Increasing Durability of Dissociated Neural Cell Cultures Using Biologically Active Coralline Matrix
Summary
Dissociated hippocampal cell culture is a pivotal experimental tool in neuroscience. Neural cell survival and function in culture is enhanced when coralline skeletons are used as matrices, due to their neuroprotective and neuromodulative roles. Hence, neural cells grown on coralline matrix show higher durability, and thereby are more adequate for culturing.
Abstract
Cultures of dissociated hippocampal neuronal and glial cells are a valuable experimental model for studying neural growth and function by providing high cell isolation and a controlled environment. However, the survival of hippocampal cells in vitro is compromised: most cells die during the first week of culture. It is therefore of great importance to identify ways to increase the durability of neural cells in culture.
Calcium carbonate in the form of crystalline aragonite derived from the skeleton of corals can be used as a superior, active matrix for neural cultures. By nurturing, protecting, and activating glial cells, the coral skeleton enhances the survival and growth of these cells in vitro better than other matrices.
This protocol describes a method for cultivating hippocampal cells on a coralline matrix. This matrix is generated by attaching grains of coral skeletons to culture dishes, flasks, and glass coverslips. The grains assist in improving the environment of the cells by introducing them to a fine three-dimensional (3D) environment to grow on and to form tissue-like structures. The 3D environment introduced by the coral skeleton can be optimized for the cells by grinding, which enables control over the size and density of the grains (i.e., the matrix roughness), a property that has been found to influence glial cells activity. Moreover, the use of grains makes the observation and analysis of the cultures easier, especially when using light microscopy. Hence, the protocol includes procedures for generation and optimization of the coralline matrix as a tool to improve the maintenance and functionality of neural cells in vitro.
Introduction
Cultures of dissociated neural cells, in this case hippocampal cells, are a valuable experimental model for studying neural growth and function by providing high cell isolation and accessibility1,2,3. This type of culture is frequently used in neuroscience, drug development, and tissue engineering due to the large amount of information that can be collected, such as rates of growth and viability, neurotoxicity, neurite outgrowth and networking, synaptic connectivity and plasticity, morphological modifications, neurites organization and wiring, etc.1,4,5,6,7.
Despite the significance of the cultures, the cultivated cells are usually forced to grow on glass coverslips in a two-dimensional monolayer. These strict environmental modifications significantly decrease the ability of neural cells to survive over time, because glass coverslips are non-nurturing substrates with a low adhesion strength, exhibiting a lower capacity to support cell growth8,9,10,11.
Because cultivated neural cells are forced to grow in challenging conditions, an essential approach to enhance their survival would be to imitate their natural environment as much as possible12,13. This could be achieved by using biomaterials that will act as matrices and mimic the extracellular matrix of the cells, enabling them to form a tissue-like structure and assist in their nourishment14.
The use of biomaterials is a promising approach in improving cell cultures, because they act as biocompatible scaffolds, providing mechanical stability and enhancing a variety of cell properties, including adhesion, survival, proliferation, migration, morphogenesis, and differentiation15,16,17. Several types of biomaterials are used to improve the conditions of the cells in vitro. Among them are biopolymers, or biological components that are usually part of the extracellular matrix of the cells. These biomaterials are mostly used as a form of polymerized coating agents or hydrogels18,19,20. On the one hand, the matrices mentioned above give the cells a familiar 3D environment to grow in, encourage their adhesion to the dish, and give them mechanical support21,22. On the other hand, their polymerized form and the confinement of the cells within hydrogels disturbs the access of the cells to nurturing components present in the growth media and also makes the follow up of the cells by microscopic methods more difficult23.
Coral exoskeletons are biological marine-originated matrices. They are made of calcium carbonate, have mechanical stability, and are biodegradable. Previous studies using the coral skeleton as a matrix for growing neural cells in culture have shown much greater adhesion, compared to glass coverslips24,25. In addition, neural cells grown on coral skeleton demonstrated their capability to intake the calcium the skeleton is composed of, which protects the neural cells in conditions of nutrient deprivation26. Moreover, the coral skeleton is a supportive and nurturing matrix that increases the survival of neural cells, encourages the formation of neural networks, elevates the rate of synaptic connectivity, and enables the formation of tissue-like structures27,28. Recent studies have also shown that the surface topography of the coral skeleton matrix plays a crucial role in the distribution and activation of glial cells8,29. Also, coral skeleton is effective as a matrix for cultivation of other cell types, such as osteocytes30,31, hepatocytes, and cardiomyocytes in culture (unpublished data).
Hence, coral skeleton is a promising matrix for cultivation of cells in vitro. Thus, the protocol detailed below describes the technique of cultivating neural cells on coral skeleton for producing more stable and prosperous neural cultures than those achieved by existing methods. This protocol may also be useful for cultivation of cardiomyocytes, hepatocytes, and other cell types.
Protocol
The use of animals in this protocol was approved by the National Animal Care and Use Committee.
NOTE: Calcium carbonated coral skeletons should be used in the crystalline form of aragonite. The coral types tested so far for neural cultures are Porites Lutea, Stylophora Pistillata, and Trachyphyllia Geoffroyi. The skeletons can be purchased whole or ground.
1. Cleaning the coral skeleton pieces
CAUTION: The following steps should be performed in a chemical hood at room temperature, because the solutions described below are hazardous and may cause burns and irritations.
- Use a hammer to break the coral skeleton and divide it into 0.5–2 cm fragments. In order to dissolve organic and non-organic residues, soak the coral skeleton fragments in 10% sodium hypochlorite solution for 10 min, then wash once with double distilled water (DDW).
