pixel size expected for the actual image (see image calibration in the acquisition software) and that the voxel size in Z corresponds to the step applied by the microscope to generate the Z-stack. If the values are not correct, modify them to ensure a correct estimation of the distances in the following steps.
- Adjust the contrast of the different channels in the menu Display Adjustment by clicking on Edit > Show Display Adjustment. Adjust each channel independently to optimize the display of each color. This step does not directly affect the image values but is essential to set precise thresholds or detect weak objects.
- Limit the analysis to a single cell by cropping the image using the options Edit > Crop 3D. To analyze another cell in the same field of view, open the same image once again, and crop it differently.
- ORP5-ORP8 spot detection
- Detect the PLA signals generated at the location of ORP5 and ORP8 interaction by clicking on the Add New Spots option, which creates a new set of objects and opens the spot detection wizard.
- Select the channel on which the spot detection should be performed.
- Adjust the Estimated XY Diameter (the range has to be adapted if the object size differs) to help the spot detection algorithm to find the objects of interest. Note that if the chosen value is too high, the nearby objects will fuse. If the value is too small, one signal may be considered as multiple objects, or aberrant signals may be detected.
- Click on Background Subtraction to remove the image background prior to spot detection to enhance the local contrast around the objects of interest.
- Adjust the spot detection threshold by keeping the quality (intensity at the object center) as the threshold parameter and the software auto-threshold, or slightly modify this value to detect all the objects. Once the spot detection is finished, save the detection parameters, and reuse them to process other images.
- Mitochondrial network detection
- Detect the mitochondrial network to generate a surface rendering by clicking on Add new Surfaces to create a new object, and open the surface detection wizard.
- Select the channel on which the surface creation should be performed.
- Apply a Gaussian filter to obtain a smoother surface by clicking the Smooth checkbox and by setting a threshold indicating the smallest details observable on the surface.
- Perform a background subtraction to enhance the local contrasts and to help with the threshold step.
- Adjust the threshold to detect the mitochondrial network based on the intensity of the signal. If it is difficult to properly set the threshold, it is recommended to go back to the previous step to adjust the degree of smoothness and background subtraction.
- If necessary, apply a filter on the surface to remove small residuals resulting from the threshold. To do this, select the Number of Voxels filter on the classify surface window, and play with the upper and lower thresholds to keep only the objects of interest. Once the surface creation is over, save the creation parameters, and reuse them to process other images.
- Generation of the 3D distance map around the mitochondria
- Generate a distance map outside of the mitochondrial surface previously created as follows. Select the mitochondria surface in the scene tree box. Click on Image Processing > Surfaces Function > Distance Transformation. This will call a Matlab XTension that asks the user to choose whether the map should be computed outside or inside the object surface.
- Select Outside Surface Object to measure the distance between the PLA spots and the surface of the mitochondria. Once generated, the distance map appears as a new channel in the display adjustment panel. In this channel, every pixel has a value corresponding to the distance to the closest mitochondria.
- Extraction of the objects' distances from the closest mitochondrion
- To measure the distance from each point to the closest mitochondrion and to identify and visualize the closest ones, select the spots previously generated in the scene tree box.
- In the statistics and detailed log, select Specific Values (to obtain one measurement for each spot). Select Center Intensity Ch=X (with X corresponding to the number of the distance map channel). This will measure the value of each spot center, which corresponds to its distance to the closest mitochondrion, in the distance map.
- Export the data as a .csv file by clicking on the Floppy disk icon at the bottom left of the window.
- To extract a subpopulation of spots based on their distances to mitochondria, select the spots in the scene tree, and click on the Filters tab.
- In this window, add a new filter based once again on the Center Intensity Ch=X, and extract the spots less than 380 nm away from mitochondria by setting the lower threshold to 0 µm and the upper threshold to 0.380 µm.
NOTE: The threshold of 380 nm was estimated based on a PLA reaction including the association between the primary and secondary antibodies (30 nm) plus half of the full width at half maximum(FWHM) of the PLA amplification signals (350 nm). - To focus on the selected spots, and for example, give them a distinct color, perform a duplication step by pressing the Duplicate Selection to New Spots button.
