滴定法简介
English
COMPARTILHAR
Visão Geral
资料来源: 实验室的博士怡北东东向谭 — — 为科学、 技术和研究机构
滴定法是一种常用的技术,用于定量确定未知确定分析物浓度。1-4它也被称为卷测定容量分析滴定中至关重要。有许多类型的滴定基于他们所利用的反应类型。最常见的类型是酸碱中和滴定和氧化还原滴定。5-11
在典型的滴定过程中,滴定滴定标准溶液逐渐应用与分析物在锥形瓶未知浓度与反应。酸碱中和滴定 pH 指示剂通常需要添加在分析物溶液中指示滴定终点。12而不是添加 ph 值指标,ph 值也可以进行监测在滴定过程中使用 pH 计和终结点以图形方式由 pH 滴定曲线。记录在终点滴定剂体积可以用于计算基于反应化学计量学分析物的浓度。
提出了一种在这个视频的酸碱中和滴定,滴定剂是标准化的氢氧化钠溶液和被测物是国内醋。醋是酸性的液体,常用的作为烹饪调味品或香料。醋主要由乙酸 (CH3COOH) 和水组成。商业醋乙酸含量变化很大,这次实验的目标是确定的商业醋乙酸含量滴定法。
Princípios
醋中的醋酸测定基于酸碱滴定法的原理。氢氧化钠和 CH3羧基之间的反应,如方程 1所示:
CH3COOH(aq) + NaOH(aq) → H2O(l) + 代表3CO2(aq) (1)
标准化的 NaOH 溶液逐步加入醋的未知的乙酸浓度直到到达结束点。在酸碱滴定中,ph 值可以被绘制为滴定液添加体积的函数。拐点在曲线上,有酸、 碱溶液中的化学计量比等量的点称为等价点。大多数的酸和碱是无色的没有明显的反应,等价点发生的。观察时已达到等值点,ph 值指示符会添加。终结点不是等价点但 pH 指示剂变色点。它是重要的是选择适当的 pH 指示剂,这样的结束点是尽可能的尽可能滴定等当点。
这种反应的终点,共轭基地代表3CO2是偏碱性。酚酞指示剂有 8.3 — — 10.0,工作 ph 值范围是无色酸性溶液和 pH 8.2 以上洋红色。因此,酚酞是首选的指标,因为它将从无色变为粉红色,在这种情况。执行实验,时,最好保持 pH 指示剂的浓度低,因为自己的 ph 值指标通常与基地反应的弱酸。
添加到结束点处的标准化 NaOH 溶液的体积可以用于计算基于上述方程的化学计量学的醋酸的摩尔浓度。在这个实验中,氢氧化钠滴定是强碱性和分析物乙酸是一种弱酸。
在执行之前的实验,它是重要的是考虑 NaOH 的吸湿性质。此属性需要其解决方案,要规范与稳定的主要标准,如邻苯二甲酸氢钾 (KHC8H4O4)。确切的摩尔浓度的氢氧化钠溶液然后可以准确地确定标准化以后。主要酸标准与氢氧化钠反应方程 2所示:
KHC8H4O4(aq) + NaOH(aq) → H2O(l) + NaKC8H4O4(aq) (2)
下面一节中提出了详细的分步滴定协议。
Procedimento
Resultados
| Unit | Trial 1 | Trial 2 | Trial 3 | |||
| Volume of diluted vinegar acid (VA) | mL | 25.00 | ||||
| Molar concentration of NaOH (cNaOH) | mol/L | 0.09928 | ||||
| Initial burette reading of NaOH | mL | 0.10 | 0. 05 | 1.20 | ||
| Final burette reading of NaOH | mL | 18.75 | 18.60 | 19.80 | ||
| Volume of NaOH dispensed | mL | 18.65 | 18.55 | 18.60 | ||
| Mean volume of NaOH dispensed (Vt) | mL | 18.60 | ||||
Table 1. Titration results.
