Efficient Isolation Protocol for B and T Lymphocytes from Human Palatine Tonsils
Summary
Palatine tonsils are a rich source of B and T lymphocytes. Here we provide an easy, efficient and rapid protocol to isolate B and T lymphocytes from human palatine tonsils. The method described has been specifically adapted for studies of the viral etiology of tonsil inflammation known as tonsillitis.
Abstract
Tonsils form a part of the immune system providing the first line of defense against inhaled pathogens. Usually the term “tonsils” refers to the palatine tonsils situated at the lateral walls of the oral part of the pharynx. Surgically removed palatine tonsils provide a convenient accessible source of B and T lymphocytes to study the interplay between foreign pathogens and the host immune system. This video protocol describes the dissection and processing of surgically removed human palatine tonsils, followed by the isolation of the individual B and T cell populations from the same tissue sample. We present a method, which efficiently separates tonsillar B and T lymphocytes using an antibody-dependent affinity protocol. Further, we use the method to demonstrate that human adenovirus infects specifically the tonsillar T cell fraction. The established protocol is generally applicable to efficiently and rapidly isolate tonsillar B and T cell populations to study the role of different types of pathogens in tonsillar immune responses.
Introduction
Tonsils are collections of incompletely encapsulated lymphoid tissues that lie under, and in contact with, the epithelium in the upper aero-digestive tract. Usually the term “tonsils” refers to the palatine tonsils situated at the lateral walls of the oral part of the pharynx. The paired palatine tonsils together with the nasopharyngeal tonsil (adenoid), paired tubal tonsils and lingual tonsils constitute the so-called “Waldeyer´s ring”. The latter is responsible for the initial contact between inhaled or ingested pathogens and the lymphoid tissues of the aerodigestive tract1,2. Indeed, numerous reports have shown that both bacterial and viral antigens can be detected in palatine tonsil tissue samples2-6.
The palatine tonsils are composed of dense lymphoid tissue covered by a stratified squamous non-keratinising epithelium. The tonsils have numerous crypts, epithelial invaginations, which penetrate the parenchyma increasing the surface area. Histologically, the palatine tonsils contain numerous lymphoid follicles with germinal centers, which are the sites for B cell maturation and differentiation (B-cell areas). Likewise, the palatine tonsils encompass T cells, which are mainly located in the extrafollicular regions (T-cell areas). In addition to the B and T cells, also various follicular dendritic cells can be detected in palatine tonsils1,2.
Due to their anatomic location, the palatine tonsils are easily accessible by surgical interventions. For example, surgical removal of tonsils, known as tonsillectomy, is routinely carried out worldwide7. In children with tonsillar hyperplasia, a partial surgical removal of the tonsils (tonsillotomy) is sometimes used, causing less postoperative pain to the patients. Considering the accumulation of various pathogens in tonsils, surgically removed tonsils provide a unique opportunity to study the influence of viral and bacterial agents on tonsillar lymphocyte functions2,8. Furthermore it is possible to study if some pathogens prefer to reside in specific cell subpopulations9. In addition, as the tonsils are rich source of B lymphocytes, isolated tonsillar B lymphocytes can be efficiently used to study the activity of different B cell subpopulations10. However, as the palatine tonsils contain a mixture of cell types an efficient method to separate the different cell subpopulations is needed.
Here, we describe a simple method for efficient and rapid isolation of tonsillar B and T cell populations from human palatine tonsils by using a magnetic-activated cell separation technique (Figure 1). The method described here is useful for scientists who want to assess the role of different infectious agents in human lymphoid organs such as palatine tonsils.
Protocol
The protocol describes the isolation of lymphoid cells from human patient material and therefore requires an ethical approval. The work done in the present study was granted by the Uppsala Ethical Review Board (Dnr. 2013/387).
1. Isolation of Mononuclear Cells (MNCs) from Human Palatine Tonsils
CAUTION: All unscreened material of human origin like blood, tissues or body fluids should be regarded as potentially infected material. Therefore, recommended biosafety practices for handling the human tissues should be followed.