Representative Results
Using the protocol described above, we detected the sites of interaction of two ER-anchored lipid transfer proteins, ORP5 and ORP8, and assessed their occurrence at ER membrane subdomains in contact with other organelles, in particular, with the mitochondria. For that, the mitochondrial network in HeLa cells was stained with a red mitochondrial marker, and ORP5-ORP8 PLA green spots were detected after fixation using the primary antibodies anti-ORP5 and anti-ORP8, whose specificity was previously tested by immunofluorescence15. Confocal images showed that endogenous ORP5-ORP8 PLA interactions in the HeLa cells occurred in the reticular ER, cortical ER, and in ER subdomains in close contact with mitochondria, commonly referred to as mitochondria-associated ER membranes (MAMs; Figure 2A). In order to quantify the ORP5-ORP8 PLA interactions occurring at MAMs, the distance of each PLA spot from mitochondria was evaluated using 3D image analysis. Using a distance threshold of 380 nm, 3D imaging analysis revealed that about 50% of endogenous ORP5-ORP8 PLA interactions were detected at MAMs (Figure 2A,B). The other 50% of interactions were distributed between the cortical and reticular ER15. To validate the specificity of the PLA, ORP5-ORP8 PLA experiments were performed either in cells treated with ORP5-targeting and ORP8-targeting siRNAs (negative control) or in cells co-overexpressing ORP5 and ORP8 (positive control). ORP5 and ORP8 downregulation induced a massive decrease in the total number of PLA signals (at MAMs, at the reticular ER, and at the cortical ER; Figure 2A,C), while their co-overexpression resulted in an increase in PLA (Figure 2A,C), confirming the specificity of the ORP5-ORP8 PLA. However, interestingly, the percentage of ORP5-ORP8 PLA interactions occurring at MAMs in cells co-overexpressing ORP5 and ORP8 was similar to that observed in cells where these proteins were expressed at endogenous levels (Figure 2B), supporting their existence as a complex at MAMs. To further confirm the presence of ORP5 and ORP8 at ER membrane subdomains in close contact with mitochondria, additional quantitative PLA analyses between ORP5 or ORP8 and the outer mitochondrial membrane protein PTPIP51, a known binding partner of ORP5 and ORP813, were carried out. PLA signals were detected in both ORP5-PTPIP51 and ORP8-PTPIP51 couples, and their average numbers were similar to the ORP5-ORP8 PLA couple, confirming ORP5 and ORP8 localization at ER-mitochondria MCSs (Figure 3).
Finally, in recent works from our lab, by using a similar approach in HeLa cells transfected with PHPLCd-RFP or mcherry-PLN1 to mark the PM or LD, respectively, we were able to analyze the occurrence of ORP5-ORP8 PLA interactions at ER-PM contacts and to identify a three-way contact site between mitochondria, the ER, and LDs (Figure 4)15,17.
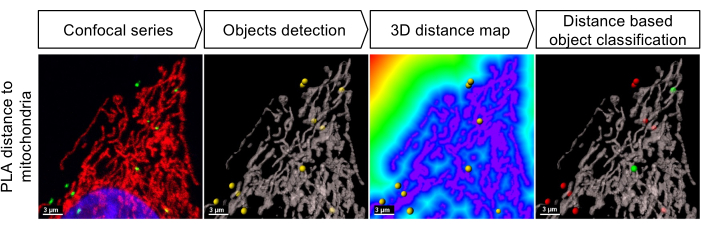
Figure 1: Workflow for the identification of PLA signals in close proximity to the mitochondria. First, the confocal stacks are segmented to identify the PLA foci (spots) and the mitochondrial network (surfaces). Then, 3D distance maps are computed toward the outside of the surfaces (mitochondria), allowing the measurement of the distance of each spot from the closest mitochondrion. Finally, PLA spots are classified into two populations (red and green) based on a proximity threshold of 380 nm established based on the precision of the detection system. This figure has been modified fromMonteiro-Cardoso et al.15. Please click here to view a larger version of this figure.
