A Point-of-Care Method with Integrated Decision Support Tool to Estimate Anemia at Population Level
Summary
An accurate hemoglobin estimation method is lacking at the point of care and may hinder population-based programs for treating anemia. Therefore, we developed a point-of-care method based on pooled capillary blood and an auto-analyzer integrated into a custom software application to categorize the hemoglobin values into different grades of anemia.
Abstract
Robust point-of-care methods are required to estimate anemia at the population level. The accurate methods are lab-based and cannot be used at the point of care. To address this caveat, a novel method based on pooled capillary blood and a portable autoanalyzer was developed for the estimation of Hb. Additionally, custom software was developed for near-real-time integration of the Hb values from the auto analyzer to the server. Moreover, a decision support tool that can immediately categorize the participants into different stages of anemia was developed. The decision support tool was designed based on the World Health Organization (WHO) cut-off for anemia at the population level and was available for all age and gender groups. This simple and user-friendly tool could easily be used by front-line health workers who have limited technical skills. Overall, the method developed could be used at the point of care and is accurate. This high-throughput method could be used for screening anemia at the population level for all age and gender groups.
Introduction
Anemia is a major public health problem globally, particularly in India. It is well known that anemia has a detrimental impact on the work productivity of the population and the economic growth of the country1. To leverage the national-level efforts to reduce anemia, the latest public health program initiated in 2018 is the Anemia Mukt Bharat program (AMB). AMB identifies 'testing' followed by tailored 'treatment' as one of the most promising approaches to reducing anemia prevalence in vulnerable age groups2. However, accurate point of care (POC) estimation of hemoglobin (Hb) for diagnosing anemia is needed to implement the 'test and treat' strategy of AMB. Moreover, robust methods are useful for accurately estimating anemia in large-scale community surveys. The current POC methods include non-invasive and minimally invasive devices, and they use capillary blood samples for Hb estimation3. However, several pre-analytical factors, such as variation in finger prick dimensions, the thickness of skin, and the stability of POC devices under environmental conditions, lead to imprecise measurements and result in large differences in prevalence estimates3,4,5. Therefore, there is a need to establish a method of Hb estimation that is mobile, has a short turnaround time (TAT), and is suited for resource-poor settings6. In order to address these needs, a pooled capillary blood collection method was developed using a touch-activated lancet (to ensure uniform prick depth and dimension) to facilitate 6-8 drops of the free-flowing blood sample into potassium ethylenediaminetetraacetic acid (EDTA) microtainer tubes. The Hb in these samples was then measured using a portable auto-analyzer placed in a vehicle at the POC equipped with an uninterrupted power supply or at a nearby center with electricity (Anganwadi, Health clinic, Panchayath, or household). A validation study comparing this method against two gold standard methods (paired venous blood samples and cyanomethamoglobin method) showed high accuracy and precision7,8.
In addition to setting up a valid and reliable POC method, there is a need for rapid decision-making to facilitate the screening and treatment of anemia at the population level. This is not currently feasible where the Hb estimation is done at the health facility, and a medical officer directly supervises the delivery of iron and folic acid (IFA) supplements. Due to the large population catered to by the medical officers at the primary health centers, there is a significant time delay in initiating the intervention. There is a need for technology that can reduce the job burden on the medical officer and empower the front-line health workers to carry out the intervention delivery without the direct involvement of medical personnel. Therefore, the study aimed to develop a custom application (eSTAR app) that can automatically transmit the data from the machine and an in-built algorithm that provides decision support to the front-line workers on the dosage of IFA based on Hb values, age, and gender groups. The software was designed using open source tools such as PHP: hypertext preprocessor (PHP) scripting language and PHP desktop chrome with Visual Studio Code as an integrated development environment. A detailed treatment protocol based on the Anemia Mukt Bharat guidelines has been integrated into the Android application2.
This integrated method addresses the ever-increasing demand to reduce the turnaround time of test results while maintaining accuracy and precision. Further, the ability to provide results within minutes enables rapid decision-making on the initiation of treatment and results in improved intervention delivery9. This integrated method can be adapted for any field-level surveys or intervention programs that include Hb testing. Additionally, it can be used at the health care facility as a job aid for the medical staff to decide on IFA treatment.
Protocol
The protocol follows the guidelines of the Institutional Review Board of the ICMR-National Institute of Nutrition, Hyderabad, India (IRB. No.08/I/2018).
