Measuring Interactions between Fluorescent Probes and Lignin in Plant Sections by sFLIM Based on Native Autofluorescence
Summary
This protocol describes an original setup combining spectral and fluorescence lifetime measurements to evaluate Förster resonance energy transfer (FRET) between rhodamine-based fluorescent probes and lignin polymer directly in thick plant sections.
Abstract
In lignocellulosic biomass (LB), the activity of enzymes is limited by the appearance of non-specific interactions with lignin during the hydrolysis process, which maintains enzymes far from their substrate. Characterization of these complex interactions is thus a challenge in complex substrates such as LB. The method here measures molecular interactions between fluorophore-tagged molecules and native autofluorescent lignin, to be revealed by Förster resonance energy transfer (FRET). Contrary to FRET measurements in living cells using two exogenous fluorophores, FRET measurements in plants using lignin is not trivial due to its complex autofluorescence. We have developed an original acquisition and analysis pipeline with correlated observation of two complementary properties of fluorescence: fluorescence emission and lifetime. sFLIM (spectral and fluorescent lifetime imaging microscopy) provides the quantification of these interactions with high sensitivity, revealing different interaction levels between biomolecules and lignin.
Introduction
Hydrolyzing lignocellulose into monomeric sugars such as glucose requires enzymes. Their activity is known to be restrained by the establishment of non-specific interactions between enzymes and lignin1,2, the latter being a hydrophobic polymer and a major constituent of lignocellulose3. Thus, quantitatively measuring such interactions directly at the cellular scale is a challenge that must be addressed to optimize enzyme catalytic activity and lignocellulose pre-treatment4.
Förster (or fluorescence) resonance energy transfer (FRET) measurement is a method of choice to asses biomolecules interaction in situ. This non radiative energy transfer can occur between a donor and an acceptor fluorophore if they fulfill different conditions. The donor emission spectrum must overlap, at least partially, the acceptors excitation spectrum. The choice of fluorophores is thus crucial for such experiments. Then, the transfer efficiency decreases with the sixth power of their distance5 and only occurs if both molecules are in close vicinity (typically in the nanometer range). Other contingency can alter the transfer efficiency such as dipole-dipole orientation but are mitigated if fluorophores have flexibility.
FRET can be measured by different techniques and detailed descriptions can be found in literature6,7. In brief, the main methods rely on: i) sensitized emission in which the variation in acceptor emission fluorescence is followed and provides mainly qualitative results; ii) acceptor photobleaching in which the donor emission fluorescence is measured before and after acceptor photobleaching (In this case, more precise FRET estimates can be achieved but require strong laser power applied to fixed samples); and iii) lifetime measurement that decreases for the donor in the presence of the acceptor. This method requires specific instruments and allows precise and sensitive interaction measurements between fluorophores. Considering the FRET measurement between fluorescent probes and lignocellulose, the main difficulty is that lignocellulose is not a single well characterized fluorophore: it has a strong native and spectrally-wide autofluorescence, mainly originating from lignin, and dependent on biomass species8,9.
In this paper, we propose a new and original procedure adapted to sections of wood/plant samples. This sFLIM based method opens the way to quantitative FRET measurements between fluorescent probes and lignin in native lignocellulose samples.
Protocol
1. Sample preparation
NOTE: Successive steps for preparing the samples are described in Figure 1.
- Plant sample preparation
- From wood trunks or fragments, cut 1 cm long samples with razor blades without damaging their structure.
- Embed samples in polyethylene glycol (PEG) medium, by dipping them in successive solutions of increasing PEG concentration diluted in water until reaching 100% PEG.
- Place sample under vacuum to remove water.
- Gently stir samples in 30% v/v PEG for 24 h.
- Gently stir samples in 50% v/v PEG for 24 h.
- Gently stir samples in 100% PEG for 24 h at 70 °C (pure PEG is solid at room temperature).
- Place samples in capsules at 70 °C (on a hot plate) and progressively decrease temperature until reaching room temperature.
- Prepare carefully 30-60 µm thickness flat sections from these PEG blocks using a microtome and disposables blades.
- Collect and wash sections in three successive baths of water for 5 min to remove PEG from the sections.
- Fluorescent probe preparation
- Prepare buffer solution of 30 mM phosphate at pH 6.0.
- Weigh PEG rhand dextran rhodamine probe powder.