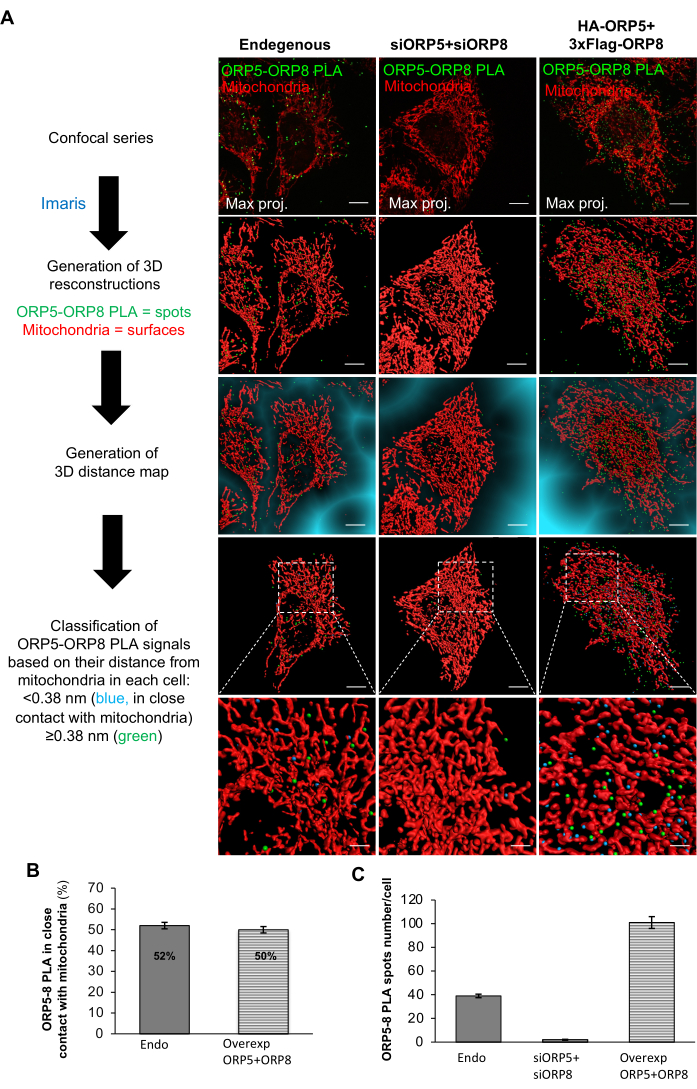
Figure 2: Representative images of the ORP5-ORP8 complex expressed at endogenous levels localizing at ER-mitochondria contacts. (A) ORP5-ORP8 PLA confocal series and respective 3D reconstitutions. Scale bars: 10 µm and 2 µm (expanded images). (B) Quantification of ORP5-ORP8 PLA foci in close contact with mitochondria. Endo, n = 33 cells, and Overexp ORP5+ORP8, n = 27 cells. Data are presented as mean ± SEM (standard error of the mean). This image has been modified from Monteiro-Cardoso et al.15. (C) Quantification of total ORP5-ORP8 PLA foci. Endo, n = 38 cells, siORP5+siORP8, n = 38 cells, and Overexp ORP5+ORP8, n = 35 cells. Data are presented as mean ± SEM (standard error of the mean). This image has been modified from Monteiro-Cardoso et al.15. Please click here to view a larger version of this figure.