1. Pooled capillary sample collection for Hb analysis using hematology analyzer10,11
- Configuring the barcode printer and scanner
- Connect the barcode printer to the laptop system.
- Install the barcode printer driver on the laptop.
- Share the printer in the printer properties.
- Test the barcode printing in the application for any errors.
- Connect the barcode scanner to the Auto analyzer, and it will configure itself automatically.
- Double-click the icon to open the application software installed on the desktop/laptop computer. Enter the authorized credentials on the login page.
NOTE: The home page of the application consists of tabs for lab result upload, barcode printing quality control (QC) reports, and the final hematology report. - To generate barcodes, click the Barcode Printing tab. The list of participants automatically appears in the barcode printing tab after entering geographic details such as village names.
- Click on the Display Selection. The list appears. Click print Barcode.
- Pooled capillary blood sample collection (Figure 1)
- Use one of the middle three fingers of the left hand for capillary blood sampling in an adult patient.
- Request the participant to sit in a comfortable position,which provides the phlebotomist access to the finger.
- Clean the finger with an alcohol wipe (containing 70% isopropyl alcohol) and allow it to dry.
- Label the microtainers with participant-related information (barcode) immediately after participant identification and before skin puncture.
- Collect the blood sample using a single-use safety lancet (contact-activated lancet 2.0 X 1.5 mm). To collect the blood sample, puncture the skin approximately 1 cm from the tip of the finger between the midpoint and the side of the finger. The puncture must be on the palm-up surface of the fingertip of the middle or ring finger.
- Wipe the first blood drop and collect the subsequent blood samples into a coated microtainer.
- Do not massage the finger.
- Place the microtainer vertically beneath the puncture site so that the blood droplets flow freely into the tube without applying pressure. Repeat until 4-6 drops (approximately 200 µL) are collected.
- Gently mix the contents by swirling and inverting only after capping the microtainer container.
- Once 3-4 blood drops are collected, press a cotton swab on the fingertip and make the participant sit still. Apply manual pressure to stop the bleeding.
- Immediately dispose of the lancet according to the biological waste disposal guidelines.
2. Analyses using a hematology analyzer
- Configuration of the autoanalyzer with the software
- Download and install the FileZilla Server components (127.0.0.1) on the laptop.
Note: Disable the Windows firewall or any third-party firewalls. - Install the custom software on the laptop and copy the path of the American Society for Testing and Materials (ASTM) file location (C/programfiles x86/destined folder/ Web/wb/astmfiles)
- In the FileZilla server application, create the user and password and map the astm file location.
- In the auto-analyzer machine, configure the same IP series (in main settings).
- In the auto-analyzer, change the astm sync option to FTP and provide the IP and credentials configured on the laptop (e.g., 192.168.1.1 and FileZilla username and password configured).
- Reboot the auto analyzer and test the file synchronization process once it is ready.
- Download and install the FileZilla Server components (127.0.0.1) on the laptop.
- Analysis of the blood samples using autoanalyzer
- Use a hematology analyzer that is fully automated and portable so that it can be used at the POC setting to give an instant measurement of Hb and other red cell indices (mean corpuscular volume [MCV], mean corpuscular hemoglobin [MCH], mean corpuscular hemoglobin concentration [MCHC], and red cell distribution width [RDW]).
- Check the levels of solvents (At least 1/4th of the reagent bottle is filled) and confirm whether they are present in sufficient quantities.
NOTE: The solvents consist of enzymatic solution, lysing solution, and buffered isotonic solution (Table of Materials). - Turn on the instrument by clicking the ON/OFF switch on the machine. Ensure that the instrument is connected to a laptop through a foil twisted pair (FTP) cable.
- Check the liquid crystal display (LCD) screen to understand whether the outside temperature is amenable to start the analysis or not. A temperature >35 °C is not advisable. Place the machine in an environment where the ambient temperature is <35 °C.
- Once the initialization is completed, allow the analyzer to run a start-up cycle automatically (the automatic start-up function is activated by default).
- After the start-up cycle, check the instrument display for reference blank counting. Ensure that the reference blank counts do not exceed the following parameter limits: Hb < 0.3 g/dL and WBC < 0.3 x 103/mm3, RBC < 0.02 x 106/mm3; HCT 0% and PLT < 10 x 103/mm3. Then, press the √ button to validate the blank results.