- Dissolve probe powder into the buffer with continuous stirring in a glass vial for 1 h in the dark at a final concentration of 0.1 g/L.
- Plant sample staining
- Incubate sections with 500 µL of the fluorescent probe (see 1.2.3) for 72 h (no stirring) in a plastic tube (no more than 3 sections per tube) in the dark.
- Pick-up a section with a brush, adsorb the buffer with a clean sheet (avoid drying the section), and then mount the incubated sections between a cover glass and a #1.5H coverslip.
2. Fluorescence lifetime and spectral measurement system calibration
NOTE: The complete workflow for sFLIM measurement is presented in Figure 2.
- Determine the spectral window width of the sFLIM detector.
- Put urea crystals in a culture box with a 0.17 µm glass bottom.
- Place the culture box on a microscope sample holder and select the 20x objective.
- Select a Ti:Sa laser with a 900 nm wavelength and 2% power, and turn on the scanning.
- Collect the second harmonic signal on the sFLIM detector.
NOTE: The second harmonic signal is emitted at exactly half excitation wavelength of 450 nm; while instantaneous, this measure also provides the instrumental response function of the system. - Gradually adjust the laser excitation wavelength from 900 to 980 nm until the second harmonic signal is collected in the second spectral channel (462.5 nm).
- Determine the spectral window length (12.5 nm).
- If the spectral range differs from what is expected, troubleshoot by doing the following.
- Adjust the laser to 910 nm.
- Move the grating position with a scroll wheel to measure the second harmonic signal on the first channel only (first channel boundary).
- Adjust the laser to 935 nm.
- Move the grating position with a scroll wheel to measure the second harmonic signal on the first channel only (second channel boundary).
- Calibration of the overall spectrometer channels
- Insert the mirror on the microscope stage.
- Select the visible continuous laser (458 nm, 1% power).
- Collect the reflection signal on the sFLIM detector and check if photons are measured in the appropriate spectral channel.
- Repeat step 2.2.2 and 2.2.3 with all available continuous lasers (514 nm, 561 nm and 633 nm).
- If the spectral channel differs from what is expected, move the grating position with the scroll wheel and repeat steps 2.1 and 2.2.
3. Sample fluorescence characterization
- In a spectrofluorometer equipped with a film holder accessory (e.g., Jasco FP-8500 instrument), place the plant section sample (not incubated with the fluorescent probe).
- Measure fluorescence emission typically on the range 300-600 nm while exciting the sample typically on the range 250-550 nm.
- Plot the fluorescence intensity versus excitation and emission wavelengths to draw a 3D fluorescence map.
- Determine the maximum excitation/emission area from the plot.
4. Spectral FRET measurement
- Autofluorescence calibration
- Select the objective 20x (NA: 0.8).
- Set the two-photon laser excitation at 750 nm.
- Place the wheat straw (WS) alone plant section between the slide and the coverslip.
- Apply the following parameters in the software. Switch to lambda mode. Select the spectral detector ChS with a 9.7 nm resolution. Select the spectral range between 420 to 722 nm.
- Acquire the image.
- Determine the donor emission peak (470 nm with this setup).
- Acceptor's calibration
- Place rhodamine-based fluorescent probes in a culture box with a 0.17 µm glass bottom.
- Acquire the image with the same setting.
- Determine the acceptor emission peak (570 nm with this setup).
- Determine the spectral range with the maximum “WS alone” signal and no acceptor signal as the donor only emission for sFLIM measurements (460-490 nm with this setup).
- Sample measurement
- Place the stained plant section between the slide and the coverslip.
- Acquire the image with the same setting.
- Save the lsm file.
- Qualitatively associate a strong FRET event with a decrease in donor emission peak compared to the “WS alone sample” and an increase in the acceptor emission peak compared to the rhodamine sample.
5. sFLIM measurements
NOTE: For the sFLIM setup, the system used is a time domain sFLIM setup as described previously10. An upright microscope and various time correlated single photon counting cards and confocal microscope manufacturers can be used and the protocol should be adapted accordingly.
- Switch the system to sFLIM mode.
- Set the confocal microscope as described in 4.1.1 and 4.1.2.
- Switch to the Non-Descanned mode in the software to send fluorescent photons to the sFLIM detector.
- Set the sFLIM acquisition to the Enable mode to allow photon counting on the SPC150.
- Select 30 s time collection on SPC150.
- Check that the CFD is between 1 x 105 and 1 x 106.