Note: To avoid contamination, all solutions and cell culture equipment must be sterile. All buffers and solutions should be pre-cooled and kept on ice. Tonsil tissues and isolated cells should be kept and handled on ice.
- Obtain fresh tonsils from patients undergoing tonsillectomy or tonsillotomy (Figure 2). Plunge the tissue clumps into a 50 ml centrifuge tube filled with ice-cold sterile Hanks balanced salt solution (HBSS) supplemented with 5% fetal bovine serum (FBS), 10 mM Glutamine, 0.05 mg/ml Gentamicin and 1% Antibiotic-Antimycotic mix (Penicillin, Streptomycin and Amphotericin B).
- Keep the tubes with the submerged tonsil tissue samples at all times on ice. Try to process the tonsils as soon as possible, and no later than 3 hr after surgical removal.
- Place the tonsil on a 60 mm plastic cell culture plate on ice and keep the tissue moistened with HBSS. Remove visible blood clots, fatty and connective tissues with a clean forceps from the tonsil surface.
- Use a new 60 mm cell culture plate containing 5 ml HBSS. Cut the tonsil tissue clump into 3-10 mm fragments using a sterile pair of scissors and/or a scalpel.
- Prepare a new 60 mm cell culture plate containing 10 ml of HBSS and place a 100 µm plastic cell strainer in the HBSS solution.
- Transfer the dissected tonsil fragments into the cell strainer with a sterile forceps. Using the plunger end of a plastic syringe, smoothly squeeze the tissue fragments through the cell strainer. Make sure that the tonsil fragments are totally immersed in the HBSS.
- Discard the cell strainer with the tissue remains and transfer the resulting cell suspension from the 60 mm cell culture plate into a 50 ml plastic centrifuge tube. Leave the tube on ice while proceeding with steps 1.8 and 1.9.
- In the case of big tonsils, larger than 2 cm in size (Figure 2), squeeze the tonsil fragments in two cell strainers to avoid clogging of the mesh in the strainer.
- Obtain the final volume of 35 ml of the cell suspension.
Note: At this step it is possible to prepare the cell suspension for cryopreservation (see section 2). - Add 10 ml of density gradient solution such as Ficoll into a new 50 ml centrifuge tube. Gently overlay the cell suspension (step 1.9) on top of the density gradient solution. Avoid mixing of the cell suspension and the density gradient solution.
Note: The density gradient solution has to be warmed up to RT. - Centrifuge the cell suspension in a swing-out rotor at 700 x g for 20 min at RT. Do not activate the brake function on the centrifuge as fast braking may disrupt the gradient. Also, use the lowest acceleration function available on the centrifuge.
Note: After this centrifugation step, observe MNCs as a fluffy white layer at the interface, and red blood cells (RBCs), fibroblasts and cell debris as the sediment at the bottom of the tube. - Carefully collect the MNCs layer by using a 10 ml pipet. Place the cell suspension into a new 50 ml centrifuge tube.
- Add 25 ml of ice-cold PBS to the cell suspension and spin the tube at 300 x g for 5 min in a swing-out rotor at 4 °C.
- Remove the supernatant using a 25 ml pipet and repeat cell pellet washing step two more times. Finally resuspend the cell pellet in 15 ml of PBS.
Note: After the final washing step, it is possible to cryopreserve the cell suspension (see section 2). - Apply 10 µl of cell suspension into hemocytometer cell counting chamber and count the cell number under a microscope. Dispense the appropriate amount of cells (e.g., 2-3 x 107) in a 15 ml centrifuge tube for subsequent magnetic bead separation. Keep this aliquot on ice until step 3.2.
2. Cryopreservation of Tonsillar Cells
- Prepare freezing medium (90% FBS and 10% DMSO) and keep it on ice. For long-term storage keep the freezing media at -20 °C.
- Determine the total number of cells using a hemocytometer cell counting chamber (step 1.15). Calculate the required amount of freezing medium according to the desired frozen cell density (e.g., 107 cells/ml of freezing medium).