- If the analyzer does not automatically run a start-up cycle after the initialization phase is completed, press the START-UP key on the instrument front panel to initiate the cycle.
- Now, the analyzer will show a login page. Enter the pre-defined username and password. Press the √ button to proceed further.
- Prior to the sample analysis, run three levels of controls (low, normal, and high) to check the precision of the analyzer. Store the control at 2-8 °C and bring to room temperature (RT) before use. Gently mix the controls several times before use.
NOTE: The controls are procured as ready-to-use reagents from the manufacturer and loaded as they are. The vials are stored at 4 °C. The usual shelf life is 15 days. The controls should be monitored regularly using Levey Jenning's plot. If the control value falls above or below 3 SD, conduct a concentrated cleaning using the wash solution provided by the supplier. - Once the quality control step is concluded successfully, prepare for sample analysis.
- Scan the barcode stuck to the sample container with the scanner connected to the analyzer, and press the X mark.
- Mention the gender of the subject (male or female) and press the √ button to proceed further.
- The instrument will show the details of the subject along with a code. Press X if the details entered are wrong.
- Before loading the blood sample to the machine, mix the blood sample gently and thoroughly.
- Remove the cap with caution not to permit the spillage of blood.
- Place the tube beneath the sampling needle and move the tube upwards until the sampling needle enters blood.
- Press the Start switch. The sampling needle will aspirate 10 µL of blood .The analysis cycle will take approximately 60 s. At the end of the cycle, the result will be displayed on the LED panel.
- Print the results by clicking on the Printer icon.
- Click on the backward arrow to proceed with the second sample.
- Once the analysis is done, upload the lab test results.
- Upload the data from the analyzer to the server.
- Connect the system to any Internet service provider that provides a minimum speed of 20 mbps.
- Double- click the icon to open the application software icon, that appears on the desktop after installation. Enter the authorized credentials on the login page.
NOTE: The home page of the application consists of tabs for lab result upload, barcode printing QC reports, and the final hematology report. The lab test result upload page shows the completed tests. The file name consists of the machine ID and the date of analysis in .astm format. - Click on the Upload Results button. Once the results are uploaded successfully, in the server, the.astm files are converted into .csv format and the converted files will appear in the hematology report. Download the files in .astm format for records.
NOTE: The page will display that the popup files are uploaded successfully. If there are duplicate files, they will be identified. - For the participants for whom ASTM files are uploaded, the decision on anemia status (based on Table 1 and Table 2) will be generated as a report in the web application.To view the decision on anemia, login to the web application, click on Reports, and then click on EDSS. A report will be visible as a table for all participants.
- To view individual test results and decisions on anemia status as well as the treatment regimen, login to the follow-up application, search for the participant using ID or name, click on the EDSS button, and view the results.
Representative Results
Testing the validity of the method
The validity of this method was established by comparing it with the gold standard, which was the venous blood autoanalyzer-based method. The validation study has been described in detail elsewhere8. Briefly, 748 apparently healthy volunteers provided a venous sample and a capillary sample consecutively on the same day. The analyses were conducted at the POC. The participants had a wide range of Hb values and belonged to categories of no-anemia, mild, moderate and severe anemia status. To establish the validity of the method, the mean differences and their confidence intervals were calculated. The bias and limits of agreement between different methods were estimated using Bland -Altman analysis.
The difference in the mean Hb values was small for the capillary blood auto-analyzer method and the gold standard venous blood auto-analyzer method, respectively (Table 3). The Bland-Altman plot for comparison between the two methods is presented in Figure 2. The mean difference and limits of agreement were 0.1 g/dL(-1.0 to 0.8).
The prevalence estimate of no anemia differed by 2.2% points between the two methods. The prevalence estimates of different grades of anemia were also very close, establishing the method's validity against the gold standard method (Table 3).
Testing the integrated software and decision support tool
The integrated method was piloted among 68 participants from Buggabai village, Ghatkesar, Narapally. The decision support tool accurately provided the anemia status and IFA doses of these participants, which were manually verified by the field medical officer based on the algorithm (Table 4).
The results show that the integrated POC method is valid and can be used for estimating the prevalence of anemia at the population level. To date, about 16,539 decisions have been generated for different grades of anemia using this method.