- To remove the remaining organic residues, soak the fragments in a 1M NaOH solution for 5 min, then wash once with DDW. Continue with the removal of the organic deposits by soaking the fragments in 30% H2O2 solution for 10 min, then wash the fragments 3x with DDW.
- Remove as much excess DDW as possible and leave the coral fragments in the hood to dry (1–8 h).
2. Cleaning the glass coverslips
- Transfer the glass coverslips into a 100 mm glass Petri dish. Add 10 mL of 95% ethanol for 15 min.
- Remove the ethanol and wash with DDW 3x, waiting 10 min between each wash. Place the dish on an 80 °C pre-warmed heating plate until the DDW evaporates. Gently stir the coverslips within the plate several times while drying to keep them from sticking to each other.
- Autoclave the coverslips.
3. Preparation of coral skeleton grains
- Grind the coral skeleton fragments using a mortar and pestle (manual grinding) until complete breakdown. The outcome is a mixture of grains with sizes ranging from 20 µm–200 µm.
- Alternatively, grind the coral skeleton fragments using an electrical grinding machine at a velocity of 1,000 rpm for 30 s (blade length = 6 cm; width = 0.5 cm–1.0 cm). The resulting grain size is similar to the size range produced by the manual grinding.
4. Purification of grains of a specific size range
NOTE: If control over the size of the grains in a matrix is desired, use the following filtration-based grain purification procedure.
- Transfer the grains onto a manual or electrical 40 µm filter mesh strainer.
- Divide the grains into two specific ranges by sieving the grains through the strainer (if using an electrical strainer, the following conditions are recommended: shaking = 600 amplitudes/min, bouncing = 6/s). This procedure produces two groups of grain sizes, one <40 µm, and the second >40 µm.
NOTE: The two sizes can be determined by using strainers of varying meshes. If a more restricted range is desired, then re-sieve each group from step 4.2 through strainers with different meshes. - Autoclave the grains.
5. Preparation of coral grain-coated dishes or coverslips
- For coating flasks, plates, or Petri dishes
NOTE: The following steps should be performed under sterile conditions.- Add the coral skeleton grains into a 20 µg/mL poly-D-lysine (PDL) solution dissolved in Hanks' solution. The concentration recommended is 5 mg/10 mg of grains per 1 mL PDL solution.
- Pour the solution into flasks and dishes (approximately 2 mL/25 cm2) and incubate overnight at 4 °C. The grains sink and attach to the bottom.
- The next day, wash the flasks and dishes once with sterile DDW. Let the flasks and dishes dry in the hood.
NOTE: It is preferable to use freshly coated flasks and dishes. The coated flasks and dishes can be used up to a week after coating if preserved at 4 °C. However, the effectiveness of the matrix may be compromised.
- Coating glass coverslips (12 mm diameter, 0.17 mm thickness)
- Add coral skeleton grains to DDW at any desired concentration. The common densities used for neural cells are 5 mg/mL and 10 mg/mL. Pour 40 µL of the grain solution onto the center of the coverslip (Figure 4).
- Place the coverslips on a heating plate prewarmed to 80 °C and wait for complete evaporation (usually 15 min). Under these conditions, the grains adhere to the coverslip. Autoclave the coated coverslips. Store in sterile conditions.
- A day before culture, place the coverslips on the lid of a sterile 24 well plate. Add 100 µL of 20 µg/mL PDL solution to each coverslip. Use the tip to ensure that the liquid covers the entire grain region and the rest of the coverslip surface.
- Cover the lid with the bottom of the plate. Wrap the sides of the plates with paraffin film. Incubate overnight at 4 °C.
- The next day, wash once with DDW and dry in the hood.
NOTE: It is preferable to use freshly coated coverslips.
6. Cultivation of hippocampal dissociated cells on coral skeleton grain-coated glass coverslips
NOTE: The method for hippocampal dissociated cell culture was modified from previously published procedures24,27. The preparation of the culture is described for four rat pups. The expected yield from each hippocampus is 1–1.5 x 106 cells.
- Setup
- Prepare an empty 60 mm Petri dish. Pour 2 mL of minimal essential medium (MEM) into a 60 mm Petri dish and put on ice.
- Pour 2 mL of MEM into a 35 mm dish and put it in an incubator at 37 °C, 5–10% CO2. Pour 2 mL of MEM into a 15 mL tube and keep it at 4 °C.
- Pour 1 mL of First Day Medium into a 15 mL tube and put it in an incubator at 37 °C, 5-10% CO2.
NOTE: The composition of the First Day Medium is described in the Table of Materials. - Thaw 200 µL of a 2.5% trypsin solution and incubate at 37 °C.
- Prepare three glass Pasteur pipettes with 0.5 mm, 0.75 mm, and 1.0 mm diameter heads using a flame.
- Prepare adequate surgical tools: one big scissor, two small scissors, one big tweezer, four small tweezers, and one scalpel.
- Prepare a stereomicroscope in the hood.
- Using a big scissor, sacrifice 0–3 day old Sprague Dawley rat pups by separating their heads from their bodies into an empty 60 mm Petri dish (step 6.1.1). Dispose of the bodies.
- Pick up the head by holding it from the mouth with a big tweezer. Cut the skin covering the skull with a small scissor.
- With a clean scissor, enter beneath the skull (on the side of the cerebellum) and cut the skull to the right and to the left to enable its removal. Using a small tweezer, peel the skull off from the brain.