CAUTION: Ensure that the number of photons measured on the TCSPC card is always less than 1% of the laser excitation frequency (in this setup, the laser excitation frequency is 80 Mhz and detection rate cannot exceed 800 kHz in any pixel to avoid a pile up effect11).
- Place the “WS alone” plant section between the slide and the coverslip.
- Set the software to Continuous mode to allow the laser scan.
- Choose the measurement area on the sample.
- Click Start on the SPC150.
- Save the sdt file.
- Repeat the process for at least 10 samples per condition.
- Place the stained plant section between the slide and the coverslip.
- Repeat steps 5.2.1 – 5.2.5.
6. sFLIM analysis
- Select sFLIM data acquired file on “WS alone” and import them to the SPCImage software using File | Import.
- Select fit parameters.
- De Option | Model, select Incomplete multi-exponentials: 12.5 ns.
- De Option | Preferences, select Calculate Instrumental Response automatically.
- From main panel's right menu, select the 2 exponential fit model:
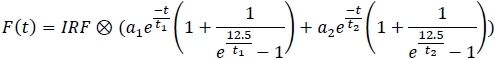
- For each channel, apply the fitting model and save the fit parameters to a spreadsheet (a1, a2, t1, t2).
- For comparison between channels and experiments, calculate the mean fluorescence lifetime in a spreadsheet (Tm):
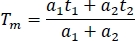
- Calculate the mean lifetime for all samples (at least 10) in all channels.
- Analyze Tm on donor channels (from channel 1 to 3: 460-490 nm) and determine the dedicated channel (Channel 2 corresponding to 467.5 – 480 nm) that shows the highest photon number and corresponds to lignin maximum emission. Values from channel 2 must be used in step 6.7.
- Select sFLIM data acquired file on stained WS and import them to the SPCImage software.
- Repeat steps 6.1-6.4.
- Calculate the FRET efficiency EFRET for the donor in the previous selected channel WS Alone (TmD) and from stained plant sample (TmDA):
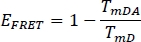
- Compare sFLIM and EFRET values between the WS and the sample.
- Consider a positive EFRET associated with a homogeneous lifetime decrease between WS and the sample to be analyzed as a FRET event (see representative results and works by Spriet et al.12 and Terryn et al.10 for details).
- Consider a lifetime decrease distributed differently to be due to a mix of FRET and lignin compaction level and do not interpret as molecular interactions.
- Consider no lifetime modification as an absence of measurable FRET signal, but not as a lack of interaction with lignin
Representative Results
To demonstrate the sFLIM capability to dissect molecular interactions between lignin and fluorescently-tagged molecules, we first used three different samples (Figure 3): native wheat straw (WS), native wheat straw incubated with PEG tagged with rhodamine (PR10), and native wheat straw with dextran tagged with rhodamine (DR10). PR10 is known to interact with lignin while DR10 is supposed to be inert13,14,15. sFLIM curves (Figure 3, top) show some modifications that can be achieved between the reference sample (WS) and two interaction cases (DR10 and PR10). Indeed, one can first easily notice the fluorescence increase in spectral regions corresponding to the rhodamine emission range. Careful observation of the three first channel photon decay curves also reveals a stronger inflection for DR10 than for PR10. After fitting the photon decay curves and calculating each channel mean fluorescence lifetime, the FRET signature becomes more obvious (Figure 3, bottom). Indeed, while the fluorescence lifetime alternatively increases and decreases along the fluorescence spectrum for the WS sample, a clear FRET signature is observed for both PR10 and DR10 with: 1) a constant lifetime value in the donor-only emission channel (3 first channels, in blue); and 2) an increasing fluorescence lifetime in the spectral channel corresponding to an increasing contribution of the high lifetime donor fluorophore.
Once unambiguous FRET has been determined, comparison of lignin fluorescence lifetime (channel 2) allows quantification of the lifetime decrease between WS (0.47 ns), DR10 (0.42 ns) and PR10 (0.36 ns) and thus, reveals molecular interactions between lignin and both PR10 and DR10 with a stronger affinity for PR10.