Sample calculations:
Mass of KC8H5O4 = 4.0754 g
Molar mass of KC8H5O4 = 204.22 g/mol
Number of moles of KC8H5O4 in 25.00 mL standard solution = 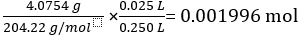
According to Equation 2,
Concentration of the diluted NaOH solution = 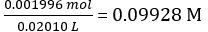
Moles of NaOH dispensed = concentration of NaOH × mean volume of NaOH dispensed = 0.09928 mol/L × 18.60 mL = 1.847 × 10-3 mol
According to Equation 1,
Number of moles of CH3COOH in 25.00 mL of diluted vinegar = 1.847 × 10-3 mol
Concentration of diluted vinegar = 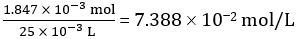
Hence concentration of undiluted vinegar = 10 × 7.388 102 mol/L = 0.7388 mol/L
The above steps are presented to illustrate the calculation procedure; we can simply apply Equation 3 to obtain the concentration of undiluted vinegar in one step.
Therefore 1.000 L of undiluted vinegar contains 0.7388 mol of CH3COOH.
Volume of CH3COOH=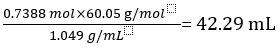
Volume percent of vinegar = 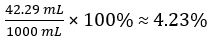
Applications and Summary
Titration is an important chemical method that is frequently applied in current chemistry research. For example, acid base titration is applied to determine amine or hydroxyl value of a sample. The amine value is defined as the number of milligrams of KOH equivalent to the amine content in one gram of sample. To determine the hydroxyl value, the analyte is first acetylated using acetic anhydride then titrated with KOH. The mass in milligrams of KOH then corresponds to hydroxyl groups in one gram of sample.13 Another example is the Winkler test, a specific type of redox titration used to determine the concentration of dissolved oxygen in water for water quality studies. Dissolved oxygen is reduced using manganese(II) sulfate, which then reacts with potassium iodide to produce iodine. Since the iodine released is directly proportional to the oxygen content, the oxygen concentration is determined by titrating iodine with thiosulfate using a starch indicator.14
Besides applications in basic chemical research, titration has also been widely adopted in industrial and everyday use. In biodiesel industry, waste vegetable oil (WVO) must first be neutralized to remove free fatty acids that would normally react to make undesired soap. A portion of WVO is titrated with a base to determine the sample acidity, so the rest of the batch could be properly neutralized.15 Benedict's method, a test for quantification of urine glucose level, is another example showing the importance of titration in healthcare. In this titration, cupric ions are reduced to cuprous ions by glucose, which then react with potassium thiocyanate to form a white precipitate, indicating the endpoint.16
Referências
- Llc, B. Titration: Ph Indicator, Thermometric Titration, Nonaqueous Titration, Equivalence Point, Acid-Base Titration, Amperometric Titration. General Books LLC, (2010).
- Jacobsen, J. J., Jetzer, K. H., Patani, N., Zweerink, G. & Zimmerman, J. Titration Techniques. J. Chem. Educ. 72, 612, doi:10.1021/ed072p612 (1995).
- Harris, D. C. Quantitative Chemical Analysis. 8th edn, W. H. Freeman, (2010).
- Mattock, G., Taylor, G. R. & Paul, M. A. pH Measurement and Titration. J. Electrochem. Soc. 110, 31C, doi:10.1149/1.2425702 (1963).
- De Levie, R. Aqueous Acid-base Equilibria and Titrations. Oxford University Press, (1999).
- Cannan, R. K. The Acid-Base Titration of Proteins. Chem. Rev. 30, 395-412, doi:10.1021/cr60097a005 (1942).
- Michalowski, T. & Lesiak, A. Acid-Base Titration Curves in Disproportionating Redox Systems. J. Chem. Educ. 71, 632, doi:10.1021/ed071p632 (1994).
- Waser, J. Acid-base Titration and Distribution Curves. J. Chem. Educ. 44, 274, doi:10.1021/ed044p274 (1967).
- Gorbikova, E. A., Vuorilehto, K., Wikström, M. & Verkhovsky, M. I. Redox Titration of All Electron Carriers of Cytochrome c Oxidase by Fourier Transform Infrared Spectroscopy. Biochemistry 45, 5641-5649, doi:10.1021/bi060257v (2006).