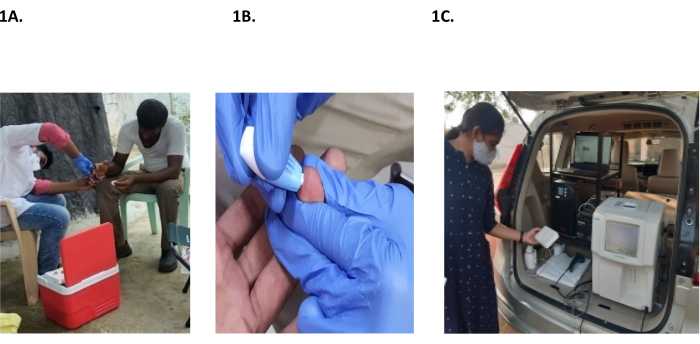
Figure 1: Sample collection and auto-analyzer setup. (A,B) Collection of a pooled capillary sample. (C) Auto analyzer setup at the point of care. Please click here to view a larger version of this figure.
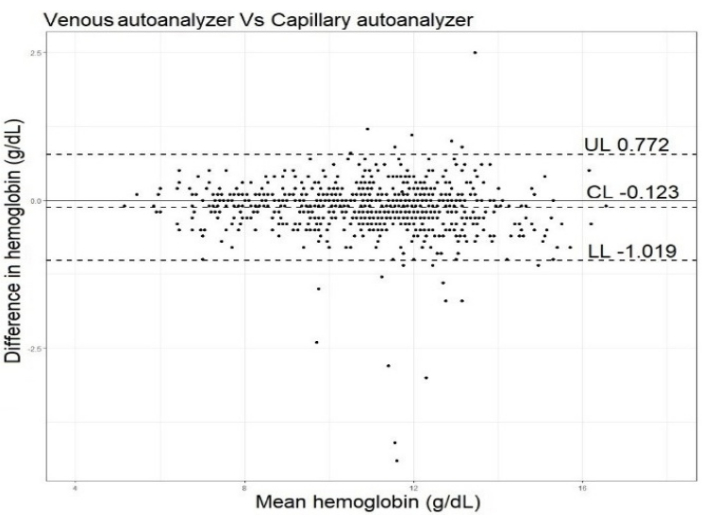
Figure 2: Bland Altman plot. The plot compares the mean difference and limits of agreement between the developed (capillary blood-auto-analyzer method) versus the gold standard (venous blood-auto-analyzer method). The mean difference between the two methods was -0.1 (95% CI, -0.2; -0.1). This figure has been modified with permission from Dasi et al.8. Please click here to view a larger version of this figure.
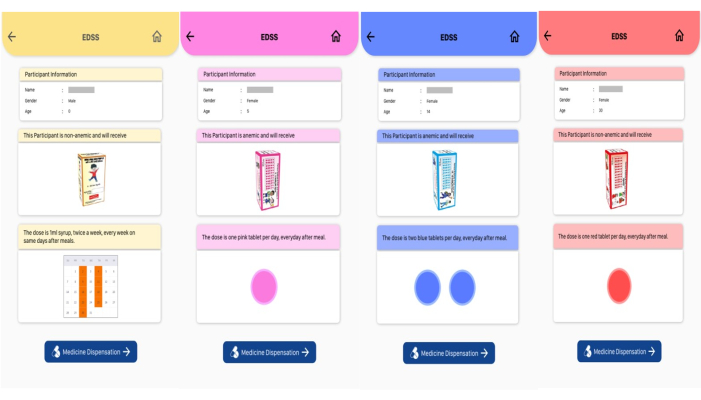
Figure 3: Decision support tool. The tool displays the grade of anemia and treatment dose with iron and folic acid. Please click here to view a larger version of this figure.