To illustrate the relevance of this method to quantify different interaction levels, we choose three other samples, mimicking enzyme accessibility upon treated plant samples: acid-treated WS (AWS), in combination with PR of two contrasted molecular weights of 5 kDa and 20 kDa (PR5 and PR20). After careful inspection of sFLIM signatures, lignin fluorescence lifetime is extracted (Figure 4). As previously stated, lignin fluorescence lifetime can be altered by its environment16,17. After acid treatment, the fluorescence lifetime measures in AWS (0.28 ns) becomes lower than previously measured for WS (0.47 ns), which confirms the requirement of the sFLIM procedure for unambiguous interpretation of lifetime decrease and the need for negative controls for each tested condition. As expected, a strong lifetime decrease is observed when adding PR to the AWS while it interacts with the lignin. Furthermore, the interaction is stronger with PR5 (0.14 ns) compared to PR20 (0.16 ns), which is consistent with their hydrodynamic radius measurement (2.3 nm and 4.8 nm for PR5 and PR20, respectively), inducing different steric constraints and thus higher accessibility of PR5 to lignin.
Both experiments demonstrate the relevancy of this method to finely assess lignin interactions with enzymes, depending on their size and on plant samples pre-treatment.
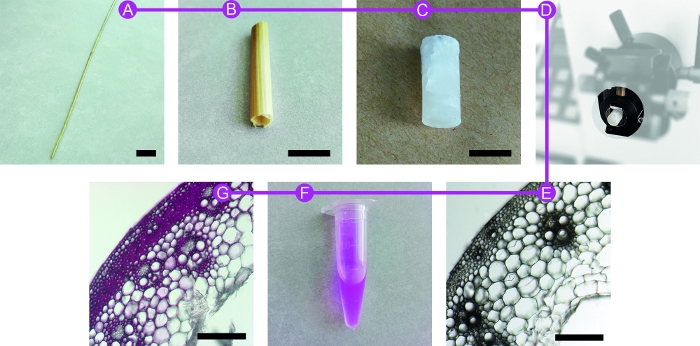
Figure 1: Different preparation steps of the samples. Wheat straw (WS) (A) is first reduced in size (B) to be embedded in PEG medium (C). The block is cut using a microtome equipped with disposable blades (D). After washing, resulting sections (E) are placed for incubation in PEG or dextran tagged-rhodamine solution (F). Labelled sections are mounted for sFLIM measurements (G). Scale bars are 2 cm (A), 1 cm (B and C), 200 µm (E and G). Please click here to view a larger version of this figure.
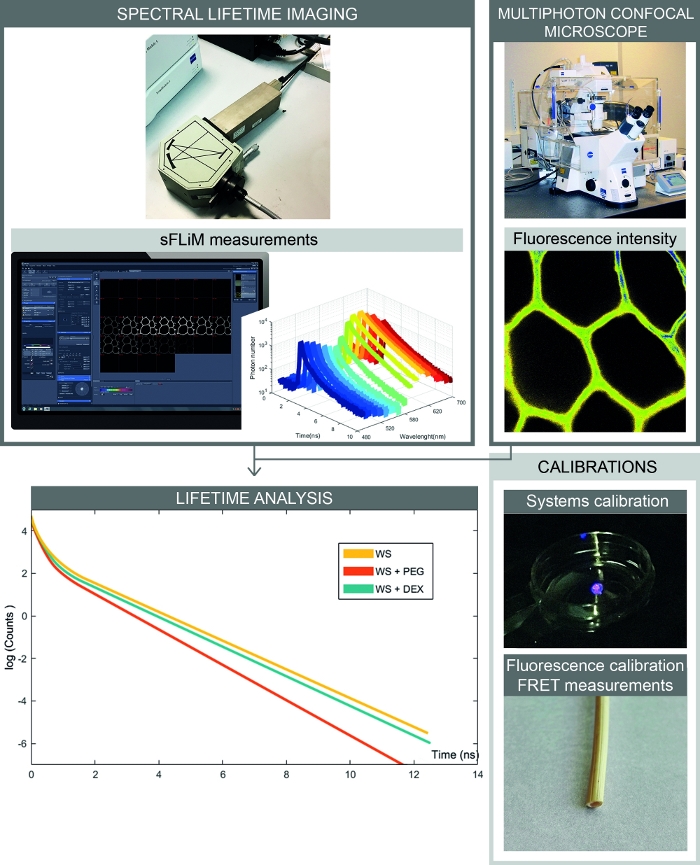
Figure 2: Complete workflow of spectral FRET- based interactions measurements. The figure presents the setup combining spectral fluorescence intensity and lifetime measurements. Spectral fluorescence images are acquired with a confocal microscope, and sequentially fluorescence lifetimes for each spectral range are measured with the sFLIM detector. Analysis of photon decay curves allows accurate determination of interactions between the sample and molecules of interest. Calibrations have to be processed to avoid artefacts. First, the sFLIM detector needs to be spectrally calibrated and its instrumental response function has to be checked. Second, the complex autofluorescence signal has to be precisely calibrated for each sample to determine the fluorescence lifetime in each channel before addition of an acceptor molecule. Please click here to view a larger version of this figure.