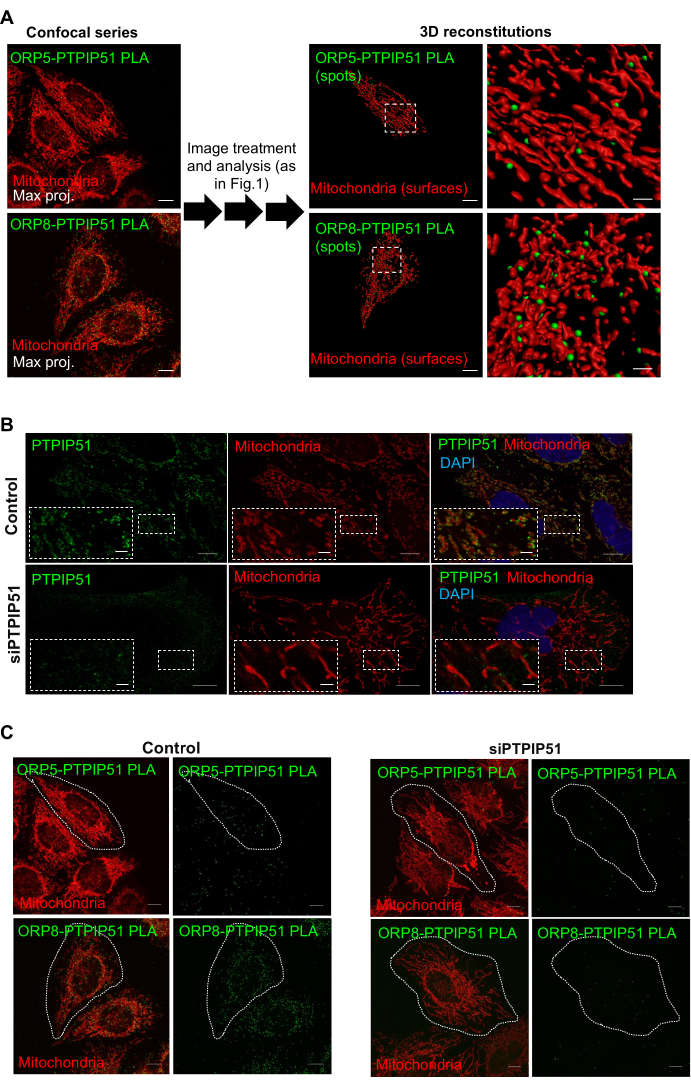
Figure 3: Representative images of PLA signals between ORP5 or ORP8 with the mitochondrial protein PTPIP51. (A) Confocal series were used to generate 3D reconstitutions and distance maps to confirm that ORPR5 or ORP8 PLA interactions with PTPIP51 occur at ER-mitochondria close contacts. Scale bars: 10 µm and 2 µm (expanded images). (B) PTPIP51 immunofluorescence confocal images (single focal plane) in control and HeLa cells treated with siRNA targeting PTPIP51. Scale bars: 10 µm and 2 µm (expanded images). (C) ORP5-PTPIP51 and ORP8-PTPIP51 PLA confocal images in control and HeLa cells treated with siRNA targeting PTPIP51. Scale bar: 10 µm. Please click here to view a larger version of this figure.
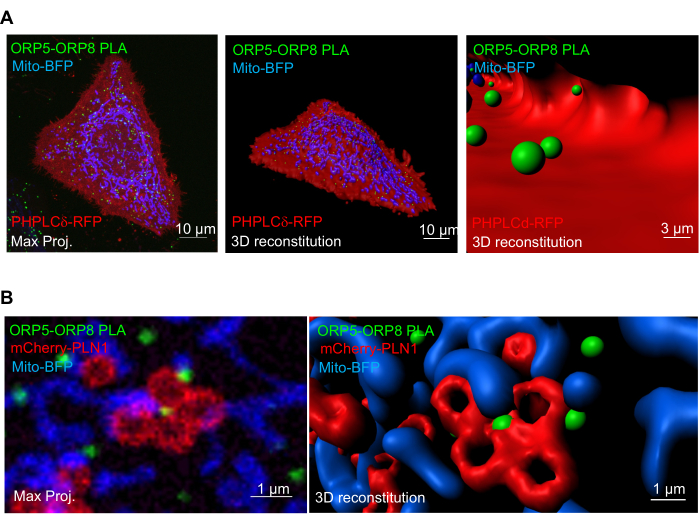
Figure 4: Examples of 3D reconstructions used to localize the ORP5-ORP8 PLA complex at ER-PM and ER-mitochondria-LD contacts. (A) Confocal image and respective 3D reconstitution of a HeLa cell with ORP5-ORP8 PLA, represented by the green spots, the PM marked with PHPLCd-RFP, represented by the red cell, and mitochondria marked with Mito-BFP, represented by blue surfaces. Left and center images: representation of the whole HeLa cell. Right image: representation of a zoomed region inside the cell. (B) Confocal image and respective 3D reconstitution of a zoomed-in region of a HeLa cell with ORP5-ORP8 PLA, represented by the green spots, LDs marked with mCherry-PLN1, represented by the red surfaces, and Mito-BFP, represented by the blue surfaces. Please click here to view a larger version of this figure.