- Centrifuge the cell suspension at 300 x g for 5 min. Decant the supernatant without disturbing the cell pellet and resuspend the cell pellet in ice-cold freezing medium (from step 2.1).
- Dispense 1 ml aliquots of the cell suspension into sterile vials designed for long-term storage in liquid nitrogen. Freeze the vials in an isopropanol chamber and store them at –80 °C O/N. For long-term preservation transfer the vials into a liquid nitrogen containing storage tank or a –140 °C cell freezer.
- To thaw vials with frozen cells, warm them rapidly in a 37 °C water bath. Immediately when thawed, disperse the cell suspension into 10 ml of pre-warmed HBSS (with supplements, step 1.1) in a 15 ml centrifuge tube. Spin the tube at 300 x g for 5 min, remove HBSS and resuspend the cell pellet at the desired cell density in HBSS (with supplements, step 1.1).
Note: The viability of the MNCs after thawing is of importance, since the presence of dead cells will decrease the final yield of purified B and T lymphocytes.
Note: According to our experience the cell viability less then 80% will reduce the cell isolation efficiency.
3. Positive Selection of T Lymphocyte Population from Tonsillar MNCs
Note 1: This protocol is based on positive selection of human CD3+ T lymphocytes from tonsillar MNCs using magnetic beads coupled to the CD3 antibody. It is possible to start this section from fresh (Section 1) or the frozen (Section 2) MNCs.
Note 2: Start with 3 x 107 MNCs. Do not exceed this cell number, since the separation columns may clog and this will reduce the isolation efficiency. Use bigger columns if more cells will be handled. The volumes used in this protocol have been experimentally optimized for the number of cells used in our experimental setup.
- Prepare the separation buffer [PBS (pH 7.2), 0.5% bovine serum albumin (BSA) and 2 mM EDTA]. Filter the separation buffer through a 0.45 µm filter and store at 4 °C.
- Spin the MNCs suspension (step 1.14) at 300 x g for 5 min at 4 °C. Resuspend the resulting cell pellet in 240 µl (80 µl per 107 cells) of ice-cold separation buffer. Transfer the cell suspension into a sterile 2 ml tube.
- Add 20 µl of CD3 magnetic antibody to the cell solution. Incubate the tubes for 1 hr at 4 °C with continuous gentle mixing to keep cells in suspension.
- Transfer all of the cell suspension into a 15 ml tube containing 5 ml ice-cold separation buffer to wash the cells.
- Centrifuge the tube at 300 x g for 10 min at 4 °C in a swing-out rotor.
- Meanwhile set up the magnetic separator and column. Attach the magnetic separator to the stand and place the column in the separator. Wash the column by applying 500 µl of ice-cold separation buffer. Discard the flow through.
- Place a new 15 ml collection tube below the column. Keep the collection tube on ice.
- Discard the supernatant (step 3.5) by pipet and gently resuspend the cell pellet in 500 μl separation buffer. Apply the cell suspension on top of the pre-washed column and let it run through.
Note: Cell clumps can clog the column and thus reduce the flow rate. To prevent this problem, place a 40 µm plastic cell strainer on top of the column. Passing the cells through this mesh will highly improve the efficiency of this method. - Wash the column 4 times with 1.5 ml of separation buffer (500 µl for 107 cells). Wait for the column reservoir to be empty (no liquid should be observed in the column) before applying the next washing step.
Note: Obtain the CD3 unlabeled cells, considered as B lymphocyte fraction, as they are eluted into the collection tube. - Remove the column from the separator and place into a new 15 ml collection tube. Pipette 2 ml of separation buffer onto the column. Using the plunger supplied with the column elute the positively selected T lymphocytes, which are regarded as the T lymphocyte fraction.
- Determine the number of purified cells (step 1.15) if necessary. Cell suspensions are now ready for downstream experiments.
4. Flow Cytometry Analysis of Isolated Tonsillar B and T Lymphocytes
CAUTION: Paraformaldehyde solution is irritant and suspected carcinogen. Wear suitable protective clothing, gloves, and eye/face protection.