| Population | Cut-off (g/dL) | Mild and moderate anemia | Severe anemia |
| Children 6–59 months | ≥11.0 | ≥7.0–<11.0 | <7.0 |
| 5–11 years | ≥11.5 | ≥8.0–<11.5 | <8.0 |
| 12–19 years (Girls) | ≥12 | ≥8.0–<12 | <8.0 |
| 12–14 y (Boys) | ≥12 | ≥8.0–<12 | <8.0 |
| 15–19 y (Boys) | ≥13 | ≥8.0–<13 | <8.0 |
| Men | ≥13 | ≥8.0–<13 | <8.0 |
| NPNL and lactating women | ≥12 | ≥8.0–<12 | <8.0 |
| Pregnant | ≥11 | ≥7.0–<11.0 | <7.0 |
Table 1: Criteria to grade participants to different grades of anemia
| Population | Per day dose (therapeutic dose) |
| Children 6–59 months | 3 mg/kg body weight (Iron syrup containing 20 mg of iron and 100 µg of folic acid) |
| 5–9 years | 45 mg of iron + 400 µg of folic acid/day (Till the body weight cut off of 18.5 kg, Pink tablet) |
| 60 mg of iron + 500 µg of folic acid /day (>18.5 kg, blue tablet) | |
| 10–11 years | 120 mg of iron + 1 mg of folic acid (Blue tablet) |
| 12–19 years (Girls) | 120 mg of iron + 1 mg of folic acid (Blue tablet) |
| 12–14 y (Boys) | 120 mg of iron + 1 mg of folic acid (Blue tablet) |
| 15–19 y (Boys) | 120 mg of iron + 1 mg of folic acid (Blue tablet) |
| Men | 120 mg of iron + 1 mg of folic acid (Red tablet) |
| NPNL and lactating women | 120 mg of iron + 1 mg of folic acid (Red tablet) |
| Pregnant | 120 mg of iron + 1 mg of folic acid (Red tablet) |
Table 2: Treatment protocol. Dosage should be given according to body weight (if the body weight is less compared to age). Severe anemic participants were referred to the local primary health center. For prophylactic doses, 1 IFA tablet per week was followed, except for pregnant women, where 1 tablet per day was given. For children, this was 1 mL biweekly. The treatment was given for 3 months.
| Methods | Mean Hb (SD) g/dL | Prevalence of anemia (Overall) (%) | Prevalence of grades of anemia (%) | ||
| Mild | Moderate | Severe | |||
| Capillary blood auto analyzer method | 11.0 (2.1) | 34.9 | 19.7 | 36.4 | 9.1 |
| Venous blood auto analyzer method | 10.9 (2.0) | 32.2 | 20.7 | 37 | 10 |
Table 3: Comparison between Hb estimates obtained by pooled capillary blood and autoanalyzer method against venous blood autoanalyzer method.
| Age group | N | Mean Hb | SD | Decisions generated by the integrated system* | ||
| Mild | Moderate | Non anemic | ||||
| 6–59 m | 11 | 9.7 | 1.41 | 4 | 5 | 2 |
| 5–11 years | 9 | 11.4 | 1.59 | 2 | 2 | 5 |
| 12–14 y | 4 | 12.4 | 1.56 | 1 | 1 | 2 |
| NPNL | 28 | 11.7 | 1.29 | 8 | 6 | 14 |
| Men | 16 | 14.7 | 1.34 | 0 | 3 | 13 |
| Total | 68 | 11.98 | 1.44 | 15 | 17 | 36 |
| *The automated decisions were verified by a field medical officer | ||||||
Table 4: Mean Hb values and decisions generated by the integrated system.
Discussion
The current paper describes a point-of-care method using a pooled capillary blood sample and an auto-analyzer. The method was integrated with custom software, which could upload the results from the analyzer automatically to the server and generate decisions on anemia. Also, it could provide the treatment doses of IFA as per the protocol of the National program2.
The custom software was designed to integrate barcode printing, hematology data export, and visualization of data. It was designed using an online PHP-based desktop application using PHP scripting language and PHP desktop chrome with visual studio code as an integrated development environment. HTML 5, CSS, and JavaScript were used to design the user interface. Web services were developed using the PHP scripting language. A user could print multiple barcodes using this desktop application through a barcode printer. A barcode scanner was used to access the barcode data and send it to the auto-analyzer. Data was stored locally using SqlLite and on the server using MySql database with MySql workbench as an environment. The hematology reports could also be downloaded in PDF format. A detailed treatment protocol based on the Anemia Mukt Bharat guidelines12 has been integrated into the Android application (Table 1 and Table 2)2. This module calculated the IFA dose (the type and number of IFA tablets) based on the participant's age, sex, physiological status, and Hb level. In the case of young children who will need iron syrup, the dose (in milliliters of syrup) was calculated based on the child's body weight. The components of the Electronic decision support system (EDSS) display included (i) participant's anemia status (grade of anemia or no anemia), (ii) visual depiction of the prescribed IFA package (pink for a 45 mg tablet, blue for a 60 mg tablet for adolescents, and red for a 60 mg tablet for adults), (iii) dose (number of tablets to be delivered for 1 month), (iv) frequency and timing (daily or weekly after the meal) (Figure 3).