Discussion
This protocol was designed to identify and quantify inter-organelle protein PLA interactions at MCSs, in particular at MERCSs. The novelty of the protocol is that it combines PLA with the labeling of multiple organelles, confocal microscopy, and 3D image analysis to localize and quantify PLA interactions between two proteins residing in the same membrane, in this case within the ER membrane in close proximity with the membrane of mitochondria (MAM) or with the MAM and LDs simultaneously. This protocol can be used as a tool to validate proteins that potentially localize at specific MERCSs but also at other MCSs.
Since its development in 2006, PLA between an ER and a mitochondrial protein has been widely used to quantify MERCSs7,9,10,11. However, the use of PLA as an indicator of MERCS abundance in cells needs to be carefully verified by high-resolution approaches. For instance, the ablation of SAM50, a mitochondrial protein that does not affect the abundance of MERCSs (as quantified by electron microscopy)15, also does not alter PLA interactions between the ER protein ORP8 and the mitochondrial protein TOM20, but it reduces PLA interactions between ORP8 and the mitochondrial protein Metaxin2 without reducing their expression levels15. These results reflect that PLA interactions can be specific to the pair of proteins used in the assay and cannot be used as the only parameter to assess the abundance of MERCS.
Nonetheless, PLA is a proven sensitive approach to detect in situ endogenous protein interactions. Contrary to other fluorescent probe-based detection methods, including split-GFP and FRET, or high-resolution imaging approaches, PLA does not require the overexpression of tagged proteins, avoiding inherent experimental artifacts, such as the alteration of the physical properties of the targeted organelles15. The detection of protein interactions by PLA also offers advantages in relation to other immunodetection methods, such as co-immunoprecipitation with western blot. While PLA allows the quantification of single fluorescence spots at the single-cell level, co-immunoprecipitation requires a high amount of biological material and only allows the relative quantification of the protein extracted from a pool of heterogenous cells, for which an appropriate loading control is difficult to obtain15. The additional advantage of the use of PLA combined with organelle staining and confocal microscopy over co-immunoprecipitation is that the first approach allows the localization of protein interactions at the subcellular level.
On the other hand, the prominent drawbacks of this protocol are associated with the PLA detection range of about 40 nm, which is larger than MERCSs, and with the maximum resolution achieved by confocal microscopy of about 250 nm, which makes it difficult to unequivocally identify contact sites. However, here, a threshold of 380 nm was used to estimate the PLA spots associated with mitochondria surfaces based on the size of the PLA reaction, including the association of the primary and secondary antibodies (30 nm) plus half of the FWHM of the PLA amplification signals (350 nm). Another limitation of PLA is related to the fact that it can only be used in fixed samples, and the acquisition of a distinct, specific, and quantifiable PLA signal requires the availability of antibodies that are highly specific to the protein of interest15. Additionally, the 3D imaging analysis for distance estimation requires specific software that may not be readily available in all laboratories.