- With a clean tweezer, separate the brain from the head and put it in a 60 mm Petri dish containing 2 mL of cold MEM (see step 6.1.2).
- Using two small tweezers, dissect the hippocampi under a stereomicroscope. Transfer the hippocampi into the prepared 35 mm dish (from step 6.1.2) containing MEM.
NOTE: Steps 6.3–6.6 should be repeated for each pup. - Cut the hippocampi to approximately 1 mm slices using the scalpel. Add 200 µL of the trypsin solution (diluted to a final concentration of 0.25%). Mix gently and incubate at 37 °C, 5–10% CO2 for 30 min.
- After incubation, add 2 mL of cold MEM (step 6.1.2) to the dish to deactivate the trypsin. Transfer the trypsinized tissue to a 15 mL tube containing 1 mL First Day Medium preheated to 37 °C (step 6.1.3) using the glass Pasteur pipette with the largest diameter (step 6.1.5).
NOTE: The best medium to tissue (v:v) ratio for further processing of the tissue is 1 mL/8 hippocampi. Avoid transferring the medium with the tissue as much as possible. - Triturate the tissue by passing it through the largest diameter glass pipette 10–15 times. Then, repeat this process using the glass pipette with the medium diameter. Continue triturating with the smallest diameter pipette. Avoid bubbling to reduce cell death.
- Let the remaining tissue pieces sink for 2–5 min, then transfer the supernatant into another 15 mL tube. Count the cells using a hemocytometer.
NOTE: The preferable cell density is 200,000–400,000 cells/mL. Use First Day Medium to dilute or centrifugation at 470 x g to concentrate the cells. - Seed 100 µL of cells on each glass coverslip. While seeding, make sure to cover the entire coverslip with cells. Incubate at 37 °C, 5–10% CO2.
- The next day, add 500 µL of Neuronal Growth Medium to the wells.
NOTE: The composition of the Neuronal Growth Medium is described in the Table of Materials. - Gently transfer each coverslip to its appropriate well using a tweezer while removing the First Day Medium by tilting the coverslip. Suction the remaining media from the lid.
- Incubate at 37 °C, 5–10% CO2. Avoid replacing the medium throughout incubation. Cultures can be maintained under these conditions up to 1 month. The major concern is humidity. Therefore, make sure to maintain the incubator maximally humidified.
Representative Results
In order to prepare the coral skeleton matrix, the entire coral skeleton (Figure 1A) was broken into 0.5–2 cm fragments using a hammer (Figure 1B) and thoroughly cleaned from organic residues through three steps (step 1 in the protocol) using 10% hypochlorite solution, 1M NaOH solution, and 30% H2O2 solution (Figure 1C). Coral fragments were well-cleaned when the skeleton color changed from brown (Figure 1D) to white (Figure 1E). Then, the cleaned pieces (Figure 2A) were ground using either a mortar and pestle (Figure 2B) or an electrical grinding machine (Figure 2C). Both procedures yielded similar outcomes: a mixture of grains ranging between 20 µm–200 µm in size (Figure 2D-G).
One of the advantages of grained matrix over soluble substrates is that several of its structural and physical properties can be modified, making it more quantitative. In this coral skeleton grain matrix, two of the grain mixture properties were manipulated: grain size and grain density. To reduce the 20 µm–200 µm size diversity generated by the above grinding procedure, a sieving approach using a filter mesh of 40 µm was chosen. Using manual (Figure 3A–D) or electrical (Figure 3E) strainers, two types of grain mixtures were produced: one with grains ranging from 40 µm–200 µm in size (Figure 3F,H) and the second with size of 40 µm or less (Figure 3G,I). The density of the grains that coated the coverslips and dishes was determined simply by applying varying amounts of grains and attaching them to the coverslips by heat (Figure 4C) or to flasks and dishes by gravity (Figure 4H). In this way, matrices of low (Figure 4D,F,I) and high densities (Figure 4E,G,J) were produced.
Figure 5 demonstrates the outcome of cultivation of hippocampal cells on coverslips coated with <40 µm (10 mg/mL) coral skeleton grains. Phase contrast micrographs show that the cells formed complex neural networks in the absence (Figure 5A) and presence (Figure 5B) of the matrix. Cell nuclei staining (Figure 5C,D) indicated that 14 days after seeding, cell density was higher on the matrix than on the uncoated coverslips. Staining with microtubule associated protein 2 (MAP2) showed that a complex neuronal and dendritic network formed on the matrix (Figure 5F) compared to the control (Figure 5E). In addition, glial cells grown on the coral skeleton matrix visualized using the glial fibrillary acidic protein (GFAP) underwent significant morphological changes. They acquired a spikier appearance with longer processes than the glial cells grown on the control substrate, where they appeared rounder and flatter (Figure 5G,H).