- Silverstein, T., Cheng, L. & Allen, J. F. Redox Titration of Multiple Protein Phosphorylations in Pea Chloroplast Thylakoids. Biochim. Biophys. Acta (BBA)-Bioenerg. 1183, 215-220, doi:10.1016/0005-2728(93)90022-8 (1993).
- Lenghor, N., Jakmunee, J., Vilen, M., Sara, R., Christian, G. D. & Grudpan, K. Sequential Injection Redox or Acid-Base Titration for Determination of Ascorbic Acid or Acetic Acid. Talanta 58, 1139-1144, doi:10.1016/S0039-9140(02)00444-7 (2002).
- Mitchell, P., Moyle, J. & Smith, L. Bromthymol Blue as a pH Indicator in Mitochondrial Suspensions. Eur. J. Biochem. 4, 9-19, doi:10.1111/j.1432-1033.1968.tb00166.x (1968).
- Perkins, E. G. Analyses of Fats, Oils and Derivatives. AOCS press, (1993).
- Spellman, F. R. Handbook of Water and Wastewater Treatment Plant Operations. 2 edn, CRC Press, (2009).
- Purcella, G. Do It Yourself Guide to Biodiesel: Your Alternative Fuel Solution for Saving Money, Reducing Oil Dependency, Helping the Planet. Ulysses Press, (2007).
- Nigam. Lab Manual Of Biochemistry. Tata McGraw-Hill Education (2007).
Transcrição
Titration is a commonly applied method of quantitative chemical analysis used to determine the unknown concentration of a solution. A typical titration is based on a reaction between a titrant and an analyte. The titrant of known concentration is gradually added to a precise volume of an unknown analyte until the reaction reaches an endpoint.
At the endpoint, the moles of titrant and analyte are equal. By manipulating the equation relating volume and concentration, the concentration of analyte can be deduced.
This video will illustrate the principles behind titration, present a protocol to determine the amount of acetic acid in commercial vinegar, and finally explore some common applications of the method.
Titrations are classified based on the type of reaction carried out. For example, redox titrations make use of an oxidation-reduction exchange between reactants which involves the transfer of electrons from one reactant to another. Complexometric titrations rely on the formation of a largely undissociated complex. However, acid-base titrations, which exploit the neutralization of an acid with a base, are one of the most widely studied. To determine the concentration of acid in an analyte, a base, such as sodium hydroxide, is used. Sodium hydroxide is hygroscopic, that is, it has the property of absorbing moisture from the atmosphere. Before it can be used as a titrant, its exact concentration in solution must be standardized.
To do this, it is first titrated with the primary standard, potassium hydrogen phthalate. A primary standard should be pure, stable, non-hygroscopic, and have a high molecular weight. Because the amount of hydronium ions contributed by the primary standard is known to a high degree of accuracy, it is used to determine the exact concentration of the hydroxide ions in the titrant. During an acid-base titration, the pH can be plotted as a function of the volume of the titrant added. The inflection point on the curve, the point at which there is a stoichiometric equal amount of acid and base in a solution, is called the equivalence point.
Most acids and bases are colorless, with no visible reaction occurring at the equivalence point. To observe when the equivalence point has been reached, a pH indicator is added. This is a pH sensitive dye that changes color in different pH environments. Its important to note that endpoint is not equal to the equivalence point, but indicates when a particular pH value has been reached. For example, phenolphthalein changes color around a pH of 8 and is commonly used as an indicator for acid-base titrations with an equivalence point around pH 7. While an accurate indicator for the titration is one that changes color as close to the equivalence point as possible, the titration curve has a steep slope around the equivalence point, leading to an acceptable level of error. At the equivalence point, the moles of base added are equal to the moles of acid initially present. An equation that utilizes the molarity and volume of each component can be used. With the other three values known, the acid concentration can be calculated. Now that you understand the principles behind the procedure, lets take a look at an actual protocol to determine the percent acetic acid in a commercial vinegar sample by reacting it with a standardized sodium hydroxide solution.