The current anemia program in India covers eight age and gender groups. The treatment doses are different, with 5 types of prophylactic doses and 7 types of treatment doses. Due to this, there is a possibility of errors in decision-making by the front-line health workers who have limited education and skills. Using this software, the errors can be eliminated, and the treatment delivery can be faster and more accurate. Therefore, the software has the potential to become a job aid for front-line workers to roll out anemia control programs at the population level. Previous studies have shown that front-line health workers could successfully use digital technologies for data collection and record keeping13. Receiving an electronic job aid would also enhance the perceived self-identity of this important first-line community health contact personnel14.
The basic cost of analysis using this method was about INR 75 per sample and included lancet, microtainer, and reagents. Even though a systematic cost analysis had not been done so far on this method, we believe that the overall cost would be comparable with other commonly used PoC methods like digital hemoglobinometer, which costs about INR 104-177 per sample analysis15. Additionally, considering the important advantages of the auto analyzer wherein markers such as RBC indices could be obtained, the auto analyzer based method could be considered promising for use in population-based surveys.
For optimal use of the integrated method in field settings, there were several critical steps that need to be followed. During blood sample collection, precautions should be taken not to press the fingers. Another critical step was sample mixing. Immediately after collection and before analysis, the sample should be mixed properly to get accurate results. During the sample upload, the firewalls installed on the laptop needed to be switched off. The system was used at the point-of-use with the help of 1 KVA batteries, which sustained the analyzer for about 4 h.
One limitation of the method was its temperature sensitivity. When the ambient temperatures were above 35 °C, the analyzer would work best inside a vehicle with an air conditioner. If the analyzed sample data was uploaded twice, the system automatically displayed the most recent upload, even though all the files would be available at the back end. The decision support tool was accurate in providing decisions. But for its use, front-line health workers needed to be digitally literate. Since the software was bilingual, it would not be difficult for the front-line health worker to understand the display language. However, if the software has to be scaled up for states other than Telangana, the new language needs to be incorporated.
With the help of an algorithm-based decision support tool and a simplified visual output, the front-line health workers will be able to treat anemics and follow them up throughout the treatment period. The integrated method has the potential for scale-up, is completely designed using open-source platforms, and is interoperable. This digital tool will provide a substantial impetus for rolling out the 'screen and treat' interventions for anemia reduction in resource-constrained settings.
Declarações
The authors have nothing to disclose.
Acknowledgements
The team acknowledges Mr. J Suresh, Mr. Medappa, Mrs. Madhu, and the entire Kavintech Corporation, Bangalore team, who successfully developed the decision support tool and the software. The authors wish to also acknowledge the Indian Council of Medical Research, Government of India for funding and the Department of public health and family welfare, Telangana for facilitating the study.