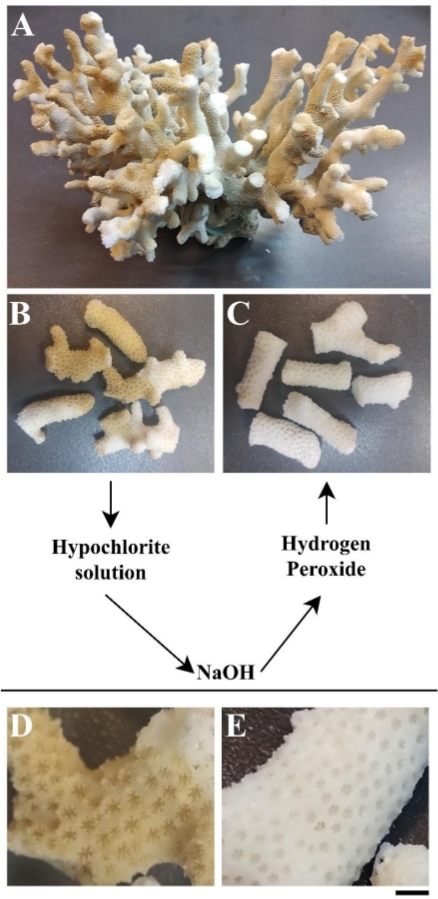
Figure 1: Cleaning the coral skeleton. (A) The skeleton of the coral Stylophora pistillata. (B) Broken down fragments of the skeleton prior to cleaning. (C) The fragments from (B) after treatment with hypochlorite solution, sodium hydroxide, and hydrogen peroxide. (D) Enlargement of uncleaned fragment shown in (B). (E) Enlargement of cleaned fragment shown in (C). Scale: A = 20 mm; B,C = 10 mm; D,E = 3 mm. Please click here to view a larger version of this figure.
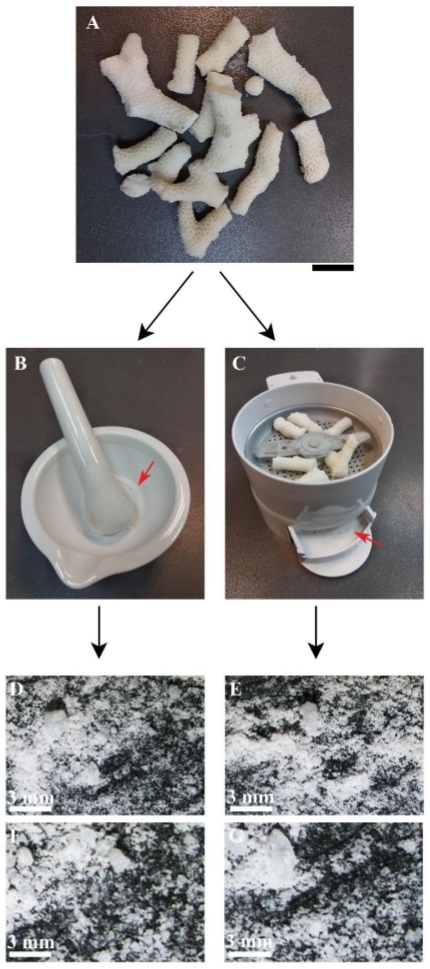
Figure 2: Grinding the coral skeleton. (A) Cleaned coral skeleton fragments. (B) Mortar and pestle used for manual grinding. Red arrow points to the resulting grains. (C) Grinding machine. Red arrow points to the resulting grains. (D) Grains after manual grinding. (E) Grains after mechanical grinding. (F) Enlargement of (D). (G) Enlargement of (E). Scale: A = 15 mm; D,E = 3 mm; F,G = 500 µm. Please click here to view a larger version of this figure.
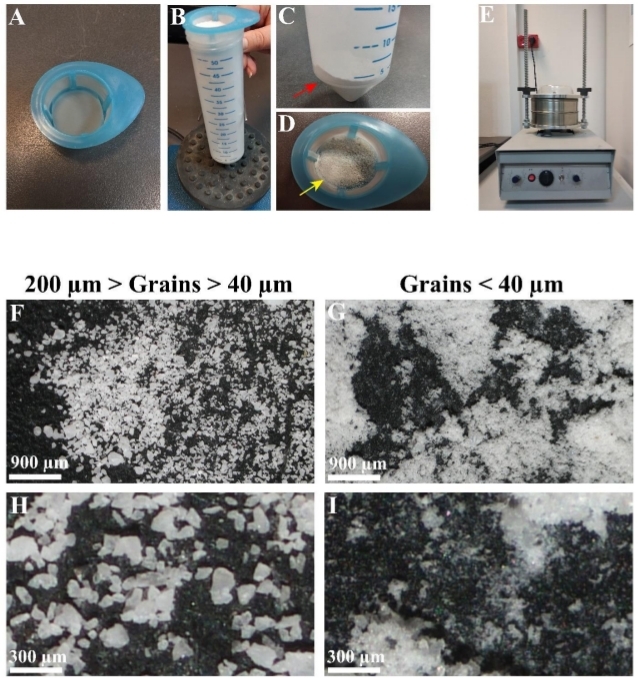
Figure 3: Control over the grain size. (A-D) Manual straining procedure. (A) Using 40 µm strainer. (B) Strainer with unstrained ground grains placed in a 50 mL tube and strained using a vortex. (C) Strained <40 µm grains at the bottom of the 50 mL tube. (D) Grains with a minimal size similar to that of the mesh (>40 µm, yellow arrow). (E) Mechanical strainer used to strain <40 µm grains from a mixture of grains 20 µm–200 µm in size. (F) Demonstration of diverse grain sizes after grinding prior to straining. (G) Demonstration of strained grains <40 µm. (H) Enlargement of (F). (I) Enlargement of (G). Scale: F,G = 900 µm; H,I = 300 µm. Please click here to view a larger version of this figure.