Notably, some of the disadvantages mentioned above can be addressed by using recently available technologies. For instance, conventional confocal microscopy can be replaced by imaging techniques at a higher resolution level, such as Airyscan or structured illuminated microscopy (SIM)17, and the lack of good primary antibodies for the proteins of interest can be overcome by stably tagging endogenous proteins using the CRISPR-Cas9 technology18. Finally, the quality and specificity of the antibodies and PLA signals can be verified by using the following controls: 1) testing the primary antibodies by immunofluorescence and western blot in control cells and in cells in which the protein of interest has been depleted or overexpressed; 2) performing PLA in cells in which one or both proteins of interest have been depleted; 3) performing PLA in cells co-overexpressing the proteins of interest; 4) performing PLA whilst omitting both antibodies for the proteins of interest; 5) performing PLA using only one of the two antibodies for the proteins of interest; 6) performing PLA with incubation with the same IgG as the species from which the primary antibodies were generated. To assess their specificity, the antibodies used in this protocol were tested by immunofluorescence and western blot in control cells and in cells that were treated with siRNA targeting ORP515, ORP815, or PTPIP51 (Figure 3B and Guyard et al.17). Furthermore, the anti-ORP5 and anti-ORP8 specificity has been assessed in cells expressing EGFP-tagged ORP5 or ORP8 by evaluating the Pearson's coefficient between the antibody detection signal and the EGFP signal15. The specificities of the anti-ORP515, anti-ORP815, and anti-PTPIP51 (Figure 1 and Figure 3C) antibodies were further analyzed in cells in which these proteins were depleted, leading to a decrease in PLA signals. Of note, the antibody concentration that is suitable for immunofluorescence staining is usually also suitable for PLA; however, depending on the antibodies used, some adjustments in the blocking solution and/or to the antibody concentrations can be made to improve the PLA staining. The ORP5-ORP8 and ORP8-PTPIP51 PLA staining was improved after adjusting the blocking solution. To obtain high-quality mitochondrial and PLA staining, it is essential to obtain high-quality confocal images with low background signals, which are important to facilitate 3D modulation. For instance, confocal images with high background impact threshold adjustments and, consequently, affect the accuracy of the 3D modulation of the mitochondrial network or PLA.
In conclusion, the protocol presented here enables the localization and quantification of PLA protein interactions at specific MERCS subdomains (interactions between proteins localized in cis within the same membrane engaged in MCSs or between proteins localized in trans within the juxtaposed membranes) by using multicellular organelle labeling and generating distance maps in 3D reconstructed confocal images. Although the procedures described here are technically simple and relatively fast compared with other methodologies to detect protein interactions at MERCSs, the data obtained are highly reproducible. Furthermore, this protocol is as versatile as the antibodies available for the proteins of interest and as the markers available to stain the organelles. Therefore, it can be applied to a wide range of pairs of proteins and several organelles and MCSs.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by the ANR Jeune Chercheur (ANR0015TD), the ATIP-Avenir Program, the Fondation pour la Recherche Medicale (n°206548), and the Fondation Vaincre Alzheimer (eOTP:669122 LS 212527), I'Agence Nationale de la Recherche (ANR-11-EQPX-0029/Morphoscope, ANR-10-INBS-04/FranceBioImaging; ANR-11-IDEX-0003-02/Saclay Plant Sciences ANR-22-CE11-0024-01/MADE to FG) and AFM Telethon (Project AFM 23778).
Materials
| 1X Dulbecco's Phosphate Buffered Saline (1X DPBS) | Gibco | 14190-094 | |
| Ammonium chloride (NH4Cl) | VWR | 21236.291 | |
| Bovine serum albumin (BSA) | Sigma | A7906 | |
| Circular glass coverslips 13mm no. 1.5 | Agar Scientific | L46R13-15 | |
| CMXRos red MitoTracker | Invitrogen | M7512 | red mitochondrial marker |
| Confocal inverted microscope SP8-X | Leica | DMI 6000 | |
| Corning Costar TC-Treated 24-Well Plates | Merck | CLS3526 | |
| Duolink In Situ Detection Reagents Green | Sigma | DUO92002 | |
| Duolink In Situ Mounting Medium with DAPI | Sigma | DUO82040 | |
| Duolink In Situ PLA Probe Anti-Mouse MINUS | Sigma | DUO92004 | |
| Duolink In Situ PLA Probe Anti-Rabbit PLUS | Sigma | DUO92014 | |
| Duolink In Situ Wash Buffers, Fluorescence | Sigma | DUO82049 | |
| Gibco Opti-MEM I Reduced Serum Medium, GlutaMAX Supplement | Gibco | 51985026 | serum free medium |
| Imaris software v 9.3 | Bitplane | N/A | cell imaging software |
| Incubator UINCU-line IL10 | VWR | 390-0384 | |
| Microscope slide StarFrost (3“ x 1“) | Knittel Glass | ||
| mouse anti-ORP8 | Santa Cruz | 134409 | |
| Paraformaldehyde (PFA) | Sigma | P6148 | |
| rabbit anti-ORP5 | Sigma | HAP038712 | |
| Saponin | Sigma | 84510 | |
| Ultra Pure Distilled Water, DNase/RNase free | Invitrogen | 10977-035 |
Referências
- Scorrano, L., et al. Coming together to define membrane contact sites. Nature Communications. 10, 1287 (2019).