Materials
| ABX Miniclean | Horiba Ltd, Japan | 23-450-004 | Enzymatic solution |
| ABX Minidil LMG | Horiba Ltd, Japan | 23-450-008 | Buffered isotonic solution for RBC/PLT dilution, sleeving and cleaning |
| ABX Minilyse | Horiba Ltd, Japan | 23-450-006 | Hb measurement; lysing solution |
| ABX Minocal | Horiba Ltd, Japan | 2032002 | Calibrator |
| ABX Minoclair | Horiba Ltd, Japan | 23-450-003 | Cleaning reagent |
| ABX Minotrol 16 – 2H | Horiba Ltd, Japan | 2042209 | Blood control |
| ABX Minotrol 16 – 2L | Horiba Ltd, Japan | 2042208 | Blood control |
| ABX Minotrol 16 – 2N | Horiba Ltd, Japan | 2042202 | Blood control |
| Autoanalyzer | Horiba Ltd, Japan | ABX Micros ES 60 | The FTP port should be functional |
| Barcode printer | Technology service corporation, USA | TSC Model TE 244 | 400 Mhz 32 bit RISC processor with 16 MB SDRAM, 8 MB Flash memory |
| Barcode Scanner | Retsol | LS-450 | Any company which can provide a scanner with the following specifications: 32 bit CPU fast decode ability, IP 54 rated, Light source – visible laser diode 650 nm, Single scan pattern with scan rate of 100scans/second, Scan width of 200 mm & precision of 4 mil, Scan angle – YAW 65 Deg, Rotation 30 Deg & Pitch 55 Deg, Scan indication – buzzer, light indicator, Scan mode both manual & continue scanning |
| BD needles holder | Becton, Dickinson and company Ltd, Dublin, Ireland | 364879 | |
| Contact activated lancet | Becton, Dickinson and company Ltd, Dublin, Ireland | 366594, 366593 | For children below 1 year, venous blood sample has been collected. |
| Custom software | Kavin Corporation, Bangalore | N/A | |
| K2-EDTA Microtainer-5 mL | Becton, Dickinson and company Ltd, Dublin, Ireland | 363706 | EDTA tube for blood profile analysis with 1.0 mg K2 EDTA, dimensions 13 x 75 mm |
| Labels | G-Technologies, Secunderabad, telangana | N/A | |
| Laptop | Any | N/A | Intel Core I3-1005G1, 8GB DDR4, 1TB HDD, 15.6 FHD LED, WIN 11 HOME and MS OFFICE H&S |
Referências
- Ministry of Women and Child Developmen. Press Information Bureau, Government of India Available from: https://pib.gov.in/Pressreleaseshare.aspx?PRID=1797687 (2020)
- . Ministry of Health and Family Welfare Government of India Available from: https://main.mohfw.gov.in/ (2018)
- Neufeld, L. M., et al. Hemoglobin concentration and anemia diagnosis in venous and capillary blood: biological basis and policy implications. Annals of New York Academy of Sciences. 1450 (1), 172-189 (2019).
- Haggenmüller, V., et al. Smartphone-based point-of-care anemia screening in rural Bihar in India. Community Medicine (Lond). 3 (1), 38 (2023).
- Neogi, S. B., et al. Diagnostic accuracy of point-of-care devices for detection of anemia in community settings in India. BMC Health Service Research. 20 (1), 468 (2020).
- Sharma, S., Zapatero-Rodríguez, J., Estrela, P., O’Kennedy, R. Point-of-care diagnostics in low resource settings: Present status and future role of microfluidics. Biosensors (Basel). 5 (3), 577-601 (2015).
- Augustine, L. F., Dasi, T., Palika, R., Pullakhandam, R., Kulkarni, B. Point of care diagnosis of anemia using portable auto analyzer. Indian Pediatrics. 57 (6), 568-569 (2020).
- Dasi, T., et al. Hemoglobin measurement in capillary blood by a portable autoanalyzer for population level screening of anemia: validation in cross-sectional and longitudinal studies. British Journal of Nutrition. 128 (6), 1108-1117 (2022).
- Briggs, C., Kimber, S., Green, L. Where are we at with point-of-care testing in haematology. British J Haematology. 158 (6), 679-690 (2012).
- Krleza, J. L., Dorotic, A., Grzunov, A., Maradin, M. Capillary blood sampling: national recommendations on behalf of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Biochemical Medicine (Zagreb). 25 (3), 335-358 (2015).
- World Health Organization. WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy). World Health Organization. , (2010).
- Iron Deficiency Anaemia. Assessment Prevention and Control. A Guide for Programme Managers. World Health Organization Available from: https://cdn.who.int/media/docs/default-source/2021-dha-docs/ida_assessment_prevention_control.pdf (2001)
- Zaidi, S., et al. Operability, usefulness, and task-technology fit of an mHealth app for delivering primary health care services by community health workers in underserved areas of Pakistan and Afghanistan: Qualitative study. Journal of Medical Internet Research. 22 (9), e18414 (2020).
- Meena, S., Rathore, M., Kumawat, P., Singh, A. Challenges faced by ASHAs during their field works: A cross sectional observational study in rural area of Jaipur, Rajasthan. International Journal of Medicine and Public Health. 10 (3), 97-99 (2020).
- Neogi, S. B., et al. Cost-effectiveness of point-of-care devices for detection of anemia in community settings in India. Clinical Epidemiology and Global Health. 14, 100995 (2022).