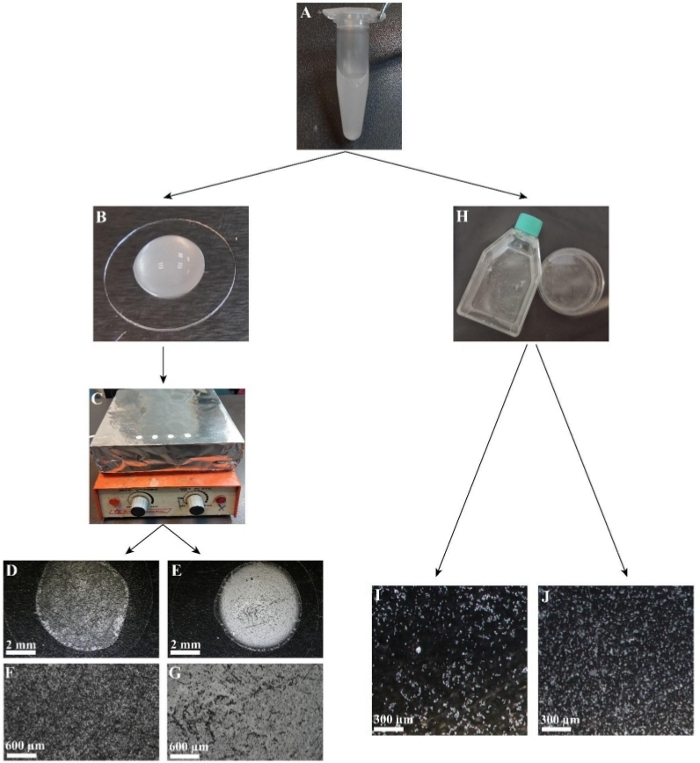
Figure 4: Controlling the density of the matrix. (A) A 1.5 mL centrifuge tube with <40 µm grains diluted to 10 mg/mL with DDW for coverslips, PDL for other dishes. (B) Glass coverslip with 40 µL of 10 mg/mL solution. (C) Coverslips with 40 µL of 10 mg/mL solution drying on a hot plate prewarmed to 80 °C, enabling the grains to adhere to the coverslip. (D) Coated coverslip with a grain concentration of 5 mg/mL after DDW evaporation. (E) Coated coverslip with a grain concentration of 10 mg/mL after DDW evaporation. (F) Enlargement of (D). (G) Enlargement of (E). (H) A 10 mg/mL solution in a T-25 flask, 60 mm plate. (I) Coated flask with grains at a concentration of 5 mg/mL. (J) Coated flask with grains at a concentration of 10 mg/mL. Scale: B,D,E,I,J = 3 mm; F,G = 600 µm. Please click here to view a larger version of this figure.
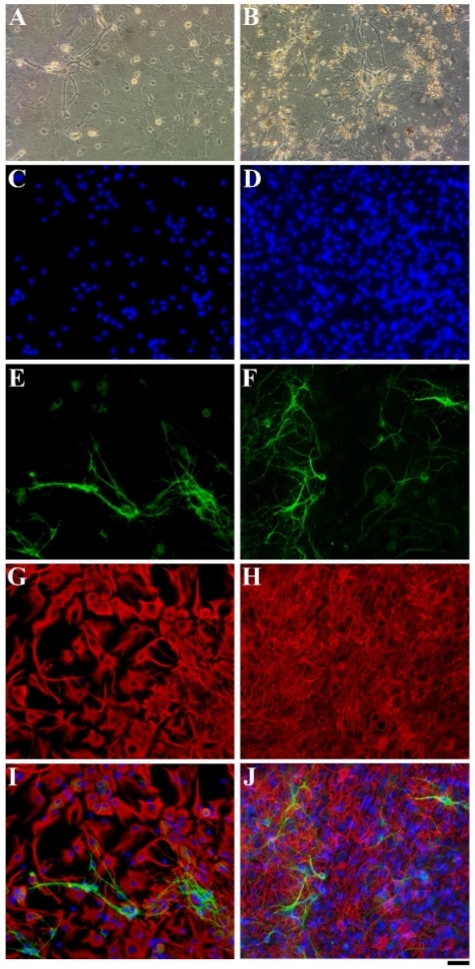
Figure 5: Cultivation of hippocampal neural cells on the coral skeleton matrix. Images show cells extracted from 0–3 days old rat pup hippocampi grown in culture for 14 days and stained with GFAP (red, glial cells), MAP2 (green, neuronal somas and dendrites), DAPI (blue, cell nuclei). (A) Phase contrast image of cells in the absence of the matrix. (B) Phase contrast image of cells grown on the coral skeleton matrix. (C) Cells grown with no matrix stained with DAPI. (D) Cells grown on the coral skeleton matrix stained with DAPI. (E) Cells grown in the absence of the matrix stained for MAP2. (F) Cells grown on the coral skeleton matrix stained for MAP2. (G) Cells grown with no matrix stained for GFAP. (H) Cells grown on the coral skeleton matrix stained for GFAP. (I) Merged image of (C),(E),(G). (J) Merged image of (D),(F),(H). Scale = 40 µm. Please click here to view a larger version of this figure.
Discussion
The technique presented here describes a way to improve the maintenance and functionality of neural cells in culture. This is achieved by adhering the cells to a matrix made of coral skeleton grains that nurtures the cells and promotes their growth and activity. Using this technique increases the capacity of the neural culture model to mimic the cells' environment in the brain.
The introduction of the matrix as a culture substrate has several advantages over other substrates used in classical neural cell culture methods. First, it increases cell adherence. The combination of coral skeleton and PDL generates a stronger adhesiveness than glass and PDL (not shown). Such an increase in adhesiveness has been shown to be essential for the survival of adherent non-floating cells in culture32. Second, the calcium carbonated crystals of the coral skeleton grains are a source of calcium ions that are actually absorbed by the cells. It is highly likely that this is the reason for enhanced durability and increased density (Figure 5C,D) of neural cells exhibited on the coral skeleton matrix, compared to their survival level in its absence26.