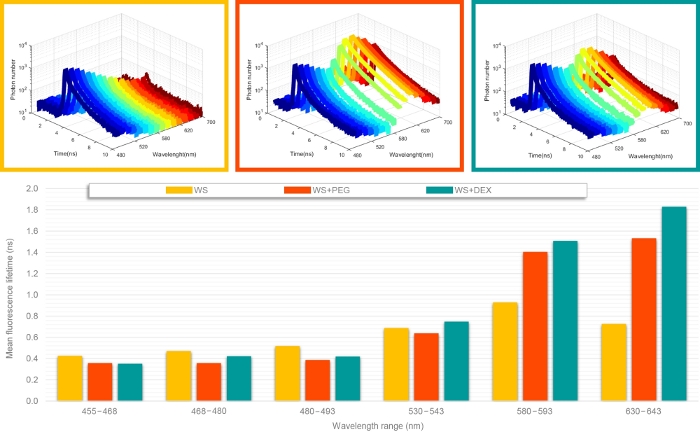
Figure 3: Representative sFLIM measurements. sFLIM curves (top panel) were acquired on native wheat straw (WS), WS incubated with PEG tagged with rhodamine (WS+PEG) and native wheat straw with dextran tagged with rhodamine (WS+DEX). For each sample, sFLIM curves were fitted with a bi-exponential decay model and the mean fluorescence lifetime was calculated for each channel (bottom panel). WS+PEG and WS+DEX samples present a decrease in the fluorescence lifetime in the channel corresponding to autofluorescence only (three first bars) associated with a lifetime increase in channel corresponding to a mixed emission of autofluorescence and rhodamine. This behavior is characteristic of a FRET event between lignin and rhodamine-tagged molecules. Please click here to view a larger version of this figure.
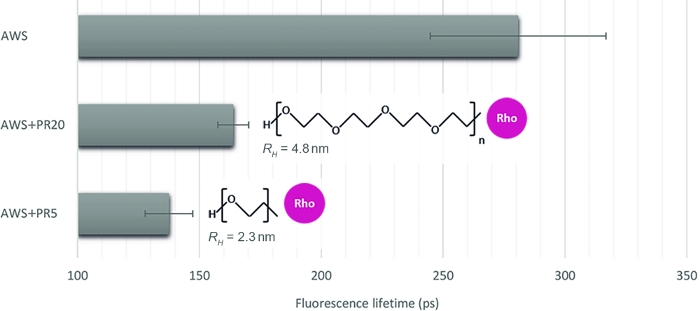
Figure 4: Fluorescence lifetime analysis of the channel corresponding to lignin autofluorescence. After validating a FRET event based on sFLIM signature, mean fluorescence lifetime was measured on acid-treated WS (AWS), in combination with PR5 or PR20 (5 kDa and 20 kDa, respectively). While both PR samples present a lifetime decrease, the smaller is characterized by a stronger lifetime reduction, revealing a stronger molecular interaction with lignin. The method is thus sensitive enough to discriminate between both (mean value and standard error are represented, n>10 per condition). Please click here to view a larger version of this figure.
Discussion
Correlating fluorescence lifetime and emission spectrum measurements allows combining advantages of both methods. Indeed, spectral measurements alone lack sensitivity and remain qualitative. On the other hand, fluorescence lifetime is the method of choice for traditional quantitative FRET measurements, but it was demonstrated that lignin autofluorescence could vary depending on lignin composition and environment16. Thus, lignin interactions with an acceptor or lignin structural changes cannot be discriminated since they both result in a lifetime decrease. As demonstrated, the developed method provides unambiguous, sensitive and quantitative measurement of interactions between lignin and acceptor molecules in plant sections. Even if the different steps of the method were rigorously optimized, caution must be particularly taken for the following points.
Regarding the samples, the quality of the plant section is critical to ensure in-focus imaging. The different steps for PEG embedding should be strictly followed. The final washing step is essential to ensure no interference from PEG remaining in the samples. Furthermore, buffer concentration and pH should be kept strictly identical for all measurements since any change can have a strong impact on fluorescence properties.