Note 1: This protocol describes the method for direct staining of the isolated B and T cells by Fluorescence Activated Cell Sorting (FACS) analysis. The main purpose of this step is to assess the purity of the isolated cell populations after CD3 magnetic antibody separation. For this purpose established B and T cell markers, fluorophore-conjugated monoclonal antibodies CD20-FITC and CD2-APC, are used. Also the inclusion of isotype control antibodies is highly recommended to distinguish the non-specific “background” binding of the CD2 and CD20 antibodies (see step 4.8).
Note 2: It is possible to keep the purified cell fractions in a 1% paraformaldehyde (PFA) solution in the dark at 4 °C until the time of staining (e.g., next day). Also the same procedure is applicable if there is a time gap between staining and FACS analysis. Remember that in both cases cells should be washed properly with PBSA (PBS containing 0.2% BSA) buffer, prior to staining or FACS analysis.
- Take out 6 x 106 cells of MNCs from step 1.15 (prior to applying to the column), B lymphocyte fraction from step 3.9 and T lymphocyte fraction from step 3.10.
- Spin the cell suspensions at 300 x g for 5 min at 4 °C using a swing-out rotor.
- Remove the supernatant with pipet and wash the cell pellet once with 1 ml of ice-cold PBSA. Spin the cell suspensions at 300 x g for 5 min at 4 °C. Remove the supernatant with pipet and resuspend the cells in 600 µl of ice-cold PBSA buffer.
- Add 100 µl of the cell suspension to the bottom of a FACS tube.
- Add 100 µl of PBS containing 10% heat inactivated human serum to each tube, mix well and incubate for ~1 min at RT or 20 min on ice.
Note: B lymphocytes carry Fc receptors. In order to block the Fc receptors the purified cell fractions are incubated with 10% heat inactivated human serum. The human serum is heat inactivated by incubation at 56 °C for 1 hr. Divide the heat inactivated serum into small aliquots and store frozen at -20 °C. - Centrifuge the cell suspension at 300 x g for 5 min at 4 °C in a swing-out rotor.
- Remove the supernatant with pipet and wash the cell pellet with 1 ml PBSA. Repeat the centrifugation (300 x g for 5 min at 4 °C).
- Add 100 µl PBSA into each tube on ice. Add the appropriate amount of fluorophore-conjugated monoclonal antibody, according to the manufacturer’s recommendation (e.g., 20 µl of anti-CD20 and/or 5 µl of anti-CD2 antibodies in 100 µl of PBSA). Note: Do not forget to assign enough control tubes for the FACS analysis. In this protocol the following controls should be included: 1) cells stained with the anti-CD2 and anti-CD20 antibodies individually, 2) cells stained with the anti-CD2 and anti-CD20 isotype control antibodies individually and, 3) cells without addition of antibodies.
- Briefly vortex the tube and incubate for 30 min at 4 °C in the dark.
- Wash 2 times with PBSA. Add 2 ml of 1% PFA solution to the samples. Let the cells remain in the PFA solution at 4 °C in dark till the time of FACS analysis.
- Immediately before running the samples in the FACS machine, wash the cells 2 times with PBSA (300 x g for 5 min at 4 °C). Resuspend samples in 1 ml of PBS and keep at 4 °C (or on ice), protected from light, prior to the separation on the flow cytometer.
- Do the FACS sorting following the protocol from the flow cytometer manufacturer.
5. PCR Detection of Adenovirus DNA in Isolated Tonsillar B and T Lymphocytes
Note 1: The adenovirus hexon gene primers are AdRJC1 (5’-GACATGACTTTCGAGGTCGATCCCATGGA-3’) and AdRJC2 (5’-CCGGCTGAGAAGGGTGTGCGCAGGTA-3’)11. These primers generate an amplicon of 139 bp. The host 18S rRNA gene can be detected with primers tp206 (5'-CCCCTCGATGCTCTTAGCTG-3’) and tp207 (5'-TCGTCTTCGAACCTCCGACT-3’). The expected amplicon size is 300 bp.