Typically, a rough estimate titration is performed to approximate where the endpoint will be. To begin, the titrant, sodium hydroxide, must be standardized. First, dissolve roughly 4 g of sodium hydroxide into 100 mL of deionized water. Make a 1:10 dilution by adding 25 mL of this stock sodium hydroxide solution to a glass container. Bring the total volume to 250 mL with deionized water and shake to mix. As sodium hydroxide absorbs carbon dioxide, it is important to use boiled, deionized water and an oven-dried bottle, and to cap the bottle quickly.
Calculate the approximate molar concentration of sodium hydroxide. Then, weigh out 5 g of the standard acid, potassium hydrogen phthalate, and place it in a drying oven. Once dried, allow the solid to cool to room temperature in a desiccator.
Weigh out 4 g of the dried potassium hydrogen phthalate to a high degree of precision, and dissolve in 250 mL of deionized water. Calculate the molar concentration of the potassium hydrogen phthalate solution.
Using a volumetric pipette, transfer 25 mL of the potassium hydrogen phthalate solution into a clean, dry Erlenmeyer flask. Add 2 drops of phenolphthalein pH indicator. Gently swirl the flask to mix. Flush a clean 50-mL burette with water and rinse at least three times with deionized water. Following this, rinse again with the diluted sodium hydroxide solution three times, making sure that the sodium hydroxide wets the entire inner surface. Mount the washed burette on a ringstand with a clamp and ensure that it stands vertically.
Fill the burette with the diluted sodium hydroxide solution. Air bubbles can affect the accuracy of volumetric readings. Gently tap the burette to free any air bubbles present, and open the stopcock to allow a few mL of titrant to flow through to release any trapped air. Read the volume of sodium hydroxide, at the bottom of the meniscus.
Place the flask containing potassium hydrogen phthalate under the burette. Add the titrant from the burette in 1–2 mL increments using one hand to control the flow rate by adjusting the stopcock, and the other swirling the flask.
When close to the endpoint, begin adding the titrant drop by drop. The endpoint is reached when the solution turns a faint, persistent pink color. Record the volume in the burette.
Repeat the titration at least two more times for consistent data and calculate the molar concentration of the diluted sodium hydroxide solution used as shown in the text protocol.
The sodium hydroxide solution is now standardized and can be used as a titrant to analyze vinegar. To reduce the pungent aroma, dilute 10 mL to a total volume of 100 mL.
Pipette 25 mL of the diluted vinegar into an Erlenmeyer flask, and add 2 drops of phenolphthalein. Fill the burette with the standardized sodium hydroxide solution and record the initial volume. Similar to the previous titration, slowly add the titrant to the analyte in the flask while swirling until the solution turns a light pink color, and record the final volume of sodium hydroxide used.
In this experiment, the titration was performed in triplicate and the mean volume of sodium hydroxide dispensed to neutralize the acetic acid in vinegar was calculated. The concentration and volume of base was used to elucidate the moles of acetic acid in the vinegar. The volume and molar mass were then used to calculate the concentration. It was determined that the vinegar had a molarity of 0.7388. Converting to percent, it was 4.23% acetic acid by volume.
Titrations are robust and easily customizable methods commonly applied in research, industry, and healthcare.
Scientists often use the measure of dissolved oxygen in freshwater bodies as an indicator of overall health that ecosystem. This is done by a redox titration. Unlike acid-base neutralizations, these titrations are based on a reduction-oxidation reaction between the analyte and the titrant. Dissolved oxygen in the water sample is reduced with chemicals in a reaction that results in the production of iodine. The amount of iodine produced and thus the level of dissolved oxygen can be determined by titration using a starch indicator. Glucose in urine can be indicative of a pathological condition like diabetes. A test to quantify urine glucose level, called Benedict’s Method, is another example of the importance of titration; in this case, in healthcare. In this titrimetric procedure, sugars from urine are first reacted with an alkali resulting in the formation of enediols with powerful reducing properties. These reduce copper two ions in Benedict’s reagent to copper one, in a colorimetric reaction that correlates with the initial concentration of glucose present in the urine sample.
You’ve just watched JoVE’s introduction to titration. You should now be familiar with the principles behind this method, know how to perform an acid-base titration, and appreciate some of the ways it is being applied in research and industry.
As always, thanks for watching!