Lifetime measurement can also be delicate. Fluorescence lifetime of the donor can be unstable, for instance because of a high excitation. In such a case, tuning the laser power and the zoom can fix the issue. Another well-known source of artefacts in TCSPC measurements is photon pulse pile-up. Indeed, TCSPC cannot measure the speed of two photons emitted between the same two laser pulses. While plant samples are highly structured, the fluorescence is not homogeneous and can lead to a hidden pulse pileup effect. While the number of photons per second remains below the 1% excitation limit, it can be higher in some sample areas. To ensure no pileup experiments, measuring the donor fluorescence lifetime at a different photon flux can be considered. If the lifetime increases while the flux decreases, a pileup effect is altering measurements. Optimal photon flux is reached with donor's lifetime stability.
Regarding sFLIM decay curve analysis, we recommend relying on each individual curve fit to ensure that the model accurately describes measurements. If it is not the case, first ensure that none of the previously mentioned artefacts have corrupted the data. Then, step 2.1.4 provides the instrumental response function (IRF) of the system. If IRF presents some anomalies, optimize the optical path to minimize them. A measured IRF for fitting during step 6.2 can also be used. Finally, the fitting model's degrees of freedom can be increased to a three-exponential decay in step 6.2. However, the number of photons required for individual lifetime and proportion determination is high and it is thus recommended only using them to achieve a more stable mean lifetime from step 6.4. More exhaustive information on FLIM, its characterization18, sFLIM and its application12 notably to lignin10, can be found in the literature.
Although challenging, FRET measurement in plant tissues using native autofluorescence shows some advantages. In comparison to FRET measurements performed on living tissues in which interaction studies between biomolecules often requires genetic engineering to express intrinsic fluorescent markers, lignified plant tissues as those presented here, offer natural autofluorescence, and extrinsic partner fluorescent probes can be easily added, saving much time. However, depending on the analyzed plant species and on possible pre-treatments carried out, autofluorescence is likely to be modified, so that it needs to be carefully characterized and fluorophore used as probes might need to be adapted.
The sFLIM method must be applied to fluorescent probes lacking catalytic activity (PEG and dextran). In the frame of plant lignocellulose hydrolysis, interactions of enzymes in various biomass samples could be performed, possibly resulting in the determination of the impact of localization (cells and tissues) on the interaction strength. The next optimization step will be automation of the analysis step. Indeed, with enough analyzed data, a machine learning-based analysis procedure could be deployed for automated phenotype analysis, which would increase its amenability to fast enzyme-biomass efficiency screening. Furthermore, sFLIM does not require fixation of the sample and can be easily applied to dynamic enzyme-lignin interaction studies. Such a unique method is likely to guide enzyme engineering strategy and biomass pre-treatment to optimize lignocellulose valorization.
Declarações
The authors have nothing to disclose.
Acknowledgements
Fanny Laurent (FARE) is warmly thanked for the preparation of the figures. The Research Federation FRABio (University of Lille, CNRS) is acknowledged for providing the technical environment conducive to achieving this work. Funding was obtained from the French National Research Agency (LIGNOPROG project ANR-14-CE05-0026).