Note 2: Every PCR run includes a negative control (distilled H2O) and a positive DNA control obtained from human B cell line (BJAB) infected with human adenovirus type 512.
- Extract DNA (from at least 1 x 106 cells, step 3.11) using silica membrane based column purification method. Extract RNA using phenol and guanidine isothiocyanate solution13.
- Add 100 ng DNA from B and T lymphocytes to a reaction mixture containing 1x HF buffer, 0.2 mM of deoxynucleotide triphosphate mix (dNTP), 0.25 µM of each primer and 1 U of High-Fidelity DNA polymerase in a total volume of 20 µl.
- Perform PCR amplification under the following cycling conditions: 30 sec denaturation at 98 °C, followed by 30 cycles of 98 °C for 10 sec, 67 °C (hexon) or 58 °C (18S rRNA) for 30 sec and 72 °C for 30 sec. The PCR reaction was ended with a final extension step of 7 min at 72 °C.
- Separate the resulting PCR amplification products on a 1.5% agarose gel in 1x TBE buffer and visualize the expected bands by staining with nucleic acid stain.
Representative Results
An efficient separation of tonsillar MNCs results in highly purified subpopulations of B and T lymphocytes. This was confirmed by FACS analysis using anti-CD20 and anti-CD2 antibodies to detect B and T lymphocyte populations, respectively (Figure 3A). In contrast, an inefficient separation of MNCs results in cell fractions that are a mixture of cells from both B and T lymphocyte subpopulations (Figure 3B).
The isolated tonsillar B and T lymphocytes (Figure 3A) are of sufficient quality for downstream analyses like nucleic acid isolation. In the current protocol, DNA and RNA extracted from the B and T lymphocytes are utilized for detection of adenovirus DNA and cellular RNA. The results show specific detection of adenovirus DNA in tonsillar T lymphocytes (Figure 4). Also isolated RNA from tonsillar B and T lymphocytes is of sufficient quality and quantity for downstream applications (Figure 5).
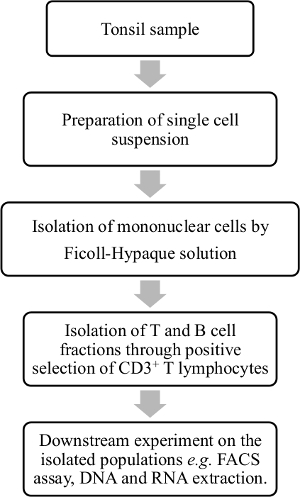
Figure 1. Overview of the tonsillar lymphocyte isolation procedure. Palatine tonsil samples are processed and tonsillar MNCs are isolated from cell suspension by density gradient centrifugation. B and T lymphocytes are purified into subpopulations by a positive selection of the T lymphocyte fraction with human CD3 magnetic antibody. This purification step makes it possible to further assess the isolated cell fractions; for example, to determine if some viral species are enriched within any of the subpopulations. Please click here to view a larger version of this figure.
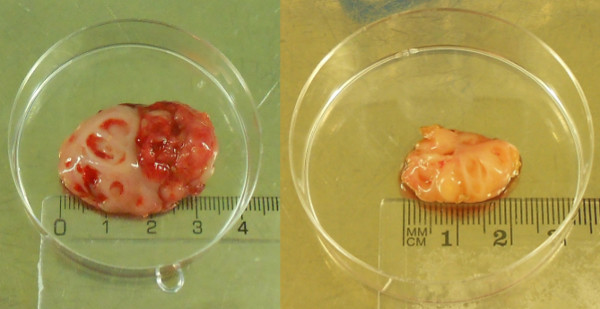
Figure 2. Surgically removed palatine tonsils. Tonsils removed by tonsillectomy (left) and tonsillotomy (right). Please click here to view a larger version of this figure.