A third advantage lies in the fact that the coral skeleton matrix is actually non-planar, unlike the glass coverslip. The grains are 3D-structured with a complex, curved surface architecture. These structural properties vary when considering that the cells face grain populations of varying morphologies, dimensions, and densities. Such a rough and non-planar culture substance better mimics the milieu of the cells in vivo than the classical flat coverslip. Indeed, we have shown that the roughness, size, and morphology of the coral skeleton affects cell distribution, survival, and activity8,26,29.
Moreover, the 3D configuration that a coral skeleton matrix provides the cells with as a microenvironment differs majorly from the conventional hydrogel matrices used for 3D growth in vitro. When using hydrogels, such as collagen and hyaluronic acid, the cells are embedded within the gel23. The gel resembles the extracellular matrix in terms of space filling, rigidity, and other attributes, but due to the viscosity of the gels, these cultures demand complicated feeding systems in order to keep the cells well-nourished21,22. By contrast, the coral skeleton 3D grains enable the cells to occupy their surface, which is completely open to the culture medium. Furthermore, in contrast to the hydrogel cultures, the cells on the coral skeleton matrix are readily monitored through phase and fluorescent microscopy.
This technique has several limitations. Despite the advantages listed above, coral skeleton is a biomaterial and cannot be fabricated but collected from dead corals, causing limitations in matrix supply, although some are available commercially. Also, the use of skeletons of multiple coral species may introduce variations in culture properties. On the other hand, the fact that coral skeleton crystals are all made of one substance, aragonite, and that the size of the grains can be controlled, eliminates most of the chemical and structural diversity among the matrices. The grains of all biomaterials tested are large enough to force the cells to grow three-dimensionally. The cells climb on the grains8, grow by attaching to the tips of the crystals33 and cross between grains27. Alternatively, geological aragonite, made of fossil sediments and synthetic calcium carbonate, is commercially available and can be used as a matrix. However, it may show lower adherence to the glass coverslips.
In view of using multiple coral species, it is important to note that some studies have shown evidence of biomineralization of corals, which may lead to the presence of diverse organic deposits among the calcium carbonate crystals. These cannot be removed using the cleaning process described in this protocol33,34. This fact is of crucial importance while identifying a biomaterial for biological applications. In the case of this study, several steps were previously performed in order to ensure as much homogeneity of the scaffold as possible. First, three types of corals were tested for matrices: Porites Lutea, Stylophora Pistillata, and Trachyphyllia Geoffroyi. There were no significant changes in terms of growth and survival of neural cells on these coral skeletons. This result may indicate that different organic deposits within the skeleton are not of a significant influence in this case. Second, Fourier-transform infrared spectroscopy of clean coral skeleton showed the absence of organic residues29.
There are some critical steps within the protocol that should be taken into consideration. First, while manually sieving the grains, it is recommended to replace the strainer from time to time, as the strainers tend to clog. The electrical strainer uses greater force while sieving. Thus, it does not tend to clog as easily. Second, while dispersing the PDL-coral grains mixture on the dishes and coverslips, it is important to frequently pipette the mixture to avoid the sinking of the grains. That will ensure a homogenous dispersion of the solution. It is important to pay attention to the attachment process of the grains to the coverslips. Incorrect heating temperatures during grain attachment to the glass coverslip, vibrations, and other factors may cause the grains to detach before or after cell seeding. It is recommended to solve this problem by carefully controlling the temperature and minimizing vibrations. Furthermore, it is preferable to perform the experiments at a set time after coating the flasks and dishes. We recommend freshly coating the dishes a day before culture. Prolonged incubation of dishes with the PDL-grain mixture can result in diversity in the amount of the attached grains to the dish.
Some modifications of the technique described above are possible according to the needs of the cultured cells. In addition to its capacity to ameliorate the growth of neural cells in culture, coral skeleton matrices support growth of osteocytes30,31, hepatocytes, and cardiomyocytes (unpublished data). To increase culture durability of other cell types, it is recommended to check for the strongest cell reaction to skeletons of different coral types, as well as optimizing the size and density of the grains.
It is also possible to grow neural cells directly on the grains, without additional coating. However, the precoating of the grains with PDL, as mentioned in this protocol (step 5) highly improves the adhesion and survival of the neural cells, above the levels obtained with PDL-coated glass. It is also possible to use other coating materials according to the preference of different cell types.
It is worth mentioning that the size of the grains in the matrix is limited. Above a certain threshold, about 200 µm, it becomes difficult to bind them to the glass coverslips. Therefore, the roughness of the matrix surface has a finite range. Experiments requiring larger grains or pieces of skeleton are not suitable for this technique. One may find an alternative way to attach larger grains, perhaps using glues or gels, but this may obscure the cell contact with the grains. Another possibility is to cultivate the cells directly on the skeleton, without grinding. This method requires a modification of the cell seeding procedure24,27. The 3D structure of the non-ground coral skeleton does contribute to neural cell survival, as we reported before26. However, such a complex and dense matrix makes microscopy work difficult. The compromised setup of the ground skeleton preserves the chemical properties of the skeleton, enables 2D microscopy analysis, and because of the large size of the grains, forces the cells to grow in 3D8.