- Vance, J. E. MAM (mitochondria-associated membranes) in mammalian cells: Lipids and beyond. Biochimica et Biophysica Acta – Molecular and Cell Biology of Lipids. 1841 (4), 595-609 (2014).
- Giordano, F. Non-vesicular lipid trafficking at the endoplasmic reticulum-mitochondria interface. Biochemical Society Transactions. 46 (2), 437-452 (2018).
- Söderberg, O., et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nature Methods. 3 (12), 995-1000 (2006).
- Gomez-Suaga, P., et al. The ER-mitochondria tethering complex VAPB-PTPIP51 regulates autophagy. Current Biology. 27 (3), 371-385 (2017).
- Han, S., et al. The role of Mfn2 in the structure and function of endoplasmic reticulum-mitochondrial tethering in vivo. Journal of Cell Science. 134 (13), jcs253443 (2021).
- Stoica, R., et al. ALS/FTD-associated FUS activates GSK-3β to disrupt the VAPB-PTPIP51 interaction and ER-mitochondria associations. EMBO reports. 17 (9), 1326-1342 (2016).
- Tubbs, E., Rieusset, J. Study of endoplasmic reticulum and mitochondria interactions by in situ proximity ligation assay in fixed cells. Journal of Visualized Experiments. (118), e54899 (2016).
- Cuello, F., et al. Impairment of the ER/mitochondria compartment in human cardiomyocytes with PLN p.Arg14del mutation. EMBO Molecular Medicine. 13 (6), e13074 (2021).
- Paillusson, S., et al. α-Synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca2+ homeostasis and mitochondrial ATP production. Acta Neuropathologica. 134 (1), 129-149 (2017).
- Tubbs, E., et al. Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes. 63 (10), 3279-3294 (2014).
- Chung, J., et al. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science. 349 (6246), 428-432 (2015).
- Galmes, R., et al. ORP5/ORP8 localize to endoplasmic reticulum-mitochondria contacts and are involved in mitochondrial function. EMBO Reports. 17 (6), 800-810 (2016).
- Ghai, R., et al. ORP5 and ORP8 bind phosphatidylinositol-4, 5-biphosphate (PtdIns(4,5)P 2) and regulate its level at the plasma membrane. Nature Communications. 8, 757 (2017).
- Monteiro-Cardoso, V. F., et al. ORP5/8 and MIB/MICOS link ER-mitochondria and intra-mitochondrial contacts for non-vesicular transport of phosphatidylserine. Cell Reports. 40 (12), 111364 (2022).
- Du, X., et al. ORP5 localizes to ER-lipid droplet contacts and regulates the level of PI(4)P on lipid droplets. Journal of Cell Biology. 219 (1), e201905162 (2020).
- Guyard, V., et al. ORP5 and ORP8 orchestrate lipid droplet biogenesis and maintenance at ER-mitochondria contact sites. The Journal of Cell Biology. 221 (9), e202112107 (2022).
- Ran, F. A., et al. Genome engineering using the CRISPR-Cas9 system. Nature Protocols. 8 (11), 2281-2308 (2013).