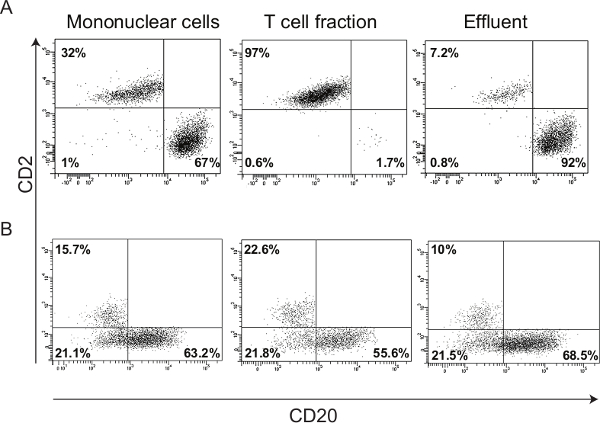
Figure 3. Representative FACS results of the enriched cell fractions. (A) Forward vs. side scatter plot showing B (CD20) and T (CD2) lymphocyte populations in tonsillar MNCs, T lymphocyte and effluent fractions. The purity of the T and B lymphocytes in enriched cell fractions is more than 90%. Since B cells comprise 92% of the total effluent cells, this fraction is named as the B lymphocyte subpopulation. (B) FACS plots showing inefficient separation of B and T cell subpopulations, due to non-optimized number of MNCs applied to the column and insufficient washing steps. Aside from the need for magnetic-activated cell sorting optimization, there is a high background of CD2– /CD20– cells, which shows that FACS staining is not well optimized. Please click here to view a larger version of this figure.
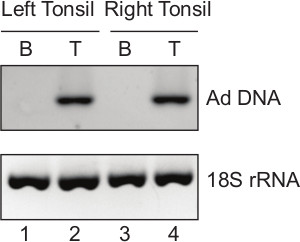
Figure 4. PCR detection of adenovirus DNA in tonsillar lymphocyte fractions. Total DNA is extracted from the B and T lymphocytes isolated from the left and the right tonsils from a patient undergoing tonsillectomy. End-point PCR is performed with 100 ng of the purified DNA. Lane 1: B lymphocyte fraction (B) of the left tonsil; lane 2: T lymphocyte fraction (T) of the left tonsil; lane 3: B lymphocyte fraction (B) of the right tonsil; lane 4: T lymphocyte fraction (T) of the right tonsil. Adenovirus (Ad) DNA is only detectable in isolated T lymphocyte fraction. Cellular 18S rRNA gene acts as an internal control to show that same amount of DNA is used for the PCR reaction in all samples. Please click here to view a larger version of this figure.
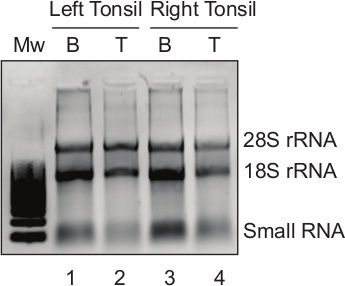
Figure 5. High quality RNA extracted from isolated B and T cell subpopulations. Total cytoplasmic RNA extracted from the isolated B and T lymphocytes was separated on a 1% agarose gel. Lane 1: B lymphocyte fraction (B) of the left tonsil; lane 2: T lymphocyte fraction (T) of the left tonsil; lane 3: B lymphocyte fraction (B) of the right tonsil; lane 4: T lymphocyte fraction (T) of the right tonsil. Migration pattern of 28S rRNA, 18S rRNA and small RNA is indicated. Please click here to view a larger version of this figure.
Discussion
One of the most important factors affecting the outcome of this protocol is the use of fresh tonsillar material as the starting material. Therefore, the tonsil samples should be processed within 3 hr after surgery. Tonsils could be obtained from both adults and children. The tonsillar material from the children is usually smaller due to the partial surgical removal of the tonsils (tonsillotomy). Therefore, the number of MNCs obtained from tonsillotomy samples (1 x 107-1 x 108) is less compared to tonsillectomy samples (1 x 108-2 x 109). However, the cell isolation process would be the same regardless of the type of surgery.