Further, an excess of coral skeleton grains may be toxic. As mentioned above, the calcium component of the coral skeleton matrix is absorbed by the cells35. Calcium excess caused by high grain density may be a burden or even inhibitory to some cell types, as in the case of neural cells36. Therefore, a reduction of grain density may be required and optimized for each cell type. Additionally, it is important to remember that coral skeleton availability may be limited. Corals grow slowly in aquariums and at their natural environment. Their growth is further stunted as a result of the global warming and ocean acidification37. Thus, the abundancy and availability of coral skeletons are limited.
The strengthening of the dissociated neural cells when in contact with coral skeleton grains in culture opens new avenues for research in neurobiology. Cellular growth, neuritic extension and ramification, as well as synaptogenesis and synaptic plasticity, can be studied with enhanced intensity and prolonged experimental duration.
Also, it seems that the coralline matrix causes an increase in some of the glial cells' cellular functions, such as growth, reactivity, and proliferation. It is therefore possible that these conditions also increase astrocytic capacity to enrich the dissociated neural culture media with secreted trophic factors, something that may be of applicability for regenerative medicine of the central nervous system.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was funded by the KAMIN program of the Israeli Trade and Labor Ministry and by Qrons Inc., 777 Brickell Avenue Miami, FL 33131, US.
Materials
| 24-well plates | Greiner | #60-662160 | |
| B-27 | Gibco | #17504-044 | |
| Bovine Serum Albumin (BSA) | Sigma | #A4503 | |
| D – glucose | Sigma | #G8769 | |
| Dulbecco's Minimal Essential Eagle (DMEM) | Sigma | #D5796 | |
| Electrical sieve | Ari Levy | #3700 | |
| Fetal Bovine Serun (FBS) | Biological Industries | #04-007-1A | |
| First Day Medium | 85.1% Minimum Essential Eagle’s medium (MEM), 11.5% heat-inactivated fetal bovine serum, 1.2% L-Glutamine and 2.2% D-Glucose. | ||
| Flasks | Greiner | #60-690160 | 25cm^2, Tissue culture treated |
| Fluoro-deoxy-uridine | Sigma | #F0503 | |
| Glass Coverslips | Menzel-Glaser | #BNCB00120RA1 | |
| H2O2 | Romical | #007130-72-19 | Hazardous |
| Ham's F-12 Nutrient Mixture | Sigma | #N4888 | |
| HANK'S solution | Sigma | #H6648 | |
| Kynurenic acid | Sigma | #K3375 | |
| L – glutamine | Sigma | #G7513 | |
| Manual strainer (40µm) | VWR | #10199-654 | |
| Minimun Essential Eagle (MEM) | Sigma | #M2279 | |
| Mortar and pestle | De-Groot | 4-P090 | |
| NaClO (Sodium Hypochlorite) | Sigma | #425044 | Hazardous |
| NaOH | Sigma | #S8045 | Hazardous |
| Neuronal Growth Medium | 45% MEM, 40% Dulbecco's modified eagle's medium (DMEM), 10% Nutrient mixture F-12 Ham, 0.25% (w/v) bovine serum albumin (BSA), 0.75% D-glucose, 0.25% L-Glutamine, 0.5% B-27 supplement, 0.1% kynurenic acid, 0.01% of 70 % uridine and 30% fluoro-deoxy-uridine. | ||
| Petri dish | Greiner | #60-628160, #60-627160 | 60mm, 35mm, respectively. |
| Poly D – Lysine | Sigma | #P7280 | |
| Smart Dentin Grinder | KometaBio | #GR101 | |
| Trypsin | Gibco | #15-090-046 | |
| Uridine | Sigma | #U3750 |
Referências
- Pan, L., et al. An in vitro method to manipulate the direction and functional strength between neural populations. Frontiers in Neural Circuits. 9, 32 (2015).
- Wellbourne-Wood, J., Chatton, J. Y. From Cultured Rodent Neurons to Human Brain Tissue: Model Systems for Pharmacological and Translational Neuroscience. ACS Chemical Neuroscience. 9 (8), 1975-1985 (2018).
- Molnár, E. Long-term potentiation in cultured hippocampal neurons. Seminars in Cell & Developmental Biology. 22 (5), 506-513 (2011).
- Silva, R. F. M., et al. Dissociated primary nerve cell cultures as models for assessment of neurotoxicity. Toxicology Letters. 163 (1), 1-9 (2006).
- Timmerman, R., Burm, S. M., Bajramovic, J. J. An Overview of in vitro Methods to Study Microglia. Frontiers in Cellular Neuroscience. 12, (2018).
- Ogata, N., Tatebayashi, H. Primary culture of mammalian brain neurons and its application to patch-clamp recording. Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica. 98 (4), 245-250 (1991).
- Lonchamp, E., Dupont, J. L., Beekenkamp, H., Poulain, B., Bossu, J. L. The mouse cerebellar cortex in organotypic slice cultures: an in vitro model to analyze the consequences of mutations and pathologies on neuronal survival, development, and function. Critical Reviews in Neurobiology. 18 (1-2), 179-186 (2006).
- Weiss, O. E., et al. Modulation of scar tissue formation in injured nervous tissue cultivated on surface-engineered coralline scaffolds. Journal of Biomedical Materials Research. Part B, Applied Biomaterials. , (2017).
- Chen, J., Herrup, K. Selective vulnerability of neurons in primary cultures and in neurodegenerative diseases. Reviews in the Neurosciences. 19 (4-5), 317-326 (2008).
- Potter, S. M., DeMarse, T. B. A new approach to neural cell culture for long-term studies. Journal of Neuroscience Methods. 110 (1-2), 17-24 (2001).