Materials
| Confocal microscope | ZEISS | LSM 710 NLO | for confocal imaging |
| fine brush | to collect sections | ||
| glass vials | for incubations | ||
| high precision microscope cover glasses 170+/-5µm n°1,5# | Marienfeld | 0107032 | saled by Dutscher s.a in France |
| Infra-red pulsed laser | COHERENT | CHAMELEON VISION | For sample excitation |
| Micropipette Research Plus monocanal 20-200µL | Eppendorf | with corresponding tips | |
| Micropipette Research Plus monocanal 2-20µL | Eppendorf | with corresponding tips | |
| Microscope slides ca. 76 x 26 mm ground edges frosted end | Thermo Fisher Scientific | LR45D | |
| microtome blades disposable (pack of 50) | Agar Scientific | T5024 | saled by Oxford Instruments in France |
| mPEG-Rhodamine, 10 kDa | Creative Peg Works | PSB-2263 | |
| mPEG-Rhodamine, 20 kDa | Creative Peg Works | PSB-22642 | |
| mPEG-Rhodamine, 5 kDa | Creative Peg Works | PSB-2264 | |
| nail polish | for mounting | ||
| pH meter five easy plus | Mettler toledo | FEP20 | with electrode |
| poly (ethylene Glycol) average mol wt 1450 | Merck (sigma-Aldrich) | P5402-1kg | for sample embedding |
| razor blades, single edges blades, stainless steel, box of 100 | Agar Scientific | T586 | for fragments preparation saled by Oxford Instruments in France |
| rotary microtome | Microm Microtech | HM 360 | |
| sFLim detector | BECKER-HICKL | SPC 150 | for lifetime acquisition |
| sodium dihydrogen phosphate dihydrate NaH2PO4, 2H2O | Merck (sigma-Aldrich) | 71500 | for buffer solution |
| sodium phosphate dibasic dihydrate Na2HPO4,2H2O | Merck (sigma-Aldrich) | 30435-1kg | for buffer solution, old reference, the new one is 71643-1kg |
| tetramethyl rhodamine isothiocyanate-dextran 10 k Da | Merck (sigma-Aldrich) | R8881-100mg |
Referências
- Inouye, H., et al. Multiscale deconstruction of molecular architecture in corn stover. Scientific Reports. 4, 3756 (2014).
- Li, X., Zheng, Y. Lignin-enzyme interaction: Mechanism, mitigation approach, modeling, and research prospects. Biotechnology Advances. 35 (4), 466-489 (2017).
- Vogel, J. Unique aspects of the grass cell wall. Current Opinion in Plant Biology. 11 (3), 301-307 (2008).
- Lynd, L. R. The grand challenge of cellulosic biofuels. Nature Biotechnology. 35, 912 (2017).
- Clegg, R. M. Fluorescence resonance energy transfer. Current Opinion in Biotechnology. 6, 103-110 (1995).
- Ishikawa-Ankerhold, H. C., Ankerhold, R., Drummen, G. P. C. Advanced fluorescence microscopy techniques-FRAP, FLIP, FLAP, FRET and FLIM. Molecules. 17 (4), 4047-4132 (2012).
- Pietraszewska-Bogiel, A., Gadella, T. W. J. FRET microscopy: from principle to routine technology in cell biology. Journal of Microscopy. 241 (2), 111-118 (2011).
- Herbaut, M., et al. Multimodal analysis of pretreated biomass species highlights generic markers of lignocellulose recalcitrance. Biotechnology for Biofuels. 11, 52 (2018).
- Chabbert, B., et al. Fluorescence techniques can reveal cell wall organization and predict saccharification in pretreated wood biomass. Industrial Crops and Products. 123, 84-92 (2018).
- Terryn, C., Paës, G., Spriet, C. FRET-SLiM on native autofluorescence: a fast and reliable method to study interactions between fluorescent probes and lignin in plant cell wall. Plant Methods. 14 (1), 74 (2018).
- Jovin, T. M., Arndt-Jovin, D. J., Kohen, E. . Cell Structure and Function by Microspectrofluorometry. , 99-117 (1989).
- Spriet, C., et al. Correlated fluorescence lifetime and spectral measurements in living cells. Microscopy Research and Technique. 70 (2), 85-94 (2007).
- Donaldson, L. A., Newman, R. H., Vaidya, A. Nanoscale interactions of polyethylene glycol with thermo-mechanically pre-treated Pinus radiata biofuel substrate. Biotechnology and Bioengineering. 111 (4), 719-725 (2014).
- Herbaut, M., Zoghlami, A., Paës, G. Dynamical assessment of fluorescent probes mobility in poplar cell walls reveals nanopores govern saccharification. Biotechnology for Biofuels. 11 (1), 271 (2018).
- Paës, G., Chabbert, B. Characterization of arabinoxylan / cellulose nanocrystals gels to investigate fluorescent probes mobility in bio-inspired models of plant secondary cell wall. Biomacromolecules. 13, 206-214 (2012).
- Donaldson, L. A., Radotic, K. Fluorescence lifetime imaging of lignin autofluorescence in normal and compression wood. Journal of Microscopy. 251 (2), 178-187 (2013).
- Auxenfans, T., Terryn, C., Paës, G. Seeing biomass recalcitrance through fluorescence. Scientific Reports. 7, 8838 (2017).
- Waharte, F., Spriet, C., Héliot, L. Setup and characterization of a multiphoton FLIM instrument for protein-protein interaction measurements in living cells. Cytometry A. 4, 299-306 (2016).