Maximizing the purity of the cell subpopulations obtained from magnetic-activated cell sorting method requires some optimization. The amount and incubation time with CD3 magnetic antibody and the number of MNCs applied to the column are the main factors that should be optimized. Applying too many cells will result in clogging of the column and inefficient enrichment of cell subpopulations. During the optimization of this protocol, we found that applying more than 3 x 107 MNCs on the separation column will cause a clogging of the column and subsequent impure cell fractions (Figure 3B). Thus, starting with 3 x 107 MNCs gives well enriched lymphocyte fractions with sufficient quality and quantity for DNA and RNA extraction (Figures 4 and 5). The final number of isolated B and T lymphocytes is expected to be 1-2 x 107 and 5-7 x 106 , respectively. Thus, usage of 20 µl of CD3 magnetic antibody per 3 x 107 MNCs appears to be optimum for successful B and T lymphocyte separation (Figure 3A).
A previous report has established that B and T lymphocytes comprise 60-70% and 30-40% of the tonsillar MNCs respectively, while other cell types like macrophages, natural killer cells and dendritic cells make up 1-8% of the tonsillar MNCs14. Similar cell proportions were achieved with our isolation protocol as shown by immunostaining of the B and T lymphocytes with the FACS technique (Figure 3A).
The number of washing steps and the washing buffer volume are critical parameters for a good result. Accordingly, excessive washing will cause the CD3 magnetic antibody associated T cells to end up in the flow-through, whereas insufficient washing will reduce the purity of the T cell fraction.
Once the critical steps in the protocol are optimized for the tissue type and the expected downstream analyses, this method is simple, efficient, straightforward and time saving. However one limitation of this method is a relatively high cost of commercial magnetic-associated cell sorting reagents, which might limit large-scale purification of tonsillar B and T lymphocytes.
In summary this protocol provides a strategy for obtaining B and T subpopulations from palatine tonsils and might be modified to isolate cell fractions from other tissues like spleen, thymus, lymph nodes and blood. We further apply this method to show that we detect an adenovirus infection specifically in the tonsillar T cell fraction (Figure 4). Thus, this protocol should be useful for a wide-area of applications, including studies on host-pathogen interactions, T cell cytotoxicity, T cell activation, cell signaling and B and T cell surface marker expression.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Swedish Cancer Society (11 0253, 13 0469), the Swedish Research Council (K2012-99X-21959-01-3), Marcus Borgströms Foundation and the Swedish Research Council through a grant to the Uppsala RNA Research Centre (2006-5038-36531-16). We are indebted to the BioVis core facility at Uppsala University for much help with the FACS analysis.
Materials
| Hanks balanced salt solution (HBSS) | Gibco | 14175-053 | Contains 5% fetal bovine serum (FBS), 10 mM Glutamine, |
| 0.05 mg/ml Gentamicin and 1% Antibiotic-Antimycotic mix | |||
| Freezing medium | 90% FBS and 10% DMSO | ||
| MACS buffer | PBS (pH 7.2), 0.5% BSA and 2mM EDTA | ||
| PBSA | PBS containing 0.2% BSA | ||
| PFA | PBS containing 1% paraformaldehyde (PFA). Make fresh. | ||
| PFA is suspected carcinogen. Wear gloves and goggles. | |||
| Antibiotic-Antimycotic mix | Gibco | 15240-062 | |
| 100-mm petridish | Nunc | 172958 | |
| Dissecting foreceps | Fisher Scientific | 1381241 | |
| Straight iris scissors | Fisher Scientific | 12912055 | |