- Kaech, S., Huang, C. F., Banker, G. General considerations for live imaging of developing hippocampal neurons in culture. Cold Spring Harbor Protocols. 2012 (3), 312-318 (2012).
- Watson, P. M. D., Kavanagh, E., Allenby, G., Vassey, M. Bioengineered 3D Glial Cell Culture Systems and Applications for Neurodegeneration and Neuroinflammation. SLAS discovery: Advancing Life Sciences R & D. 22 (5), 583-601 (2017).
- Karimi, M., et al. Microfluidic systems for stem cell-based neural tissue engineering. Lab on a Chip. 16 (14), 2551-2571 (2016).
- Murphy, A. R., Laslett, A., O’Brien, C. M., Cameron, N. R. Scaffolds for 3D in vitro culture of neural lineage cells. Acta Biomaterialia. 54, 1-20 (2017).
- Walker, P. A., et al. Advances in Progenitor Cell Therapy Using Scaffolding Constructs for Central Nervous System Injury. Stem Cell Reviews. 5 (3), 283-300 (2009).
- Pettikiriarachchi, J. T. S., Parish, C. L., Shoichet, M. S., Forsythe, J. S., Nisbet, D. R. Biomaterials for Brain Tissue Engineering. Australian Journal of Chemistry. 63 (8), 1143-1154 (2010).
- Lu, T., Li, Y., Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. International Journal of Nanomedicine. 8, 337-350 (2013).
- Maclean, F. L., Rodriguez, A. L., Parish, C. L., Williams, R. J., Nisbet, D. R. Integrating Biomaterials and Stem Cells for Neural Regeneration. Stem Cells and Development. 25 (3), 214-226 (2016).
- Drury, J. L., Mooney, D. J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 24 (24), 4337-4351 (2003).
- Woerly, S., Marchand, R., Lavallée, G. Intracerebral implantation of synthetic polymer/biopolymer matrix: a new perspective for brain repair. Biomaterials. 11 (2), 97-107 (1990).
- Dillon, G. P., Yu, X., Sridharan, A., Ranieri, J. P., Bellamkonda, R. V. The influence of physical structure and charge on neurite extension in a 3D hydrogel scaffold. Journal of Biomaterials Science, Polymer Edition. 9 (10), 1049-1069 (1998).
- Carballo-Molina, O. A., Velasco, I. Hydrogels as scaffolds and delivery systems to enhance axonal regeneration after injuries. Frontiers in Cellular Neuroscience. 9, (2015).
- George, J., Hsu, C. C., Nguyen, L. T. B., Ye, H., Cui, Z. Neural tissue engineering with structured hydrogels in CNS models and therapies. Biotechnology Advances. , (2019).
- Shany, B., et al. Aragonite crystalline biomatrices support astrocytic tissue formation in vitro and in vivo. Tissue Engineering. 12 (7), 1763-1773 (2006).
- Baranes, D., López-García, J. C., Chen, M., Bailey, C. H., Kandel, E. R. Reconstitution of the hippocampal mossy fiber and associational-commissural pathways in a novel dissociated cell culture system. Proceedings of the National Academy of Sciences of the United States of America. 93 (10), 4706-4711 (1996).
- Peretz, H., Talpalar, A. E., Vago, R., Baranes, D. Superior survival and durability of neurons and astrocytes on 3-dimensional aragonite biomatrices. Tissue Engineering. 13 (3), 461-472 (2007).
- Shany, B., Vago, R., Baranes, D. Growth of primary hippocampal neuronal tissue on an aragonite crystalline biomatrix. Tissue Engineering. 11 (3-4), 585-596 (2005).
- Baranes, D., et al. Interconnected network of ganglion-like neural cell spheres formed on hydrozoan skeleton. Tissue Engineering. 13 (3), 473-482 (2007).
- Morad, T. I., et al. Gliosis of astrocytes cultivated on coral skeleton is regulated by the matrix surface topography. Biomedical Materials. 14 (4), 045005 (2019).
- Green, D. W., et al. A Therapeutic Potential for Marine Skeletal Proteins in Bone Regeneration. Marine Drugs. 11 (4), 1203-1220 (2013).
- Neto, A. S., Ferreira, J. M. F. Synthetic and Marine-Derived Porous Scaffolds for Bone Tissue Engineering. Materials. 11 (9), (2018).
- Ahmad Khalili, A., Ahmad, M. R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. International Journal of Molecular Sciences. 16 (8), 18149-18184 (2015).
- . Visualization of the ultrastructural interface of cells with the outer and inner-surface of coral skeletons Available from: https://www.ncbi.nlm.nih.gov/pubmed/19218486 (2019)
- Drake, J. L. Proteomic analysis of skeletal organic matrix from the stony coral Stylophora pistillata. Proceedings of the National Academy of Sciences of the United States of America. 110 (10), 3788-3793 (2013).
- Ramos-Silva, P., et al. The skeletal proteome of the coral Acropora millepora: the evolution of calcification by co-option and domain shuffling. Molecular Biology and Evolution. 30 (9), 2099-2112 (2013).
- Peretz, H., Blinder, P., Baranes, D., Vago, R. Aragonite crystalline matrix as an instructive microenvironment for neural development. Journal of Tissue Engineering and Regenerative Medicine. 2 (8), 463-471 (2008).
- Morad, T. . CaCO3 Matrix Dictates Astrocytes Transition to Astrogliosis. , (2019).
- Prada, F., et al. Ocean warming and acidification synergistically increase coral mortality. Scientific Reports. 7, (2017).