| disposable scalpels | Swann-Morton | REF 0501 | |
| 100μm plastic cell strainer | Corning Life Sciences | 352360 | |
| 40μm plastic cell strainers | Corning Life Sciences | 352340 | |
| 2-ml plastic syringe | BD Biosciences | 300185 | |
| 15-ml conical centrifuge tubes | SARSTEDT | 62554502 | |
| 50-ml conical centrifuge tubes | SARSTEDT | 62547254 | |
| Low-speed centrifuge with fixed-angle or swinging-bucket rotor | Thermo Scientific Heraeus Megafuge 16R | ||
| Ficoll–Hypaque | Sigma-Aldrich | F5415-50ML | Ficoll solution |
| Fetal calf serum | Biological industries | 040071A | |
| Dimethyl sulphoxide (DMSO) | SIGMA | D2650-5X5ML | |
| MACS MS columns | Miltenyi Biotec | 130-042-201 | |
| MACS human CD3 MicroBeads | Miltenyi Biotec | 130-092-881 | |
| MACS separator (Octo MACS) | Miltenyi Biotec | 130-042-109 | |
| Bovine Serum Albumin (BSA) | Merck Millipore | 1120180100 | |
| EDTA | AnalaR NORMAPUR | 20302.293 | |
| CD2 conjugated to allophycocyanin (CD2-APC) | BD Biosciences | 560642 | |
| CD20 conjugated to fluorescein isothiocyanate (CD20-FITC) | BD Biosciences | 556632 | |
| Human serum | Rockland Immunochemicals | D119-0100 | |
| Phusion High-Fidelity DNA Polymerase | Thermo Scientific | F-530L | |
| FACS tubes | BD Falcon | 352003 | |
| Cryotube | SARSTEDT | 72379 | |
| BD LSRII flowcytometer | BD Biosciences | ||
| BD FACSDiva 4.1 software | BD Biosciences | ||
| Nucleospin Blood | Macherey-Nagel | 740951.50 | DNA isolation kit |
| TRIzol reagent | Life technologies | 15596 | RNA isolation reagent |
| Hemocytometer | The Paul Marienfeld GmbH & Co. KG | 0610030 | cell counter device |
| GelRed | Biotium | 41003 | Nucleic acid gel stain |
Referências
- Nave, H., Gebert, A., Pabst, R. Morphology and immunology of the human palatine tonsil. Anat Embryol (Berl). 204, 367-373 (2001).
- Perry, M., Whyte, A. Immunology of the tonsils. Immunology today. 19, 414-421 (1998).
- Alkhalaf, M. A., Guiver, M., Cooper, R. J. Prevalence and quantitation of adenovirus DNA from human tonsil and adenoid tissues. J Med Virol. 85, 1947-1954 (2013).
- Brandtzaeg, P., Halstensen, T. S. Immunology and immunopathology of tonsils. Adv Otorhinolaryngol. 47, 64-75 (1992).
- Imai, S., et al. Epstein-Barr virus (EBV)-carrying and -expressing T-cell lines established from severe chronic active EBV infection. Blood. 87, 1446-1457 (1996).
- Veen, J., Lambriex, M. Relationship of adenovirus to lymphocytes in naturally infected human tonsils and adenoids. Infect Immun. 7, 604-609 (1973).
- Friedman, M., Wilson, M., Lin, H. C., Chang, H. W. Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 140, 800-808 (2009).
- Garnett, C. T., et al. Latent species C adenoviruses in human tonsil tissues. J Virol. 83, 2417-2428 (2009).
- Garnett, C. T., Erdman, D., Xu, W., Gooding, L. R. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol. 76, 10608-10616 (2002).
- Perez, M. E., Billordo, L. A., Baz, P., Fainboim, L., Arana, E. Human memory B cells isolated from blood and tonsils are functionally distinctive. Immunol Cell Biol. 92, 882-887 (2014).
- Cooper, R. J., Yeo, A. C., Bailey, A. S., Tullo, A. B. Adenovirus polymerase chain reaction assay for rapid diagnosis of conjunctivitis. Invest Ophthalmol Vis Sci. 40, 90-95 (1999).
- Zhang, Y., Huang, W., Ornelles, D. A., Gooding, L. R. Modeling adenovirus latency in human lymphocyte cell lines. J Virol. 84, 8799-8810 (2010).
- Chomczynski, P., Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. biochem. 162, 156-159 (1987).
- Watanabe, T., Yoshizaki, K., Yagura, T., Yamamura, Y. In vitro antibody formation by human tonsil lymphocytes. J Immunol. 113, 608-616 (1974).

